Abstract
Introduction: Extended-spectrum beta-lactamase (ESBL) producing bacteria have been increasingly reported as causal agents of nosocomial infection worldwide. Resistance patterns vary internationally, and even locally, from one institution to the other. We investigated the clinical isolates positive for ESBL-producing bacteria in our institution, a tertiary care hospital in Madrid (Spain), during a 2-year period (2007–2008).
Methods: Clinical and microbiological data were retrospectively reviewed. Two hundred and nineteen patients were included in the study.
Results: Advanced age, diabetes, use of catheters, previous hospitalization and previous antibiotic treatment were some of the risk factors found among patients. Escherichia coli was the most frequent isolate, and urinary tract the most common site of isolation. Internal Medicine, Intensive Care Unit (ICU) and General Surgery presented the highest number of isolates. There were no outbreaks during the study period. Antibiotic patterns showed high resistance rates to quinolones in all isolates. There was 100% sensitivity to carbapenems.
Conclusion: Carbapenems continue to be the treatment of choice for ESBL-producing bacteria. Infection control measures are of great importance to avoid the spread of these nosocomial infections.
The emerging problem of extended spectrum beta-lactamase (ESBL) producing bacteria has become of great importance during the last decades. Since the first report of an ESBL-producing organism in the 1980s, there has been a growing interest due to their wide spread and constant evolution, becoming increasingly resistant to most of the commonly used antibiotics Citation1. Enterobacteriaceae are naturally present in the intestinal tract of humans and animals, but they can occupy various other ecological niches. In the hospital environment, they can contaminate medical apparatus and devices such as catheters. They can also colonise patients with a prolonged hospitalization, who can become symptomatic or asymptomatic carriers Citation2.
Risk factors that have been related to infection by these microorganisms include advanced age and patients’ previous co-morbidities (such as neoplasia, renal failure, immunosuppression, etc.), long hospital stay, use of invasive devices (urinary catheters, venous catheters, endotracheal tubes) and previous therapy with wide-spectrum antibiotics Citation3.
The pattern of acquired resistance in these microorganisms is in constant shift, and the evolution of resistance enzymes points to a worldwide distribution of the most successful clones, but with a very different national and international epidemiology Citation4, Citation5. This includes infections by ESBL-producing bacteria now occurring in the community as well as in the hospital environment Citation6, Citation7. Therefore, it is important to know the national and even local/institutional incidence in order to adjust antimicrobial therapies and try to avoid a further increase of resistance rates.
While it is unlikely that hospital-acquired infection by antimicrobial resistant microorganisms can be completely eradicated, there is evidence suggesting it can be prevented by the application of strict control measures. Various institutions and National Health systems have developed evidence-based protocols and guidelines to reduce the infection rates in the hospital environment Citation8, Citation9. In Spain, several multicenter studies have been performed on the subject, and different institutions have participated in global surveillance programs such as the SMART (Study for Monitoring Antimicrobial Resistance Trends) Citation10–Citation13. According to this study, the estimated national prevalence of ESBL-producing bacteria in the hospital environment is between 5–10%.
Hospital de La Princesa is a 500-bed, university-affiliated, adult tertiary care teaching hospital located in central Madrid, Spain. Major teaching programs in both surgical and medical specialities are developed, as well as trauma and intensive care. There are no obstetrics–gynaecology and paediatric departments, which are located in different buildings and run separately. The aim of this study was to elucidate the epidemiologic trends of ESBL-producing bacteria from clinical isolates throughout the hospital, and determine if isolation measures had been applied in these cases to avoid transmission.
Methods
We conducted a retrospective, 2-year study (1st January 2007 to 31st December 2008) in order to determine the characteristics of patients with infection by ESBL-producing bacteria in our institution, the local resistance patterns and bacterial susceptibility to the most commonly used antibiotics.
