Abstract
Background : Supplementary immunization activity (SIA) campaigns provide children with an additional dose of measles vaccine and deliver other interventions, including vitamin A supplements, deworming medications, and oral polio vaccines.
Objective : To assess the cost-effectiveness of the full SIA delivery platform in South Africa (SA).
Design : We used an epidemiologic cost model to estimate the cost-effectiveness of the 2010 SIA campaign. We used province-level campaign data sourced from the District Health Information System, SA, and from planning records of provincial coordinators of the Expanded Programme on Immunization. The data included the number of children immunized with measles and polio vaccines, the number of children given vitamin A supplements and Albendazole tablets, and costs.
Results : The campaign cost $37 million and averted a total of 1,150 deaths (95% uncertainty range: 990–1,360). This ranged from 380 deaths averted in KwaZulu-Natal to 20 deaths averted in the Northern Cape. Vitamin A supplementation alone averted 820 deaths (95% UR: 670–1,040); measles vaccination alone averted 330 deaths (95% UR: 280–370). Incremental cost-effectiveness was $27,100 (95% UR: $18,500–34,400) per death averted nationally, ranging from $11,300 per death averted in the Free State to $91,300 per death averted in the Eastern Cape.
Conclusions : Cost-effectiveness of the SIA child health delivery platform varies substantially across SA provinces, and it is substantially more cost-effective when vitamin A supplementation is included in the interventions administered. Cost-effectiveness assessments should consider health system delivery platforms that integrate multiple interventions, and they should be conducted at the sub-national level.
To access the supplementary material to this article ‘Supplementary data’ please see Supplementary files under Article Tools online.
The World Health Organization (WHO) reported a substantial decrease in global measles mortality, with an estimated 140,000 measles-related deaths in 2010 and a significant 85% decrease from 2000 to 2010 in the African region Citation1. The WHO strategy to reduce measles mortality includes maintaining high coverage of routine measles immunization and ensuring all children receive a second dose Citation2. In many low- and middle-income countries, where the second dose is not routinely delivered through primary health care services, this second opportunity is offered during supplementary immunization activities (SIAs) Citation2 that take place either nationally or sub-nationally Citation3. This approach was first successfully implemented by the Pan American Health Organization Citation4. Over the past decade, the strategy has been exported to sub-Saharan Africa and is credited with the recent reduction in measles mortality Citation1, Citation5.
As demonstrated in Mexico, a package of interventions can be provided through National Health Weeks Citation6. SIAs can be expanded to deliver vitamin A supplementation, deworming medicines, oral polio vaccines (OPV) Citation7, and in malaria endemic countries, insecticide-treated bed nets (ITN) Citation8, Citation9. SIAs, which are periodic (usually every 3 years in South Africa) are often integrated into ‘Child Health Days’ Citation10.
South Africa (SA) has integrated SIAs into polio national immunization days at the provincial level since 1996 Citation11. Unlike most sub-Saharan African countries, which receive aid, SA's SIAs are entirely funded by the government. The SA campaigns deliver vitamin A supplementation, deworming medicines (Albendazole), and oral polio vaccines, in addition to measles vaccines (MCV). The last poliovirus case in SA was identified in 1989 Citation12, and measles-related deaths have dropped in SA since the mid-1990s, from about 500 deaths in 1993 to less than 10 in 2007 Citation13. Prevalence of vitamin A deficiency among 1- to 9-year-olds is prominent, with 14% having a serum vitamin A concentration under 10 µg/L Citation14. Soil-transmitted-helminth infections among school-aged children remain prevalent in SA coastal regions Citation15.
Little is known about the cost-effectiveness of the integrated SIA delivery platform Citation16 Citation17. Most research to date has focused only on the cost-effectiveness of the measles vaccination component of the platform Citation18–Citation23. We analyze the cost-effectiveness of the full SIA delivery platform, which we call the child health campaign (CHC) platform. To assess the cost-effectiveness of the platform, we look at effectiveness and costs of the interventions included (measles vaccination, polio vaccination, vitamin A supplementation, deworming medicines). Our study takes the entire delivery platform as the unit of analysis. Because budgets are set by provincial authorities and because provinces differ substantially in geography and epidemiology from one another, assessing cost-effectiveness in SA at the provincial level is important.
