Abstract
A range of factors can lead to situations where university courses have to be taught with a very small number of students. In this paper, we report on our experiences of a chemistry course that was especially designed to encourage learning in small groups of students (four to five per course). The course design included inquiry-based approaches, case methodology and problem-based learning concepts. The main goal was to enhance student motivation and to support them to become active agents (‘pro-sumers’). Technology was adopted to support students in their inquiry-based learning processes by using online logs, group wikis and quizzes and with sections of laboratory work. We explored student perceptions during the first 2 years in two courses. The students were, in general, very positive about the course and communicated that the technological tools along with the pedagogical design decisions had assisted them to different extents in their learning. Our conclusion is that such a design is appropriate for an advanced-level chemistry course with small numbers of students, and the course will continue to be given in this form.
Introduction
The “shift from teaching to learning” (Barr and Tagg Citation1995) argues that higher education needs more learner-centred approaches. Instead of offering teacher-centred concepts, such as traditional lectures, student-centred learning approaches design teaching and learning from the student's perspective. Because learning is “an active process of constructing rather than acquiring knowledge” (Duffy and Cunningham Citation1996), higher education courses require concepts in which students learn to become active producers and creators of their own knowledge.
At Umeå University in Sweden, many courses in the later stages of educational programmes are conducted with low numbers of students – fewer than 10 students per course. Such a small number of students is a challenge and calls for rethinking the design for learning. During a restructuring of chemistry courses at the master's level, two pilot courses were identified as subjects for developing more learner-centred approaches instead of having lectures only. This article describes one of these courses. The aim was to maintain high quality and create courses that would inspire learning in small class sizes. The teachers also hoped to turn a rather lecture-centred education into more student-active learning. The pedagogical concept of problem-based learning (PBL) gave inspiration and with the help of the university's pedagogical centre (UPC), a workshop for chemistry teachers was organised to help the teachers think about learning in different ways. During this workshop the methodologies of PBL, inquiry-based learning and case methodology were introduced. The reason for selecting these pedagogies was that they had already successfully been applied, for example in engineering education at Umeå University. Thus, a critical mass of teaching staff familiar with these methods existed that could assist the teacher team if needed.
Working with the UPC, the teachers created a new course design in chemistry in accordance with PBL philosophy. The assumption was that this newly designed course (how it was designed for learning) would support the students in learning, being engaged and being motivated – these goals were the objectives of the course design. We use the student perspectives and data from the course evaluation to study and illustrate whether this kind of PBL course design applied in chemistry for small groups is useful for achieving the course aims and design objectives.
This paper discusses the course design, student responses and student perceptions. The development of a PBL course enhanced by collaborative technology in an advanced-level course in chemistry in higher education is described in detail. The findings from the student course evaluation are discussed.
Theoretical framework
PBL in chemistry
The use of inquiry-based learning, PBL and case methodology in chemistry courses has been described in several publications. For example, PBL has been described by Abate et al. (Citation2000), Hicks and Bevsek (Citation2012), Ram (Citation1999) and case methodology has been discussed by Azzawi and Dawson (Citation2007), Belt, Overton and Summerfield (Citation2003), and Cam and Geban (Citation2011). The existing studies show that PBL can be used to teach chemistry and can affect student learning in a positive way on different levels (e.g., Tarhan and Acar Citation2007; Tarhan et al. Citation2008; Tosun and Taskesenligil Citation2013). For instance, the method of PBL motivates students (Ellis and Gabriel Citation2010; Senocak, Taskesenligil and Sozbilir Citation2007) and helps them to develop particular higher-order thinking skills such as analysing, evaluating and creating, including social skills (Alcazar and Fitzgerald Citation2005; Bilgin, Senocak and Sozbilir Citation2009), whereas the PBL design in general does not focus on lower-order thinking skills such as remembering, understanding or applying knowledge (Anderson and Krathwohl Citation2001).
Other studies show that students performed equally well in assessments when comparing PBL approaches and other forms of teaching and learning (Abate et al. Citation2000; Senocak, Taskesenligil and Sozbilir Citation2007; Williams et al. Citation2010).
One of the benefits of PBL is that it can support students to make a shift from not only being consumers of content such as in traditional lectures but also becoming producers of knowledge (‘pro-sumers’). In addition, it has been reported in several studies that PBL is inspiring and enjoyable for both students and teachers (Gurses et al. Citation2007; Kelly and Finlayson Citation2009; Ram Citation1999). It also prevents students from losing motivation in their studies.
The benefits reported for PBL approaches led to a change of course design. The overall aim in changing the traditional course towards a more PBL-designed course was to see whether, to what extent and in what ways enquiry-based learning works in a chemistry course on advanced level that contains and combines large modules of theory connected to experimental chemistry laboratory work. In relation to what has previously been published on this subject, we aimed to investigate the effect on small student groups and how technology could be used to assist the students in their learning. The main research questions were as follows:
To what extent is a mix of PBL, inquiry-based learning and case methodology an appropriate design for ‘hands-on’ chemistry classes at the master's (MSc) level for helping students in their learning process?
Does the course design for learning encourage and motivate students to become active agents in very small classes? What benefits and challenges emerge?
How is technology useful to enhance this type of student-active learning?
Digital didactical design as a foundation to plan a course
To create, plan and conduct a course that focuses on designs for learning rather on teaching designs, different elements require change (Jahnke Citation2016; Jahnke, Norqvist and Olsson Citation2014; McCormick and Srimshaw Citation2001). The five main elements of every course in higher education that turns from a teacher-centred into a learner-centred model are as follows.