Data collection and variables
Data were retrospectively collected using the Microbiology Department's database. The main inclusion criterion was a positive culture for ESBL-producing bacteria in any clinical isolate from hospitalized patients. Successive cultures from the same patient were excluded to avoid duplicating data. If multiple sites of isolation occurred in the same patient, all were registered. The date used for classification of a positive culture was that of the first isolation.
For each patient, medical histories were reviewed to record clinical data, including demographics (age, sex, department of hospitalization, etc.), co-morbidities, previous (<6 months) hospitalization or ICU admission, use of invasive devices during present hospitalization and previous antimicrobial therapy. The site of isolation, species of bacteria and antimicrobial resistance pattern were also recorded, as well as if other microorganisms (bacteria/fungi) were isolated in the same patient and if correct contact isolation measures had been undertaken to avoid transmission.
Samples and microbiologic methods
Isolates were obtained from clinical samples and classified as: urine, blood, surgical wounds, pus, sputum and other respiratory samples, faeces, pressure ulcers, drains, catheter, and other sterile body fluids. The specimens were collected and processed following conventional microbiological procedures for correct management of clinical samples.
Identification and susceptibility tests were determined using the MicroScan Neg MIC panel type 32 (SIEMENS©). ESBL confirmatory tests were performed by Broth microdilution following CLSI recommendations Citation14 using the MicroScan ESBL plus panel (SIEMENS©) that includes cefpodoxime (0.5–64 µg/ml), cefotaxime (0.5–128 µg/ml), cefotaxime plus clavulanate (0.12/4–16/4 µg/ml), ceftazidime (0.5–128 µg/ml) and ceftazidime plus clavulanate (0.12/4–16/4 µg/ml). A <3-fold concentration decrease in a MIC for either antimicrobial agent tested in combination with clavulanic acid versus its MIC when tested alone, was considered as ESBL positive. Double-disk method Citation15 and E-Test Citation16 were also used in those cases in which results were not conclusive.
Statistical analysis
All data were introduced in a database, and processed using SPSS 15.0 software package for Windows. As there were no groups to compare, only descriptive statistics were performed.
Results
During the study period, a total of 219 hospitalized patients (107 in 2007 and 112 in 2008) presented non-duplicate clinical isolates positive for ESBL-producing bacteria. Of these, 124 (56.6%) were patients admitted to medical wards, 61 (28%) to surgical wards and 34 (15.5%) to ICU.
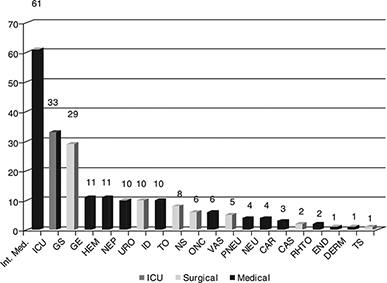
The distribution of isolates by hospital department is detailed in .
Fig. 1 Distribution of isolates (patients with infection by ESBL-producing bacteria) by hospital department. Departments: Int. Med, Internal Medicine; ICU, Intensive Care Unit; GS, General Surgery; GE, Gastroenterology; HEM, Hematology; NEP, Nephrology; URO, Urology; ID, Infectious Diseases; TO, Thoracic Surgery; NS, Neurosurgery; ONC, Oncology; VAS, Vascular Surgery; PNEU, Pneumology; NEU, Neurology; CAR, Cardiology; CAS, Cardiac Surgery; RHTO, Rheumatology; END, Endocrinology; DERM, Dermatology; TS, Trauma Surgery.
Distribution of patients by sex was 88 (40%) male and 131 (60%) female. The mean age was 71 years (range 18–98 years). The average duration of hospitalization from the date of admission to the date of a positive culture for ESBL-producing bacteria was 25 days. The mean hospital stay was 47 days.
The risk factors and clinical characteristics of patients are shown in Table 1.