Methods
Effectiveness of the SIA platform
We used coverage data collected for the nine SA provinces in the 2010 CHC, held in the District Health Information System (DHIS), SA. The data included the number of children reached per province by each intervention: measles vaccination for 6-month- to 15-year-olds, polio vaccination for kids aged below 5 years, vitamin A supplementation and deworming for 12–59 months. By intervention and by province, we assessed the burden of disease averted by the one-time 2010 CHC, in deaths averted. shows the values of all parameters and references.
Table 1. Input values used in the study
Measles vaccination
We estimated the number of measles deaths averted over 3 years, chosen for the typical 3-year cycle for SA SIAs Citation3
Citation5.Footnote1
We used a Poisson count model relying on national measles-related deaths data from SA's vital registration system for 1993–2007, adjusted for underreporting and completeness, and corrected for misclassified HIV/AIDS deaths; national routine coverage data for the first dose of measles vaccine; SIA's national coverage data Citation3
Citation5
Citation13
Citation14
Citation24
Citation25. The model is as follows:1
2
where D
t
counts the measles-related deaths in year t, Sia
t−j
is the SIA coverage in year t−j (j=0, 1, 2) (measles deaths averted are estimated over three subsequent years because, in recent times in SA, SIAs have been implemented every 3 years), Cov
t−7
and Births
t−7
are the routine coverage and birth cohort in year t−7, respectively. The choice of a 7-year lag provides the best fit to the data (explained in supplementary data, section 1).Footnote2
The number of measles deaths averted in province k is derived as:3
where Cov k is the provincial routine coverage of the first dose of measles, Births k is the size of the provincial birth cohort, Sia k is the provincial SIA coverage (DHIS, SA). The number of measles cases averted is calculated by dividing the number of deaths averted by the case fatality rate. Details are provided in the supplementary data (section 1).
Vitamin A supplementation
We determined the number of deaths averted by vitamin A supplementation over 1 year (vitamin A campaigns occur annually), using diarrhea-related death estimates from the Global Burden of Disease study for SA Citation13. Vitamin A supplementation reduces diarrhea-related mortality in 6- to 59-month-olds, shown in the results of seven randomized controlled trials (RCTs) and cluster RCTs reported in a systematic review (RR=0.72; 95% CI: 0.57–0.91) Citation26. In a given province k, we determined the number of deaths averted as a function of number of individuals reached, pre-existing diarrhea deaths among 12–59 month-olds, and reduction of diarrhea mortality due to vitamin A supplementation as documented by RCTs. Details are provided in the supplementary data (section 2).
Polio vaccination and deworming
The polio vaccination averted no cases, making the impact of the intervention null (details are provided in the supplementary data, section 3). For anti-helminthics, the SA Medicines Control Council did not give distribution approval in time for the 2010 CHC, so most provinces left out deworming. Consequently, very few children were reached, the burden of worm disease averted was tiny, and impact of the intervention was disregarded in the estimates that follow (details are provided in the supplementary data, section 4).
Each death averted was estimated to correspond to 31 Disability-Adjusted Life Years (DALYs) (discounted at 3%). Years of Life with Disability were disregarded in the calculations, as they represented a tiny fraction of the total DALYs (details are provided in the supplementary data). All the health gains were in Years of Life Lost (none in Years of Life with Disability), hence results are reported in deaths averted.
Costs
shows the costs incurred by the 2010 CHC per province by item categories. Data were obtained from the Expanded Programme on Immunization coordinators. In preparation for the campaign, each province presented a pre-campaign micro-planning document to the National Department of Health, including a budget for campaign items. The items were categorized as follows: vaccines and medicines (MCV and OPV doses were $0.6 and $0.4, respectively), injection materials and consumables, cold chain equipment, waste management supplies, transport, social mobilization materials, training, campaign personnel (registered nurse and other health worker salaries were $109/day and $70/day, respectively). There were gaps in the province budget data; therefore, costs were not available for all items. We used the more complete data available for some provinces (Eastern Cape, Gauteng, KwaZulu-Natal, Limpopo, Mpumalanga, and Western Cape) and extrapolated them for the provinces with missing data (Free State, Northern Cape, and North West). The unit of extrapolation is the number of clinics in the province for some elements (e.g. cold chain equipment) and the number of vaccine doses administered for others (e.g. injection material). Cost data were recorded in 2010 SA Rand (ZAR) then converted to US$ with 1 ZAR=0.14 US$ (Google Finance, accessed 19 July 2011).