Teaching aims and learning intentions
The teacher uses the curriculum to define the intended learning outcomes and brings the curriculum into life in his or her teaching practice. In such a course, the teacher creates a learning intention with regard to a problem that the students need to work on. This is also the foundation for the evaluation of the course in the end: Could the students achieve the intended learning outcomes by engaging with the problems? Were the teaching aims clear to the students or were they hidden?
Learning activities
The teacher creates a plan for the entire course, including assignments and timelines. He or she creates a plan for when the students will be active and what resources are required. The teacher also decides about individual and collaborative learning phases.
Process-based assessments
Before the course starts, the teacher creates a plan describing when, and how often, to give feedback. Process-based assessments support students in becoming better learners and improving their learning progress. The teacher plans for process-based assessment activities such as reflections on learning activities that might be useful in the beginning, middle and end of a course. This planning includes the organisation of peer-reflective learning, feedback given by the teachers and student self-assessment. The feedback is essential for letting the students know what they have achieved so far and what they need to work on.
Social relations and roles
The teacher plans how to support social interaction such as student–student communication. Usually, the students have learnt the role of consumers and how to consume course content, but in a PBL course they need to learn to become producers of knowledge. Because some students may not know how to become engaged in this way, the teacher plans assistance, for example, through collaborative activities and group assignments. Students may often have limited experience in handling the role of active agents, and they may have trouble with the group dynamics in learning situations. For this purpose, PBL can be useful if and when it gives clear instructions for students.
Technology integration
The benefit of technology, for example in the form of learning management systems (LMS), wikis or online blogs, is that all members of a course are able to see and read what the others did – in other words learning as a process is made visible: students produce, learn and share. Students often do not know that their own problems might be ‘normal’ problems that all students face; however, technology can be used to support students in sharing their awareness about different types of problems and how to solve them.
Research methods and context
When university teachers work together with academic developers and researchers in order to redesign existing courses or to create new courses, design-based research (DBR) is a useful methodology (Reeves, Herrington and Oliver Citation2005; Wang and Hannafin Citation2005). DBR consists of several phases of designing to improve learning and continuous reflections by the teachers about their actions during the course, as well as reflections supported by research data, during the course and when the course is finished.
For the current project, the data came from different sources such as student evaluations and teachers’ observations. The data-gathering methods included subjective data from the student course evaluation with standardised and open questions, as well as informal participant observations by the teachers involved (Bauer and Gaskell Citation2000; Bryman Citation2008). In addition, objective data were used, such as data from student learning outcomes, performances and assessments that showed how the students fulfilled the learning outcomes of the different assignments during and after an advanced chemistry course given during 2 consecutive years. The data, which were collected during and at the end of the course, informed the teachers how appropriate their ideas, specific teaching methods and learning approaches were and how they supported student engagement and student learning.
The course evaluation sheet for students
In 2012 the online student course evaluation consisted of 65 standardised questions, including 38 open questions (Likert scale 1–5; 1=lowest value, 5=highest value, 0=not applicable/not used); see Supplement for a complete list of all questions.
The evaluation sheet contained 12 parts.
Part 1 was about the course in general, for example, “How do you rate the overall quality of the course?” (Q1–Q5).
Part 2 consisted of items focusing on the intended teaching aims, learning outcomes and student expectations (Q6–11); for example, “Was the aim clear to you?” and, “Did the content of the course match its aim?”
In part 3, the questions focused on the course material, the content and its quality (Q12–15).
Then, in part 4, questions were asked about the technology integration, such as the quality of the Moodle site and the interactive tools (Q16–19).
Part 5 focused on feedback, formative assessment and examinations, for example, “How do you rate the supervision you received during the course?” (Q20–24).
Part 6 contained Q25–Q32, which asked about the perceived quality of different course modules, for example, the “organic trace analysis module” and the “surface analysis module.”
Part 7 contained questions about the PBL in detail (Q33–38), for example, “Did the introduction to problem based learning and case methodology the first day help you during the course?”
Part 8 (Q39–Q41) consisted of items concerning the social relationship and group dynamics, for example, “Did group dynamics and group roles affect your work and your learning?”
Part 9 included questions about the student individual reflections (self-reflections guided by the teachers), such as, “To what extent did the individual log help you to critically evaluate new knowledge?” (Q42–48).
Part 10 focused on group work and student learning activities, lab work and group feedback (Q49–56), for example, “Did the group wiki help you to go more into depth in your learning?”
Part 11 asked specifically about the final project, which the students completed as the final assignment (Q57–Q60).
Finally, part 12 was about possible improvements: “How can we improve the course” and “What should we definitely not change?” (Q61–65).
In the second year, three new questions were added related to how motivating the new design was for the students.
Ethical considerations
The student course evaluation is a well-established method for the development of every course at the university. The students are aware that such data is used by teachers and departments to reflect on teaching and learning for different reasons, for example, creating a new course design, learning from existing courses. The data are always anonymous. The course evaluations are generally summarised and published on the university course website to enable students to see what their peers thought about the course. In order to protect the students, in this article, we do not use any comments that could be linked to a specific person.
The environmental analytical chemistry course
The higher education course Environmental Analytical Chemistry (EAC) was conducted in the autumn semester of 2012 (10 weeks) and a second time in 2013 (10 weeks). The course was given as a full-time class, which means that the students were expected to dedicate 40 hours per week to the course, divided between independent work, scheduled assignments and group time (). The course had not been offered previously using any other teaching pedagogy.