Table 1. Risk factors and clinical characteristics of patients with a positive culture for ESBL-producing bacteria
One hundred and fifty-eight isolates (72%) out of 219 were Escherichia coli, 40 (18%) were Klebsiella pneumoniae, 9 (4%) were Enterobacter cloacae, 7 (3%) Enterobacter aerogenes, 3 (2%) Klebsiella oxytoca and 1 (0.5%) Proteus vulgaris. The sites of isolation are shown in Table 2.
Table 2. Location of isolates obtained from clinical samples
Bacterial and fungal polimicrobial infection was observed in 106 (48%) and 39 (18%) of the patients, respectively. The antimicrobial susceptibility test results by species are detailed in Table 3. Application of correct isolation measures by preventive medicine (registered in medical records) was 57.1% (125 patients).
Table 3. Antibiotic susceptibility patterns of the ESBL-producing isolates
Discussion
This study delineates the microbiological spectrum of ESBL-producing Enterobacteriaceae, their antimicrobial resistance patterns and the clinical characteristics of patients associated with these infections in our institution, during a 2-year period. The departments with the highest number of positive ESBL-producing isolates were internal medicine (61; 49%) among the medical, and general surgery (29; 13%) among the surgical, which were also the departments with a higher number of in-patients during the study period. The ICU was the second in total number of registered cases (34; 15.5%). ICU patients are among the most susceptible to infection by multi-resistant microorganisms, with multiple risk factors Citation17,Citation18.
Advanced age has demonstrated to be an independent risk factor for infection in many studies Citation19. In our institution, most of the patients with infection were of advanced age (mean of 71 years). Diabetes mellitus was the most frequent co-morbidity, present in 33% of our hospitalized patients. The altered metabolism and associated immune deficiency may have led to the higher risk of infection, particularly those related to wound, catheter and bacteremia. Another factor related to infection in our patients was the existence of a previous/actual neoplasia (31%). In most cases this was the main condition for hospitalization. The risk of infection can be increased due to the neoplastic process itself or the chemotherapy received Citation20. An altered immune condition was registered in 17.4% of patients. Two hundred and eleven patients (96.3%) had vascular/urinary catheterization at some point during hospitalization. Up to 33% had also an endotracheal tube (mainly surgical or ICU patients) Citation21. Other risk factors which can condition a specific, higher risk of infection by ESBL-producing bacteria, were previous hospitalization (58%) and previous antibiotic therapy (73%). In 39.7% of patients, the antibiotic treatment received included two or more different antibiotics. Previous therapy with antibiotic drugs, especially if lengthy or inappropriate, allows bacterial mutations to become the dominant strains and consolidate resistance Citation22. Enterobacteriaceae are the main bacteria found to develop ESBLs. In Spain, a subanalysis from the SMART study, with data from the 13 participating Spanish hospitals found a prevalence of 6%. E. coli was the most frequently isolated species (61%) followed by Klebsiella spp. (20%) and Enterobacter spp. (8%) Citation13. In our study, E. coli was the most common pathogen (72%), 10% over the national percentage given by SMART, followed by Klebsiella (20%) and Enterobacter (7.3%) with percentages of isolation very similar to the national study.
The most common site of isolation was urine, followed by blood (bacteremia). Urinary tract infections (UTI) are the most frequent infections worldwide among hospitalized patients, and Enterobacteriaceae (mainly E. coli) are generally the causal agents. The microbiological spectrum of nosocomial UTI is growing, and ESBL bacteria appear as a problem because of their resistance to all of the most commonly used antibiotics, including quinolones. While in medical wards urine continued to be the first site of isolation, in the surgical wards pus/wound specimens were the most frequent sites of infection Citation23,Citation24.
In our study, correct application of isolation measures to avoid transmission was 57.1%. Therefore, there is still a margin for improvement. Isolation measures included correct use of sterile gloves and aprons, systematic hand decontamination before and after visiting the patients, single room and restricted visitors Citation9.