Table 2. Selected costs (in 2010 1,000 US$) incurred by the 2010 Child Health Campaign by province and nationally
We estimated the costs avoided due to measles hospitalizations averted, calculated by taking into account age-specific probability of complications, length of hospitalizations, and related direct costs (). Each patient not hospitalized was assumed to have one outpatient visit. No costs were averted by vitamin A supplementation because vitamin A supplementation was not found to reduce the number of hospitalizations Citation26.
Cost-effectiveness
Net health benefits and costs are estimated over 3 years. The 2010 CHC added to pre-existing levels of measles vaccination and vitamin A supplementation interventions. The baseline scenario to which the 2010 CHC platform is compared is the scenario where the 2010 CHC platform is not implemented: no supplemental measles vaccine, vitamin A supplementation, polio vaccines, or deworming medicines were distributed. We defined an incremental cost-effectiveness ratio (ICER) in dollars per death averted for the CHC in each province as ICER=NC / (Death M +Death V ), where NC is the total net cost, and Death i is the deaths averted by the intervention of measles vaccination (M) or vitamin A supplementation (V). We defined one ‘hypothetical’ sub incremental cost-effectiveness ratio, ICER M =NC /Death M , where NC M includes the net programmatic costs associated with CHC implementation (transport, social mobilization, personnel, others categories in ) and the measles vaccination intervention (MCV, injection materials, cold chain equipment, waste management categories).
At the real exchange rate, SA's GDP per capita is $7,274 (International Monetary Fund, www.imf.org/external/pubs/ft/weo/2011/02, accessed 8 November 2012). Therefore, the CHC platform would be qualified cost-effective, if the ICER is <$7,274 per DALY.
Sensitivity analysis
We conducted a Monte Carlo multivariate sensitivity analysis to estimate aggregate uncertainty from model inputs. Key parameters were given values, using specific distributions capturing uncertainty simultaneously in 10,000 iterations (details are provided in the supplementary data, section 5). This allows the determination of 95% uncertainty ranges.
Analyses were conducted with the R statistical package (www.r-project.org).
Results
The results of the Poisson regression model (equations 1 and 2) are given in the supplementary data (Table S.1). In this case, the goodness of fit was substantially higher than other models with R2=0.94 and RMSE (Root Mean Square Error)=34. a and present the burden of disease averted. The burden of disease averted by measles vaccination alone was: 327 deaths (95% uncertainty range: 283–371) nationally; the least was 10 deaths averted in the Northern Cape, the most was 144 deaths averted in KwaZulu-Natal. The burden of disease averted by vitamin A supplementation alone was substantial: 818 deaths (95% UR: 672–1,035) nationally; the least was 8 deaths averted in the Northern Cape, the most was 237 deaths averted in KwaZulu-Natal. A combination of high burden of disease and large population reached makes KwaZulu-Natal the greatest beneficiary in terms of burden of disease averted. Overall, the 2010 Child Health Campaign averted 1,145 deaths (92% UR: 994–1,363), including 381 deaths in KwaZulu-Natal and 185 deaths in Gauteng. The lowest number was in the Northern Cape with 18 deaths.
Fig. 1. Deaths averted (a) and cost-effectiveness (b) for the 2010 Child Health Campaign by province.
EC, Eastern Cape; FS, Free State; GP, Gauteng; KZN, KwaZulu-Natal; LP, Limpopo; MP, Mpumalanga; NC, Northern Cape; NW, North West; WC, Western Cape.
ICER: incremental cost-effectiveness ratio (in US$ per DALY, assuming 1 death corresponds to 31 DALYs).