Figure 1. Outline of the EAC course in 2012. The course started with five modules where the theory of the course was given. The course ended with a final lab project that started with a planning session followed by lab work. Activities where teachers were present were as follows: course introduction lectures (I), base group meetings (B), case seminars (C), laboratory work (L), feedback session about lab report writing (G), question sessions (F), workshops (Ws) and oral presentations by the students (O). Deadlines for lab reports (D), computer lab sessions (W) and course evaluations (E) were also scheduled. Apart from these activities the students needed to manage their time to enable time for their own theory acquisition, preparing scheduled activities and writing assignments (log, group wiki, lab reports) (blue areas of the course schedule).

Course outline and information
The course consisted of five subject modules () covering the content: sampling, organic trace analysis, inorganic trace analysis, modelling and surface analysis. These five modules gave the theoretical and practical subject content and were followed by a large final project in which the students applied the knowledge they had been learning.
The students were divided into smaller working groups, called ‘base groups’, where they collaboratively worked with the subject content of each module. Each module had its own PBL scenario, which was discussed during three base group meetings, and the scenarios overlapped, so that at the end of each base group meeting the next scenario was introduced.
Individual theory acquisition from the course literature and streamed lectures was complemented with practical lab exercises and/or case scenarios as shown in . Participation in lab work, activity at case seminars, activity in base group work, lab reports and completion of the individual log and group wiki were compulsory and formed the reporting and assessment structure of the course.
The course was given in the second year of the master's programme, the first half of the autumn semester, and represented the last course before the final master thesis project for the majority of students. The master courses were, with one exception, taught with traditional pedagogy with classroom lectures. However, before starting the EAC course some of the students had taken a course in environmental chemistry that also used PBL as a teaching method (Jansson et al. Citation2015).
The course had eight students in total over the 2 years, from seven different nations worldwide (four European countries, including Sweden, and three Asian countries). One student base group was formed each year that also functioned as lab group for the final project. The course language was English. In total, five of the students had, to our knowledge, been exposed to PBL as a teaching strategy before starting the EAC course. There were female and male teachers in both of the years and the student group consisted of both male and female students. One teacher held all base group meetings and gave feedback on the individual logs both years. The lab exercises, case seminars, group wiki and lab reports were led and evaluated by one to three teachers (university lecturers) per module, depending on expertise. The whole course was led by a teacher team of three teachers plus the course leader. To protect the identity of the students, the exact numbers of male/female students and their exact countries of origin are not mentioned here. During and after these 2 years, the data collected were used to analyse the student perspective.
Teaching objectives and expected learning outcomes
The core of the course is to develop competencies in performing an environmental study in chemistry, more specifically, an environmental study of a contaminated site that includes sampling and analysing different types of contaminants, and a critical evaluation of data. The study also includes writing a report. Students learn to evaluate and discuss different sampling and analysis methods and to choose the appropriate methods for different contexts. At the end of the course they should independently create their own environmental study and carry it out. Thus, the course includes many skills labelled as higher-order cognitive skills, such as analysing, evaluating and creating a study (Anderson and Krathwohl Citation2001).
Learning activities organised into four steps
The learning activities were designed to support the students in their process of active learning. Within the course, the activities were divided into four steps (explained in more detail below): step 1, assignments to construct the theory; step 2, practical experience in standard experiments; step 3, assignments for applying, evaluating and analysing theory and data; and step 4, synthesis.
To support group and open learning, the open source Moodle LMS was introduced. It helped the students visualise the modular structure of the course and enabled them to easily navigate between course material and their tasks. Content such as information, material, e-books, streamed lectures, and so forth was available through the LMS. All writing assignments such as individual and group wikis, quizzes and anonymous surveys (course evaluation), and reports were completed, uploaded and/or graded through the LMS. During the uploading process, the students’ drafts and products were automatically sent through a web-based service that checked all documents for possible cases of plagiarism. The LMS also made it possible to monitor the deadlines for lab reports and to make material available for students at specific time points. Thus, the LMS assisted the logistics of the course.
Learning activities, step 1 – Assignments to construct the theory
The course organised base group meetings on campus twice a week (). In step 1, the theories were introduced together with skills that the students needed later to conduct the experimental study. Throughout the entire course, the students wrote an individual log, as well as a group wiki to collect and cover the material of the course and to reflect on the course content.
The role of base group meetings
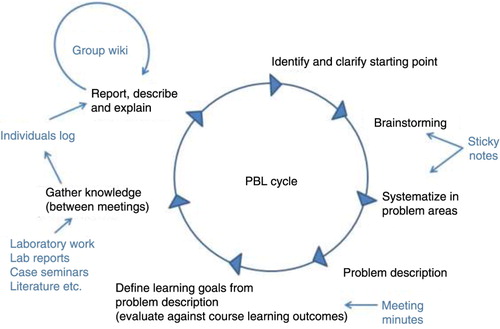
At the very first base group meeting the students discussed, agreed and made a contract of how they would work and treat each other in the base group based on a design that we inherited from other teachers at our university. Each base group meeting was started by the base group supervisor (a university lecturer), who functioned as chairperson for the meeting. The supervisor only rarely intervened, for example, asking questions that could challenge the students to probe deeper into their understanding. She did not participate in the base group work or answer questions about the theory. The same lecturer functioned as base group supervisor throughout the course both years. Each meeting, one student was selected as secretary and was responsible for taking notes and posting them in a ‘base group minutes’ wiki in the LMS. gives an overview.