The study of antimicrobial sensitivity globally demonstrated high resistance rates to quinolones (up to 83% ciprofloxacin resistance in E. coli). The results for amoxicillin–clavulanic presented some sensitive/intermediate strains, when by definition ESBL-producing bacteria are resistant to betalactams. Interpretation of these results must be cautious. While ‘in vitro’ results show sensitivity, amoxicillin–clavulanic is usually ineffective ‘in vivo’ due to the ‘inoculum effect’ associated to ESBL-producing bacteria Citation25. When used as empirical therapy this can lead to a lack of response and treatment failure. Amikacin presented a good rate of sensitivity in all species, maybe because the use of aminoglycosides has been reduced institutionally over the last years to avoid renal toxicity. Enterobacter spp. presented the highest rate of global resistance to the antibiotics tested. Fortunately, no carbapenem-resistant strains were identified. Carbapenems have been the most successful antibiotics against ESBL-producing bacteria because of their beta-lactamase stability, and continue to be the treatment of choice. Nevertheless, the emergence of new resistance mechanisms such as carbapenemases, and the abuse or under-dosing of these antibiotics represent a constant threat to their efficacy Citation26.
In conclusion, the knowledge of institutional resistance patterns can help physicians select adequate empirical antibiotic regimens, so that antibiotics with high resistance rates can be avoided. Treatment can be tailored in each patient, considering individual risk factors and ESBL-targeting if necessary. This can help reduce morbidity and mortality, and achieve a better control over hospital infections.
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study.
Acknowledgements
The authors would like to thank Marta Ruiz, Antonio Valdivia and Angels Figuerola, (Department of Preventive Medicine, Hospital Universitario de La Princesa, Madrid) for providing data on institutional application of isolation measures, and for their constant collaboration with our departments in the prevention of nosocomial infections.
References
- Gómez J, García Vázquez E, Ruiz Gómez J. Clinical relevance of bacterial resistance: a historical approach (1982–2007). Rev Esp Quimioter. 2008; 21: 115–122.
- Rodríguez-Baño J, López-Cerero L, Navarro MD, Díaz de Alba P, Pascual A. Faecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J Antimicrob Chemother. 2008; 62: 1142–1149. 10.1093/jac/dkn293.
- Graffunder EM, Preston KE, Evans AM, Venezia R. Risk factors associated with extended-spectrum β-lactamase-producing organisms at a tertiary care hospital. J Antimicrob Chemother. 2005; 56: 139–145. 10.1093/jac/dki180.
- Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G. CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother. 2007; 59: 165–170. 10.1093/jac/dkl483.
- Coque TM, Baquero F, Canton R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 2008; 13: pii: 19044.
- Rodríguez-Baño J, Alcalá J, Cisneros JM, Grill F, Oliver A, Horcajada JP. Escherichia coli producing SHV-type extended-spectrum beta-lactamase is a significant cause of community-acquired infection. J Antimicrob Chemother. 2009; 63: 781–784. 10.1093/jac/dkp028.
- Denholm JT, Huysmans M, Spelman D. Community acquisition of ESBL-producing Escherichia coli: a growing concern. Med J Aust. 2009; 190: 45–46.
- Owens RC, Rice L. Hospital-based strategies for combating resistance. Clin Infect Dis. 2006; 42: 173–181. 10.1086/500664.
- Howard DP, Williams C, Sen S, Shah A, Daurka J, Bird R. A simple effective clean practice protocol significantly improves hand decontamination and infection control measures in the acute surgical setting. Infection. 2009; 37: 34–38. 10.1007/s15010-008-8005-3.
- Hernández JR, Martínez-Martínez L, Cantón R, Coque TM, Pascual A; Spanish Group for Nosocomial Infections (GEIH). Nationwide study of Escherichia coli and Klebsiella pneumoniae producing extended-spectrum beta-lactamases in Spain. Antimicrob Agents Chemother. 2005;49:2122–5. 10.1128/AAC.49.5.2122-2125.2005.