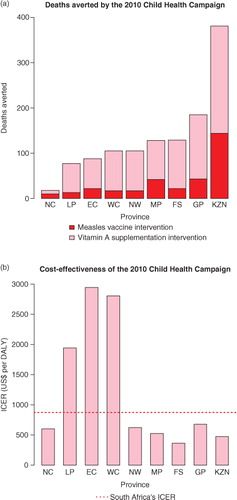
Table 3. Burden of disease averted, in deaths averted (95% uncertainty range in parentheses), by the 2010 Child Health Campaign by province and nationally
and list the costs and net costs, respectively; ; b give the cost-effectiveness results. The total cost of the platform was $37 million nationally, ranging from about $8 million each in the Eastern Cape and KwaZulu-Natal to $0.5 million in the Northern Cape. The CHC platform's ICER is $27,063 per death averted (95% UR: 18,476–27,063) nationally, ranging from $11,284 per death averted in the Free State to $91,264 per death averted in the Eastern Cape, reflecting the substantial heterogeneity within SA. A combination of high burden of disease, large population reached, and lower costs (about $1.8 million) makes the intervention most cost-effective in the Free State; a combination of lower burden of disease and higher costs (about $8 million) makes the intervention least cost-effective in the Eastern Cape. Gauteng and KwaZulu-Natal present ICERs of $21,018 per death averted and $14,725 per death averted, respectively. These are the two most populous provinces with a high prevalence of inadequate vitamin A status among children (89% in KwaZulu-Natal, 65% in Gauteng) and high burden of diarrhea (Citation13, Citation14). In the hypothetical scenario, where measles vaccination would be the sole intervention delivered by the CHC platform, the sub ICER would be $88,102 per death averted (95% UR: 64,387–110,174) nationally, ranging from $33,418 per death averted in the Northern Cape, with a smaller population immunized and lower costs, to $367,722 per death averted in the Eastern Cape with higher costs.
Table 4. Net costs (in million 2010 US dollars), and incremental cost-effectiveness ratio ICER in US$ per death averted (95% uncertainty range in parentheses) for the 2010 Child Health Campaign by province and nationally
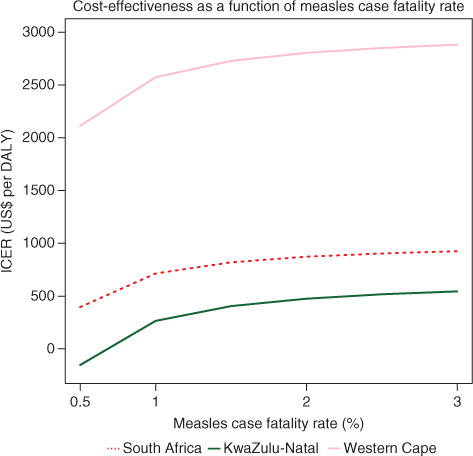
The extent of the confidence intervals is mainly due to the uncertainty in the number of measles cases averted and related hospitalization costs, a consequence of the values available and used for the measles case fatality rate (CFR). CFRs vary widely between settings, as the criteria of diagnosis are not constant, and measles cases are largely underreported. Measles cases reporting efficiency is also often higher among those hospitalized with complications, which may bias the CFR values Citation27. Therefore, plots the 2010 CHC's ICER for South Africa and two provinces (KwaZulu-Natal and the Western Cape) for selected CFR values. Though more sensitive to the lowest value of CFR, overall, the results remained stable: the CHC remains cost-effective nationally (400<ICER<$1,000/DALY); the Western Cape's ICER remains high (>$2,000/DALY) whereas KwaZulu-Natal's ICER remains low (< $600/DALY, with net savings for a CFR of 0.5%).
Discussion
The 2010 Child Health Campaign delivery platform is cost-effective nationally with a cost-effectiveness of $27,063 per death averted ($873/DALY), but with significant heterogeneity in the results across provinces (; ): cost-effectiveness was best for the Free State ($11,284 per death averted or $364/DALY) and worst for the Eastern Cape ($91,264 per death averted or $2,944/DALY) with a high price tag – about $8 million. These disparities have implications in terms of efficiently allocating resources across provinces. Looking at the hypothetical scenario, where the CHC would only deliver MCV, cost-effectiveness would become worse: $88,102 per death averted ($2,842/DALY) nationally, ranging from $33,418 per death averted ($1,078/DALY) in the Northern Cape to $367,722 per death averted ($11,862/DALY) in the Eastern Cape. Integration of vitamin A supplementation therefore makes the CHC platform more attractive, confirming previous findings Citation28. The expansion of the platform to vitamin A supplementation substantially increases its economies of scope, with high effectiveness and low cost.