Figure 2. How different learning activities and technical tools were used to support the PBL process cycle. Sticky notes were used in the silent brainstorm and during the process of systematising the outcome from the brainstorming session. In the base group minutes the students recorded their learning goals and what they had agreed upon as a group during the base group meeting. Case seminars, lab exercises and lab reports were constructed to support the students in gathering and evaluating knowledge. In their individual logs they were to report their new knowledge and reflect on it. In the group wiki they were to process the new knowledge in the group to enhance learning and promote higher order learning in learning taxonomies (modified PBL cycle from Hård af Segerstad et al. Citation1997).
Individual log for seeing the learning progress
Each log entry in the individual log was designed to contain two parts. The first part was a summary of the most important things in the literature, streamed lectures and other materials that the student had been working on between the base group meetings. This part of the log was used later, during the base group meetings. The second part of the log entry contained a reflection on the material, for example, whether different sources agreed, whether the sources were reliable and so on. To guide the students, a set of questions and instructions were developed. In addition, informational material was provided, such as the structure of the log, why students should keep the log and for whom they were writing, and what was expected to pass this part of the course (Varner and Peck Citation2003). Each log was open to read and visible to all students in the course, as well as the course teachers.
The individual log was designed to support the students in becoming active agents. This was relevant because it has been reported from existing studies of PBL courses that students feel insecure in their active role; for example, they are concerned that they are not learning the ‘right’ things and they are not sure that they are learning anything at all when a teacher is not presenting them with the ‘right’ material (Hård af Segerstad et al. Citation1997).
This log was also expected to help teachers; they could follow the students’ learning progress and notice if students went too far off the course goals. This gave the teacher time to think of questions that could guide the students in the right direction during base group meetings, if necessary. The log was also designed to help the teachers to see what types of material the students were using in their knowledge building process. The reflection in the log also provided some feedback to the teachers about the progress of the course. The individual log was compulsory and served as part of the assessment of the course. It was graded ‘pass’ or ‘fail’ for each student. The assessment was made based on the content of the log: it was to contain entries about the theory covered, a reflection about the students’ own learning and knowledge-building process, as well as a critical evaluation of the sources used and the theory gathered.
Group wiki for collaborative learning
In the group wiki the students were assigned to gather, summarise and discuss the material they covered during the course in a written document. The aim of the wiki was to prompt the students to deepen their understanding of the course subject and to better integrate what they had learned during the work individually and in the base group. The structure was left very open to enable students to be creative and give them freedom to decide how to write the group wiki. The students received the information that the wiki was there to help them summarise and discuss their newly found knowledge, draw conclusions and record it for future use, but that they should not copy and paste material from their individual logs. The group wiki was graded at the end as a summative group assessment with specific feedback on parts that the teachers wanted to stress. However, some formative feedback was given continuously throughout the course orally during base group meetings, with a focus on the theory content. The individual input from the students into the wiki was not assessed, nor was the wiki process. To support this group wiki and other writing assignments in 2013, we designed an obligatory plagiarism quiz in the LMS. This quiz was constructed to illustrate for the students several clear cases of plagiarism as well as subtler variants, and it was aimed at helping the students understand the concept of plagiarism so that they could avoid it.
Learning activities, step 2 – Practical experience in standard experiments
To prepare the students for the practical experience needed later in step 4, the students had lab classes where they performed predefined experiments on different types of advanced analytical instrumentation covered during the course. Before performing the laboratory work, students read through a booklet and completed an obligatory lab safety quiz created in the LMS Moodle. The laboratory work during the first five modules was classical lab exercises designed by the teachers to give the students a way to practise and learn the range of methods they could select from and use in the final project at the end of the course (, step 4 –synthesis below).
Learning activities step 3 – Assignments for applying and analysing theory and data
In order to document and reflect their practice in step 2, the students wrote individual and extensive lab reports after each laboratory exercise. For this activity a template was prepared for them, inspired by templates available from scientific publishers. The students also had two case seminars, in which they practised evaluating different sampling and analysis methods, and they were encouraged to use the group wiki with the aim of discussing the theory that they developed in step 1. After the first report the students got detailed feedback about how lab reports should be written and structured in order to assist those students that were less used to this type of writing. Each lab report could be seen as a summative individual assessment of one module but as a formative assessment in the method of constructing and presenting written lab reports.
Learning activities step 4 – Synthesis
The course ended with a final project aimed at synthesising all parts of the course into an environmental study that the students planned and conducted themselves as a group (Ram Citation1999). The students were given a problem site and had to plan, design and execute an environmental study, using appropriate sampling, sample handling and analysis techniques. Thus, the final project functioned as a formative assessment. As a group, the students evaluated the analysis techniques previously covered and decided which ones would be most suitable with regard to the environmental study objectives, expected contaminants, available time and budget. The students created a timetable, booked the instrumentation for carrying out the study and later also performed all steps by themselves. The base group supervisor and another teacher gave feedback after the first draft of the study plan. In the report, the students were requested to justify the choices they made regarding analysis methods and give a recommendation for further treatments addressed to (imaginary) city council staff. The hope of the teachers was that such a real-life context would be inspiring to the students and make them perform at their best.
Findings and discussion
Over the 2 years, five out of eight students passed the course (both students that had used PBL previously and students that had not). The first year's group collaborated to a much larger extent than the group in year 2. The students in the first year also, in general, appeared to be much more positive to the course and all of its parts. We present the detailed findings with a focus on the student perspective. More specifically, we were interested to see to what extent technology-enhanced PBL was suitable for this type of course in chemistry education.