- Guembe M, Cercenado E, Alcalá L, Marín M, Insa R, Bouza E. Evolution of antimicrobial susceptibility patterns of aerobic and facultative gram-negative bacilli causing intra-abdominal infections: results from the SMART studies 2003–2007. Rev Esp Quimioter. 2008; 21: 166–173.
- Rossi F, Baquero F, Hsueh PR, Paterson DL, Bochicchio GV, Snyder TA. In vitro susceptibilities of aerobic and facultatively anaerobic gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: 2004 results from SMART (Study for Monitoring Antimicrobial Resistance Trends). J Antimicrob Chemother. 2006; 58: 205–210. 10.1093/jac/dkl199.
- Baquero F, Cercenado E, Cisterna R, De la Rosa M, García-Rodríguez JA, Gobernado M. Patrones de sensibilidad a antimicrobianos de Enterobacteriaceae causantes de infecciones intraabdominales en España: resultados del estudio SMART 2003. Rev Esp Quimioter. 2006; 19: 51–59.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 20th informational supplement. M100-S20 CLSI. Wayne PA: Clinical and Laboratory Standards Institute, 2010.
- MacKenzie FM, Miller CA, Gould IM. Comparison of screening methods for TEM and SHV-derived extended-spectrum beta-lactamase detection. Clin Microbiol Infect. 2002; 8: 715–724. 10.1046/j.1469-0691.2002.00473.x.
- Drieux L, Brossier F, Sougakoff W, Jarlier V. Phenotypic detection of extended-spectrum betalactamase production in Enterobacteriaceae: review and bench guide. Clin Microbiol Infect. 2008; 14: 90–103. 10.1111/j.1469-0691.2007.01846.x.
- Wroblewska MM, Rudnicka J, Marchel H, Luczak M. Multidrug-resistant bacteria isolated from patients hospitalised in Intensive Care Units. Int J Antimicrob Agents. 2006; 27: 285–289. 10.1016/j.ijantimicag.2005.11.015.
- Mulvey MR, Bryce E, Boyd D, Ofner-Agostini M, Christianson S, Simor AE. Ambler class A extended-spectrum B-lactamase producing Escherichia coli and Klebsiella spp. in Canadian hospitals. Antimicrob Agents Chemother. 2003; 48: 1204–1214. 10.1128/AAC.48.4.1204-1214.2004.
- Kaye KS, Schmader KE, Sawyer R. Surgical site infection in the elderly population. Clin Infect Dis. 2004; 39: 1835–1841. 10.1086/425744.
- Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 2009; 10: 589–597. 10.1016/S1470-2045(09)70069-5.
- Saint S, Savel RH, Matthay MA. Enhancing the safety of critically ill patients by reducing urinary and central venous catheter-related infections. Am J Respir Crit Care Med. 2002; 165: 1475–1479. 10.1164/rccm.2110035.
- Paterson DL, Ku WC, von Guttberg A, Mohapatra S, Casellas JM, Goossens H. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum β-lactamases. Clin Infect Dis. 2003; 39: 31–37. 10.1086/420816.
- Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol. 2001; 14: 244–269. 10.1128/CMR.14.2.244-269.2001.
- Owens CD, Stoessel K. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect. 2008; 70: 3–10. 10.1016/S0195-6701(08)60017-1.
- Queenan AM, Foleno B, Gownley C, Wira E, Bush K. Effects of inoculum and beta-lactamase activity in AmpC- and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae clinical isolates tested by using NCCLS ESBL methodology. J Clin Microbiol. 2004; 42: 269–275. 10.1128/JCM.42.1.269-275.2004.
- Treviño M, Moldes L, Martínez-Lamas L, Varón C, Regueiro BJ. Carbapenem-resistant Enterobacter cloacae and the emergence of metallo-beta-lactamase-producing strains in a third-level hospital (Santiago de Compostela, NW Spain). Eur J Clin Microbiol Infect Dis. 2009; 28: 1253–1258. 10.1007/s10096-009-0765-x.