Our numbers differ from a previous study that looked at the cost-effectiveness of measles SIAs alone, in two SA provinces Citation18. The time horizon and methodology in our study were significantly different: we looked at a one-time comprehensive delivery platform integrating multiple interventions with a shorter time horizon. Our results thus differ from other cost-effectiveness studies on measles SIAs in sub-Saharan African countries, including Uganda and Ethiopia, which also have substantially higher measles burdens Citation22 Citation23. These studies looked at the broader cost-effectiveness of measles eradication over a 40-year period Citation22 Citation23 Citation29, and did not consider any other interventions except for measles vaccination. The Child Health Campaign delivery platform appears in the range of high cost interventions in sub-Saharan Africa compared to other interventions in the region against diarrheal diseases (US$500–1,700/DALY), HIV/AIDS (US$600–1,500/DALY), and cardiovascular diseases (US$600–27,000/DALY) Citation30.
The cost-effectiveness model here presents several limitations. The estimation of burden of disease averted (both morbidity and mortality) is difficult as data on measles and diarrhea incidence and deaths are limited. Hence, epidemiological interactions between measles, diarrhea, and vitamin A deficiency were not incorporated. Measles complications that are serious but considered rare events such as encephalitis Citation23 were not included. Potential non-specific beneficial effects on survival from immunization Citation31 were not included because these effects, often estimated upon non-randomized studies, are subject to selection bias Citation32. The Poisson regression did not model the effectiveness of SIAs against measles that potentially varies with province and time, due to lack of data. The regression model also presents limitations due to the small time series used (1993–2007), which may then be subject to serial correlations. However, we checked the robustness of our findings and found consistent estimates of measles deaths averted when different time lags (other than 7 years) for routine coverage were tried in equation 2. We did not incorporate the full spectrum of helminth-associated morbidity Citation33, because the population reached with deworming medicines in 2010 was so small that their inclusion would not affect results. In relation to costs, we had an incomplete recording of projected costs and expenditure on costs was not available. However, projected costs from campaign planners provide a reasonable estimate. Also, we did not discount health benefits and costs over the 3 years, as this would not greatly affect results over a 3-year time frame Citation34. Finally, polio and measles vaccination interventions may be considered global goods. In the larger context of polio eradication Citation35 and measles elimination Citation22 Citation23, eradication not only reduces infections but also eliminates the need for future vaccinations. However, our analysis uses sub-national data toward describing the SA situation, a step toward these global goods.
These results have important implications for SA's decision makers in terms of how to optimize the current Child Health Campaign platform. SA's routine health services are currently free of charge to all children 6 years and under. Spending US$37 million on a nationwide campaign may not be the best allocation of scarce government resources given that child mortality is distributed heterogeneously in the country. As shown on , a campaign selectively targeting the provinces of KwaZulu-Natal, Gauteng, the Free State, Mpumalanga, and North West may be more economically attractive while addressing up to 80% of the burden of disease that can be averted by a nationwide CHC. A comparison of campaign costs with the costs and projected benefits (from improved coverage) of expanding routine child health services in areas where they are especially weak should also be made. In the comparison, the current performance of the SA health system must be considered, as routine immunization coverage is widely heterogeneous among districts, with some districts, particularly in KwaZulu-Natal, presenting low immunization rates Citation36. In addition, routine activities rarely achieve universal coverage especially when the population is diverse and remote rural areas are difficult to reach. Delivery platforms periodically delivered directly to communities such as Child Health Campaigns may raise overall coverage and reduce coverage heterogeneity, while targeting those not reached by inadequate routine health services Citation37–Citation39. Child Health Campaigns may be therefore required for equitable access to basic child health services, and can also be implemented in conjunction with WHO's ‘Reaching Every District’ strategy which focuses on building national capacity from district level upward to maximize universal vaccine access Citation40.