Quality of the course and student expectations
Considering the low number of students during the 2 years but a response frequency of 62% (five out of eight students replied), the course evaluation can be seen as an indication of a general student response to the new course design and the changes that the teachers made. The data serve as an indicator of improvement and support a positive change from a traditional course towards a more learner-centred education.
When asked how they would rate the quality of the course (from 1 to 5, where 5 is the highest rating) four students rated it a 4 and one gave it a 5. The students rated the specific modules between 4 and 5 with an average of 4.4 over the 2 years (five modules). One student commented: “All modules were really good. PBLs and labs were complementary, which made learning easier. The final project was quite hard.”
Regarding what expectations of the course had been before starting, one student said, “Actually my expectations were high, because I had some info from students which studied in Umeå before me.” Moreover, to the follow-up question of whether the course lived up to expectations this student answered, “I was surprised, it was even better than I expected. For me: pass with distinction.” To the question about prior expectations, another student answered, “I thought that this course would be taught ‘normally’, i.e. having lectures and labs. But I think PBL worked fine on this course,” and, to the question of how the course lived up to these expectations, “really good, even though it was sometimes quite stressful to write the reports on time.”
It was also interesting to see that the students rated their own workload between 35 and 50 hours per week the first year and between 30 and 60 the second year. The numbers are close to a full work day, with 8 hours per day over 5 days. The higher hours indicate that some students worked very hard during the course or that they overestimated their effort. A time investment of 60 hours per week would correspond to a bit more than 8.5 hours per day (including on weekends); such an intense work effort should have resulted in excellent results, unless the student spent a lot of time covering content that he or she was not expected to learn for this course.
Overall, the comments that the students gave in the course evaluation were positive. In answer to the question about what they felt should definitely not be changed in the course, the students mentioned the following: the course outline, lab sessions, the final project, the online books and PBL. All the students that filled out the survey stated that they would recommend this course to a friend.
“More time is needed”
There were some negative responses. To the question of how the course could be improved, two students gave suggestions for improvements in the lab section, such as more time between labs, to have more time for writing the final report. Other comments were about time limitations, and one student identified problems with the large amount of literature. One student suggested more feedback from teachers on the lab reports. Some of these comments have been reported as commonly raised in PBL (Hård af Segerstad et al. Citation1997). Although these results are not new, teachers face the challenge of how to improve this situation. A difficult task for teachers is to estimate how much time the students will need. One possibility is to reduce the amount of literature, but there is currently no chance to allocate more time to the entire course. A solution could be to adjust deadlines during the course in order to give the students more structure for their self-organised writing assignments; this change might help them to solve the assignments more efficiently in the same amount of time.
When students change from consumers to producers
The data show that when students have to change roles from consumer to active producer, it is not always easy for them and they may miss support. An example of such a wish for support is that they asked about more teacher-led time. For example, one student wrote, “Maybe add some lectures.” However, this student's wish for more teacher-led time cannot be solved by including more lectures, which would change the students’ role back to consumer. A solution could be for the teachers to create coaching sessions where they ask questions such as, “How was the learning process so far? What did you learn so far?” Teachers may also give hints to students, such as, “It is normal in research to change your experimental design; you have ups and downs and go back and forth.” A positive feedback culture is helpful to guide and support student learning. The PBL concept, in general, needs a more elaborate design for “the social relations and roles,” as mentioned in Digital Didactical Design by Jahnke (Citation2016). For future PBL courses, that means that the design for social relationships, which is often neglected, needs special design attention.
In the EAC course, the overall student performance during different assessments suggests that the majority of students did manage to perform the PBL tasks. They learnt how to gather information and extract what they needed from it. However, when it comes to group work, the data show a more mixed picture and some problems. For example, one student said that he or she did not see a benefit from the group work but preferred to work on his or her own, whereas others appreciated the input from other students. Despite this, the student that preferred to work alone still completed the group assignments. This suggests that teachers might need to give more positive feedback on group efforts, encouraging group work and showing the benefits of working in a group to increase motivation.
It is also worth noting that students who are less well-trained to handle larger amounts of literature in limited time periods, for example due to reading difficulties, might be discriminated against by the PBL design compared to traditional lectures, where they can rely on listening. This possibility suggests that it is important to vary the forms in which information is presented; instead of using text material only, streamed lectures could be used, giving a mix of different forms. The benefit of having several types of information available was highlighted by one student.
Different forms of assessment and student motivation
In the second year, the students were asked whether the PBL learning approach was more or less motivating compared to traditional lectures. Two students replied to this question and both stated that the PBL scenarios and case seminars had a motivating effect. In the first year, some students made entries in the reflective part of their individual log and in course evaluations suggesting that they found the final project motivating. The students also expressed that they had learned other skills from PBL such as working in a group and managing to overcome group conflicts. These skills were listed by some students as the most important experience during the course. What often seems to motivate students to study during a course is the assessment and, consequently, it might be expected that the form of assessment would influence how students work with course material. The students expressed that they were in favour of having different types of reports (lab reports, group wiki and individual logs) as different forms of assessment during the course compared to traditional essay questions, which are generally only given at the end of a course. (The assessment followed the positioning of the lab exercises: in the first year, the students were examined approximately once every one to 2 weeks, whereas in the second year most of the assessment occurred in the second part of the course.) The students also thought that the reports were a fair assessment of what they had learned during the course and that it gave them a chance to practise using their knowledge in a more real-life context. One student wrote: “The examination was in the form of reports. It is a good way to show what we have learnt and how we can use this knowledge in a real situation.” The students were also asked to rate the assessments as a learning opportunity and four out of five of the students rated them 4 out of 5 (5=highest).