Further work in sub-Saharan Africa could determine country-specific cost-effectiveness of Child Health Campaign expansion, and which combination of child health interventions and which size and groups of beneficiaries, it is optimal to target. In particular, there could be an emphasis on the economies of scope, including the estimation of which costs are apportioned to which interventions (not available for this paper), when there is expansion of the delivery platform. Important considerations are the burden of disease, as well as issues of health rights and entitlements in targeting children who are often overlooked by the health system. Our approach to economic evaluation in SA can be used to evaluate other countries’ Child Health Campaign platforms. It could aid Ministries of Health at both national and provincial level in designing a Child Health Campaign platform that achieves better outcomes at lower costs: it enables a comparison of tradeoffs with other delivery platforms, such as routine immunization services. In particular, there may be a transitional period when a particular country moves away from a CHC platform to deliver health care toward strengthening its routine health services. Finally, our approach emphasizes the consideration of health system delivery platforms and the importance of integrating and combining multiple interventions onto the same delivery platform.
Conflict of interest and funding
The authors declare they have no conflicts of interest. This study was funded by the Bill & Melinda Gates Foundation through the Disease Control Priorities Network grant to the University of Washington and by the Fogarty International Center at the US National Institutes of Health.
Acknowledgements
This study was undertaken under the auspices of PRICELESS SA (Priority Cost Effective Lessons for Systems Strengthening – South Africa) at the University of the Witwatersrand School of Public Health, Johannesburg. This work would not have been accomplished without the commitment of many people. In particular, we are indebted to Calle Hedberg and Sonja Venter at the Health Information Systems Programme SA; Thulani Masilela (Chief Director: Strategic Planning Department of Health, SA); Johann van den Heever, Lindsay Botham, and Milani Wolmarans at the South African National Department of Health; Victoria Pillay-van Wyk at the Medical Research Council (SA) Burden of Disease Unit; Candy Day at the Health Systems Trust; Cheryl Cohen, Claire von Mollendorf, and Sibongile Walaza at the National Institute for Communicable Diseases (SA); Bafedile Chauke at the World Health Organization (SA); Mandy Maredza, Julia Moorman, Nonkululeko Mthembu, Shan Naidoo, Haroon Saloojee, and Heinrich Volmink at the University of the Witwatersrand School of Public Health. We thank Gretchen Stevens, Rachel Pullan, Jason Peat, Demetre Labadarios, Slim Haddad, and Mira Johri for helpful suggestions. Finally, we thank Meg Stalcup and two anonymous reviewers for providing valuable and constructive comments on the manuscript.
Notes
1The number of measles deaths averted by the CHC platform is estimated at the provincial level, though the Poisson regression model is estimated at the national level, as data on measles-related deaths were available at the national level only.
2Only one time lag (i.e. 7 years) was used for the routine coverage covariate because its time series presents substantial correlation.
References
- Simons E, Ferrari M, Fricks J, Wannemuehler K, Anand A, Burton A, et al.. Assessment of the 2010 global measles mortality reduction goal: results from a model of surveillance data. Lancet. 2012; 379: 2173–8.10.3402/gha.v6i0.20056.
- World Health Organization. Measles vaccines: WHO position paper. Weekly Epidemiological Record. 2009; 84: 349–60.
- World Health Organization. 2012Retrospective measles data on supplementary immunization activities 2000–2010. Available from: http://www.who.int/immunization_monitoring/data/Summary_Measles_SIAs_2000_to_2011.xls [cited 6 July 2012]..
- de Quadros CA, Izurieta H, Venczel L, Carrasco P. Measles eradication in the Americas: progress to date. J Infect Dis. 2004; 189(Suppl. 1):S227–35.10.3402/gha.v6i0.20056.
- Biellik R, Madema S, Taole A, Kutsulukuta A, Allies E, Eggers R, et al.. First 5 years of measles elimination in southern Africa. Lancet. 2002; 359: 1564–68.10.3402/gha.v6i0.20056.
- Sepúlveda J, Bustreo F, Tapia R, Rivera J, Lozano R, Oláiz G, et al.. Improvement of child survival in Mexico: the diagonal approach. Lancet. 2006; 368: 2017–27.10.3402/gha.v6i0.20056.
- World Health Organization/UNICEF. (2009). Joint annual measles report 2009. Strengthening immunization services through measles control. Geneva: WHO.
- Grabowsky M, Nobiya T, Ahun M, Donna R, Lengor M, Zimmerman D, et al.. Distributing insecticide-treated bednets during measles vaccination: a low-cost means of achieving high and equitable coverage. Bull World Health Organ. 2005; 83: 195–201.
- Grabowsky M, Farrell N, Hawley W, Chimumbwa J, Hoyer S, Wolkon A, et al.. Integrating insecticide-treated bednets into a measles vaccination campaign achieves high, rapid and equitable coverage with direct and voucher-based methods. Trop Med Int Health. 2005; 10: 1151–60.10.3402/gha.v6i0.20056.
- Doherty T, Chopra M, Tomlinson M, Oliphant N, Nsibande D, Mason J, et al.. Moving from vertical to integrated child health programmes: experiences from a multi-country assessment of the Child Health Days approach in Africa. Trop Med Int Health. 2010; 15: 296–305.10.3402/gha.v6i0.20056.
- Uzicanin A, Eggers R, Webb E, Harris B, Durrheim D, Ogunbajo G, et al.. Impact of the 1996–1997 supplementary measles vaccination campaign in South Africa. Int J Epidemiol. 2002; 31: 968–76.10.3402/gha.v6i0.20056.
- World Health Organization. 2012Health topics/poliomyelitis. Polio incidence time series. Available from: http://apps.who.int/immunization_monitoring/en/globalsummary/timeseries/tsincidencedip.htm [cited 8 November 2012].
- Institute for Health Metrics and Evaluation. (2012). IHME database. Seattle WA: IHME.
- Labadarios D. The National Food Consumption Survey – Fortification Baseline (NFCS – FB): the knowledge, attitude, behavior and procurement regarding fortified foods, a measure of hunger and the anthropometric and selected micronutrient status of children aged 1–9 years and women of child bearing age: South Africa, 2005. Pretoria: Department of Health, Nutrition Directorate. 2007.
- Brooker S, Clements ACA, Bundy DAP. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006; 62: 221–61.
- Mueller DH, Wiseman V, Bakusa D, Morgah K, Daré A, Tchamdja P, et al.. Cost-effectiveness analysis of insecticide-treated net distributions as part of the Togo Integrated Child Health Campaign. Malar J. 2008; 7: 73.10.3402/gha.v6i0.20056.
- Fiedler JL, Chuko T. The cost of child health days: a case study of Ethiopia's enhanced outreach strategy (EOS). Health Policy Plan. 2008; 23: 222–33.10.3402/gha.v6i0.20056.
- Uzicanin A, Zhou F, Eggers R, Webb E, Strebel P. Economic analysis of the 1996–1997 mass measles immunization campaigns in South Africa. Vaccine. 2004; 22: 3419–26.10.3402/gha.v6i0.20056.
- Acharya A, Diaz-Ortega JL, Tambini G, de Quadros C, Artia I. Cost-effectiveness of measles elimination in Latin America and the Caribbean: a prospective analysis. Vaccine. 2002; 20: 3332–41.10.3402/gha.v6i0.20056.
- Dabral M. Cost-effectiveness of supplementary immunization for measles in India. Indian Pediatr. 2009; 46: 957–62.
- Dayan GH, Cairns L, Sangrujee N, Mtonga A, Nguyen V, Strebel P, et al.. Cost-effectiveness of three different strategies against measles in Zambian children. Vaccine. 2004; 22: 475–84.10.3402/gha.v6i0.20056.
- Levin A, Burgess C, Garrison LP, Bauch C, Babigumira J, Simons E, et al.. Global eradication of measles: an epidemiologic and economic evaluation. J Infect Dis. 2011; 204(Suppl. 1):S98–106.10.3402/gha.v6i0.20056.
- Bishai D, Johns B, Nair D, Nabyonga-Orem J, Fiona-Makmot B, Simons E, et al.. The cost-effectiveness of supplementary immunization activities for measles: a stochastic model for Uganda. J Infect Dis. 2011; 204(Suppl. 1):S107–15.10.3402/gha.v6i0.20056.