Technology-enhanced learning for chemistry courses?
In response to questions about the reading material and information found in the LMS, the students gave high marks for the course material and the majority commented that they found all they needed there. One student also added the following comment: “It was good to have textbooks available on Moodle. Probably I wouldn't have found them by myself, while searching information.” This comment showed that the online support through the platform for finding good source material was appreciated, even if this consisted of a fairly large amount of textbooks and journal articles. Another student wrote that the information was enough and if more was needed he or she could search for it on the Internet. Only one student stated that there was not enough material in Moodle and that the material he or she had found was not focused enough on the subject: “The time spent on finding information and compiling it was long – too long. Several thousands of pages of nonsense had to be sieved through before a grain of truth could be extracted.” It is interesting to note that this was the same student that preferred to work alone. However, this student appreciated the textbooks that were present as online books and, in answer to the question of what should not be changed in the course, indicated that the textbooks should be kept. This could reflect a difficulty with extracting information from journal articles and other types of material that were not especially written for educational use at the undergraduate/graduate level. The online books available on our course site were written as ‘open-learning’ books, with the explicit intention that they should be easy to access and use as self-study books.
Individual log
One student mentioned that he or she learned a lot from the other students’ logs, and this student also expressed this perception in one base group meeting. In the final evaluation the individual log got high ratings (4 or 5) from the majority of the students on the following points: whether the log made the learning more visible to the student (average 4.2), whether it helped the student reflect on their learning (3.8), whether it helped them to critically evaluate new knowledge (3.6), how useful it had been (4.2) and whether it helped in the group learning process (all answered ‘yes’ except for one student). In answer to the question of whether the log helped them in their learning, one student wrote, “Totally! It helped me to summarise my thoughts, I also learnt how to work with literature, how to do proper quotes etc.” Two other students answered, “Yes, they [the log entries] forced me to do my reading and writing before going to class,” and “It helped, because I had to write and present things I had learned. Re-constructing of new knowledge is really hard, but learning is more structured.” This suggests that apart from assisting the students to learn theory content, the log also gave them practice in referencing and reference handling connected to information retrieval. In the individual logs, sources were assessed critically but not referencing. However, correct referencing was assessed in the group wiki, which led the students to carefully record sources in their individual logs as well.
There were a few challenges with the individual log. One student made an additional comment that it was good but had sometimes been a bit stressful, which probably reflects that all the writing tasks during the course took time for the students. One student expressed that the log had not been at all helpful in any aspect and gave very low ratings for all questions concerning the log. However, no explanation was given. From the course evaluation it appears this student preferred to work on his or her own, so perhaps the purpose of writing a log that in part had the purpose of sharing knowledge in the base group was not appealing to this student. As a counterpoint to this view, summarising literature content would still have been useful for a student who prefers to work by himself or herself. Consequently, we as teachers need to make the benefit here clearer to the students, for example, by stressing the added benefits of the collected critically reflected knowledge. Instructors could make it clear that this is the first step, completed in preparation for the next step, that is, that after collecting data individually in the logs students would later share their data and combine them in the group wiki to create a larger whole; both these processes are tools constructed for learning the course content.
Group wiki
The group wiki got high ratings from many of the students, with statements describing how it helped the group to merge ideas and knowledge. The students wrote, “It was very helpful to evaluate all our knowledge to one output,” and “The information in the group wiki is written in discussion within our group so it is more reliable.” Two students claimed that the group wiki had helped them go further in-depth with their learning, whereas a third student stated that it had not added more than the individual logs. The student who preferred to work alone wrote, “No. I did all the learning by myself.” This is an interesting difference and might reflect that the students were at different levels of understanding. For some students the process of summarising the logs seems to have helped them to learn more and feel more confident that they were learning the ‘right’ material. However, others seem to not have needed or even wanted this help – again, illustrating that the different learning activities were not equally appreciated by all students.
Combining the comments for the different tools constructed to support the students, it can be concluded that the sentiments towards the individual log and group wiki were mixed but that the large majority of students found both tools useful and that these tools were supporting them in their learning. Moreover, it was probably not just the tools themselves but how the tools were integrated into the learning activities, the self-reflections and group reflections that constructed the student learning experience. The integration of technology and the assignments for the learning activities seem to have merged into a positive student group experience, serving as a step towards a student-led classroom. From comments it seems that the individual log was slightly more appreciated than the group wiki by some students, perhaps because the individual log was used for a concrete purpose close in time, that is, to share information at the start of the group meeting. The purpose of the group wiki was not clear enough to the students, which we intend to change the next time the course is given. Consequently, the motivation for students to work on the group wiki is something we will work on for the upcoming course sessions, in order to better define the purpose of the group wiki to the students (apart from just being a type of course assessment). From the course evaluations it is evident that several students appreciated the sense of security that the combined work effort gave them when they collected and assembled the group wiki as a team. We as teachers could perhaps make this benefit more visible to the students. Another thing that could be pointed out is the possible future use of the document created. As one student commented in year 1: “It is good way to summarise knowledge; it is also good for future use.” Interestingly, in the first year, all students answered that they had been helped by the group wiki during the final project, whereas the students in year 2 did not consider it to have been helpful during the final project. Perhaps the students in year 2 could have been helped in their motivation by a description of what previous students had thought of the group wiki and how they had also used it for the final project.
This method of working with the course material in a PBL course might not suit every student, although it was helpful to and appreciated by the majority of our students. There is no method that suits all students; there will, likely, always be some students who are less satisfied and who do not feel supported enough. However, with PBL we anticipate that we can reduce dissatisfaction to a minimum and make it an enjoyable and attractive learning experience for almost all students.