- Murray CJL, Rajaratnam JK, Marcus J, Laakso T, Lopez AD. What can we conclude from death registration? Improved methods for evaluating completeness. PloS Medicine. 2010; 7(4):e1000262.10.3402/gha.v6i0.20056.
- Birnbaum JK, Murray CJL, Lozano R. Exposing misclassified HIV/AIDS deaths in South Africa. Bull World Health Organ. 2011; 89(4):278–85.10.3402/gha.v6i0.20056.
- Imdad A, Herzer K, Mayo-Wilson E, Yakoob MY, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. Cochrane Database Syst Rev. 2010. Art. No.CD008524.
- Wolfson LJ, Grais RF, Luquero FJ, Birmingham ME, Strebel PM. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. Int J Epidemiol. 2009; 28: 192–205.
- Caulfield LE, Richard SA, Rivera JA, Musgrove P, Black RE. Stunting, wasting, and micronutrient deficiency disorders. Disease control priorities in developing countries2nd ed. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al..The World Bank. Washington DC, 2006; 351–367.
- Babigumira JB, Levin A, Burgess C, Garrison LP, Bauch CT, Braka F, et al.. Assessing the cost-effectiveness of measles elimination in Uganda: local impact of a global eradication program. J Infect Dis. 2011; 204(Suppl. 1):S116–23.10.3402/gha.v6i0.20056.
- Laxminarayan R, Mills AJ, Breman JG, Measham AR, Alleyne G, Cleason M, et al.. Advancement of global health: key messages from the Disease Control Priorities Project. Lancet. 2006; 367: 1193–208.10.3402/gha.v6i0.20056.
- Aaby P, Bhuiya A, Nahar L, Knudsen K, de Francisco A, Strong M. The survival benefit of measles immunization may not be explained entirely by the prevention of measles disease: a community study from rural Bangladesh. Int J Epidemiol. 2003; 32: 106–15.10.3402/gha.v6i0.20056.
- Fine PEM. Commentary: non-specific effects of measles vaccine – more grist for the mill. Int J Epidemiol. 2003; 32: 116–7.10.3402/gha.v6i0.20056.
- Hotez PJ, Bundy DAP, Beegle K, Brooker S, Drake L, de Silva N, et al.Helminth infections: soil-transmitted helminth infections and schistosomiasis. Disease control priorities in developing countries2nd ed. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al..The World Bank. Washington DC, 2006; 467–482.
- Musgrove P, Fox-Rushby J. Cost-effectiveness analysis for priority setting, Chapter 15. Disease control priorities in developing countries2nd ed. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al..The World Bank. Washington DC, 2006; 271–285.
- Kahn MM, Ehreth J. Costs and benefits of polio eradication: a long-run global perspective. Vaccine. 2003; 21: 702–5.10.3402/gha.v6i0.20056.
- Verguet S, Jassat W, Hedberg C, Tollman S, Jamison DT, Hofman KJ. Measles control in sub-Saharan Africa: South Africa as a case study. Vaccine. 2012; 30: 1594–600.10.3402/gha.v6i0.20056.
- Bhutta ZA, Chopra M, Axelson H, Berman P, Boerma T, Bryce J, et al.. Countdown to 2015 decade report (2000–10): taking stock of maternal, newborn, and child survival. Lancet. 2010; 375: 2032–44.10.3402/gha.v6i0.20056.
- Christie A, Gay A. Response toHeymann DL, Fine PE, Girffiths UK, Hall AJ, Mounier-Jack S. Measles eradication: past is prologue. Lancet. 2010; 376:1719–20. Lancet 2011;377:808.10.3402/gha.v6i0.20056.
- Goodson JL, Kulkarni MA, Vanden Eng JL, Wannemuehler KA, Cotte AH, Desrochers RE, et al.. Improved equity in measles vaccination from integrating insecticide-treated bednets in a vaccination campaign, Madagascar. Trop Med Int Health. 2012; 17: 430–7.10.3402/gha.v6i0.20056.
- World Health Organization. 2012The reaching every district strategy. Available from: http://www.who.int/immunization_delivery/systems_policy/RED-FactSheet.pdf [cited 14 August 2012].