Was the technology-enhanced PBL design suitable for this type of course?
Analysis of the written reports and the different forms of assessment shows that the students developed competencies towards the intended teaching aims: The first learning aim was that the students be able to individually perform an environmental study of a contaminated site, including sampling and analysis of different types of contaminants and critical evaluation of data, as well as report writing. Six of eight students reached this goal. The second learning aim was that the students learn to individually evaluate and discuss different sampling and analysis methods and chose the appropriate methods for different contexts. Five of eight students reached this goal. Finally, the overall learning aim was that at the end of the course the students, as a group, be able to independently create their own environmental study of a contaminated area and carry it out. All students reached this goal. All students achieved the overall intended course aim – the students developed the skills and competences necessary to plan and conduct an environmental study. This result points to a positive result of the course design and suggests that PBL is an appropriate learning concept for this type of a chemistry course.
The whole course was aimed at giving the students the knowledge to make, plan and conduct an environmental study. Their learning outcomes and attitudes towards the final project is therefore one important key to answer the question of whether PBL is suited for this type of chemistry course, and here the student course evaluation provides a more detailed picture than the written reports. The final project was appreciated by all students; however, many of them expressed that they had needed more time. All the students gave very high marks to the quality of the final project as an assessment: three students rated it 4, and two students gave it a 5. One student commented that the final project was quite difficult and another that the final project had been similar to a real research project: “In this course we did not have a final paper examination. Instead we did the final project together. I think the final project was good. We had to do everything from A to Z. We had to plan everything, based on the budget; we evaluated and chose the analysis methods, sampling plan, analysis and then wrote a report and gave an oral presentation. What we did felt exactly like real research.”
Problematising the generalisation of the results
Because the course was given in a highly international context with participants from several different countries, we do not consider this study to be specific but rather as inspiration for all subjects within higher education.
Critically, it should be stated that the number of students was very low. Furthermore, not all students filled out the course evaluation at the end of the course. It should also be noted that the comments from the students were given from a student group with large differences in cultural background (Europe and Asia). This difference could have affected how the answers were given, if students were less used to working with course feedback and were too polite to bring forward negative criticism.
However, because this study was not conducted in a laboratory but out in the real life of a university course and the course has already been given twice with this design, we argue that the case illustrates how chemical education can be modified towards learner-centred concepts such as in a PBL course.
Conclusions
The results of this study of redesigning an advanced-level chemistry course show that the students felt motivated and were engaged by the PBL design; they appreciated the real-life context embedded into the course, including the assignments constructed to support their learning activities and the different forms of assessments. All the students indicated that they would recommend the course to a friend. The students expressed that the adopted social-interactive technology enhanced their learning, made their learning more visible to them, guided them to solve learning problems in new ways (e.g., by summarising knowledge in the individual log and group wiki) and led them further to develop skills such as reflection and source criticism.
Although the number of students was very low, the course, which was conducted over 2 years on two separate occasions, illustrates how chemical education can be modified towards learner-centred concepts such as in a PBL course. The study suggests a new way to support students in their role change from that of a consumer with a passive attitude to a more active producer role. A role reflection and role discussion could perhaps be integrated in the individual log and group wiki for the future, to help make this change more visible to the students.
To conclude, technology-enhanced PBL could successfully be used to teach the course content and assist a small group of students in their learning while simultaneously motivating them. From this study, PBL seems to be a very beneficial way to support learning in small groups and in subjects such as advanced analytical chemistry courses covering large amounts of practical laboratory work. Consequently, we will continue to give the course following this design, improving it slightly as mentioned. We anticipate that the knowledge acquired from this process will be transferrable to other courses at the department.
Additional information
Notes on contributors
Madeleine Ramstedt
Madeleine Ramstedt Associate Professor, has worked as a research group leader and teacher at the Department of Chemistry, Umeå University, since 2009. She has been actively involved in introducing new teaching methods at the department and developing master’s degree education programmes. She has been the course leader of the Environmental Analytical Chemistry (EAC) course.
Tomas Hedlund
Tomas Hedlund Associate Professor, is a senior lecturer at the Department of Chemistry, Umeå University, having received his PhD degree in 1988. His research is mainly focused on metal speciation in environmental systems. He has been programme coordinator for the engineering programmes Chemical and Bioresource Engineering, and is actively involved in teaching and developing the EAC course.
Erik Björn
Erik Björn Associate Professor, has worked as a research group leader and senior lecturer at the Department of Chemistry, Umeå University, since 2009. He is active in teaching and teaching administrative work including course coordination, development of master’s degree education programmes and examination. He has been actively involved in teaching and developing the EAC course.
Jerker Fick
Jerker Fick Associate Professor, has worked as a researcher and teacher at Department of Chemistry, Umeå University, since 2004. He has been actively involved in teaching several courses and has developed a new course in pharmaceutical analytical chemistry. He has been actively involved in the teaching and development of the EAC course.
Isa Jahnke
Isa Jahnke Professor, is leader of the research group Digital Didactical Designs at Umeå University in Sweden. In 2015, she started as research director of the Information Experience Lab and associate professor of information science and learning technologies, iSchool/SISLT, at the University of Missouri, Columbia, MO, USA. She was assistant professor at TU Dortmund University (2008–2011).
References
- Abate M.A., Meyer-Stout P.J., Stamatakis M.K., Gannett P.M., Dunsworth T.S., Nardi A.H. Development and evaluation of computerized problem-based learning cases emphasizing basic sciences concepts. American Journal of Pharmaceutical Education. 2000; 64: 74–82.
- Alcazar M.T.M., Fitzgerald V.L. An Experimental design to study the effectiveness of PBL in higher education, in first year science students at a University in Peru, South America. College Quarterly. 2005; 8(2): 1–19.
- Anderson L.W., Krathwohl D.R. A taxonomy for learning, teaching, and assessing: A revision of Bloom's taxonomy of educational objectives. 2001; New York: Longman.
- Azzawi M., Dawson M.M. The effectiveness of lecture-integrated, web-supported case studies in large group teaching. Bioscience Education Journal. 2007; 10(article 4): doi: http://dx.doi.org/10.3108/beej.10.4.
- Barr R., Tagg J. From teaching to learning. A new paradigm for undergraduate education. Learning from change: Landmarks in teaching and learning in higher education from Change magazine. 1995; 198–200. Sterling, VA: Stylus Publishing.. D. DeZure (ed.).
- Bauer M., Gaskell G. Qualitative researching with text, image and sound. 2000; London: Sage.
- Belt S., Overton T., Summerfield S. Problem solving case studies in analytical and applied chemistry. New Directions. 2003; 1(1): 12–15.
- Bilgin I., Senocak E., Sozbilir M. The effects of problem-based learning instruction on university students’ performance of conceptual and quantitative problems in gas concepts. EURASIA Journal of Mathematics, Science & Technology Education. 2009; 5(2): 153–164.[PubMedAbstract] [PubMedCentralFullText]
- Bryman A. Social research methods. 2008; Oxford: Oxford University Press.
- Cam A., Geban Ö. Effectiveness of case-based learning instruction on epistemological beliefs and attitudes toward chemistry. Journal of Science Education and Technology. 2011; 20(1): 26–32.
- Duffy T.M., Cunningham D.J. Constructivism: Implications for the design and delivery of instruction. Handbook of research for educational communications and technology. 1996; New York: Simon and Shuster Macmillan. D.H. Jonassen (ed.), 170–198.
- Ellis R., Gabriel T. Context-based learning for beginners: CBL and non-traditional students. Research in Post-Compulsory Education. 2010; 15(2): 129–140.
- Gurses A., Acikyildiz M., Dogar C., Sozbilir M. An investigation into the effectiveness of problem-based learning in a physical chemistry laboratory course. Research in Science & Technological Education. 2007; 25(1): 99–113. [PubMedAbstract] [PubMedCentralFullText].
- Hård af Segerstad H., Helgesson M., Ringholm M., Svedin L. Problembaserat lärande. 1997; Stockholm: Liber AB.
- Hicks R.W., Bevsek H.M. Utilizing problem-based learning in qualitative analysis lab experiments. Journal of Chemical Education. 2012; 89(2): 254–257.
- Jahnke I. Digital didactical designs. Teaching and learning in CrossActionSpaces. 2016; New York: Routledge.
- Jahnke I., Norqvist L., Olsson A. Digital didactical designs of learning expeditions. Open learning and teaching in educational communities. The 9th European Conference on Technology Enhanced Learning, EC-TEL 2014. 2014; New York: Springer. 165–178. C. Rensing et al. (eds.).
- Jansson S., Söderström H., Andersson P.L., Nording M.L. Implementation of problem-based learning in environmental chemistry. Journal of Chemical Education. 2015; 92(12): 2080–2086. doi: http://dx.doi.org/10.1021/ed500970y.
- Kelly O., Finlayson O. A hurdle too high? Students’ experience of a PBL laboratory module. Chemistry Education Research and Practice. 2009; 10: 42–52.
- McCormick R., Srimshaw P. Information and communications, technology, knowledge and pedagogy. Education, Communication and Information. 2001; 1(1): 37–57.
- Ram P. Problem-Based learning in undergraduate education. Journal of Chemical Education. 1999; 76(8): 1122–1126.
- Reeves T., Herrington J., Oliver R. Design research: a socially responsible approach to instructional technology research in higher education. Journal of Computing in Higher Education. 2005; 16(2): 97–116.
- Senocak E., Taskesenligil Y., Sozbilir M. A study on teaching gases to prospective primary science teachers through problem-based learning. Research in Science Education. 2007; 37: 279–290.
- Tarhan L., Acar B. Problem-Based learning in an eleventh grade chemistry class: “Factors Affecting Cell Potential”. Research in Science & Technological Education. 2007; 25(3): 351–369. [PubMedAbstract] [PubMedCentralFullText].
- Tarhan L., Ayar-Kayali H., Urek R.O., Acar B. Problem-Based learning in 9th grade chemistry class: “Intermolecular Forces”. Research in Science Education. 2008; 38: 285–300.
- Tosun C., Taskesenligil Y. The effect of problem-based learning on undergraduate students’ learning about solutions and their physical properties and scientific processing skills. Chemistry Education Research and Practice. 2013; 14: 36–50.
- Varner D., Peck S.R. Learning from learning journals: the benefits and challenges of using learning journal assignments. Journal of Management Education. 2003; 27(1): 52–77.
- Wang F., Hannafin M.J. Design-based research and technology-enhanced learning environments. Educational Technology Research and Development. 2005; 53(4): 5–23.
- Williams D.P., Woodward J.R., Symons S.L., Davies D.L. A tiny adventure: the introduction of problem based learning in an undergraduate chemistry course. Chemistry Education Research and Practice. 2010; 11: 33–42.
