Abstract
Vibrio cholerae is a Gram-negative bacterium that occurs naturally in aquatic environment. Only V. cholerae O1 and V. cholerae O139 produce cholera toxin and cause cholera, other serogroups can cause gastroenteritis, open wounds infection, and septicaemia. V. cholerae O1 and V. cholerae O139 grow and survive inside Acanthamoeba castellanii. The aim of this study is to investigate the interactions of the Swedish clinical isolates V. cholerae O3, V. cholerae O4, V. cholerae O5, V. cholerae O11, and V. cholerae O160 with A. castellanii. The interaction between A. castellanii and V. cholerae strains was studied by means of amoeba cell counts, viable counts of the bacteria in the absence or presence of amoebae, and of the intracellularly growing bacteria, visualised by electron microscopy. These results show that all V. cholerae can grow and survive outside and inside the amoebae, disclosing that V. cholerae O3, V. cholerae O4, V. cholerae O5, V. cholerae O11, and V. cholerae O160 all can be considered as facultative intracellular bacteria.
Vibrio is a genus of Gram-negative bacteria found in water. The genus comprises nearly 70 species such as Vibrio parahaemolyticus, V. vulnificus, and V. cholerae (Citation1).
V. cholerae species are comprised of serogroups depending on antigen O (O1–O206) (Citation2), but only V. cholerae O1 and V. cholerae O139 produce cholera toxin and cause cholera. The other serogroups can cause vibriosis symptoms such as gastroenteritis, open wound infections and septicaemia. Vibriosis is increasing globally, with an estimated 80,000 cases and 300 deaths annually in the United States (Citation3).
V. cholerae and free-living amoebae (FLA) are present in aquatic environments, including drinking water (Citation4–Citation6). FLA are unicellular eukaryotic protozoa found in all soil and aquatic environments (Citation7). The FLA belong to the genera Balamuthia, Naegleria, and Acanthamoeba and are responsible for opportunistic and non-opportunistic infections in mammals (Citation8). Acanthamoeba causes three main types of diseases: keratitis, encephalitis and disseminated infection (Citation8–Citation10).
Sea living animals may carry the vibrio, and the combination of increased water temperature and salinity may contribute to increased association rates of the bacteria with sea-living animals or protozoa (Citation11). The ability of FLA to act as reservoirs for many bacteria has been studied (reviewed in (Citation12)). However, output of the interaction between bacteria and amoeba is dependent on whether the interacting bacterium is extracellular or intracellular and on whether it possesses a type three secretion system (TTSS), since TTSS effector proteins are observed to strongly affect output of the interaction (Citation12). The amoeba may become a host or predator to the interacted bacteria but, on the other hand, many bacterial species are able to kill the amoeba (Citation12). V. cholerae O1, V. cholerae O139, and V. mimicus have been found to be able to grow inside A. castellanii (Citation13–Citation15). In 2004, more than 50 cases of vibriosis were reported in Sweden after exposure to water from the Baltic Sea or swimming outdoors in summer (Citation16–Citation19).
The aim of this study is to investigate the interactions of the Swedish clinical isolates V. cholerae O3, V. cholerae O4, V. cholerae O5, V. cholerae O11, and V. cholerae O160 with A. castellanii.
Materials and methods
Microorganisms
A. castellanii ATCC 30234 was obtained from the American Type Culture Collection, Manassas, VA. The clinical isolated strains: V. cholerae 03 (wound infection, 2006, Ronneby), V. cholerae 04 (sepsis, 2006, Karlskrona), V. cholerae 05 (blood, 2006, Stockholm), V. cholerae 011 (ear infection, 2004, Karlskrona), and V. cholerae 0160 (blood, 2006, Gävle) were obtained from the Public Health Agency of Sweden.
Culture media, growth conditions and analyses
V. cholerae strains were grown on blood agar plates overnight at 37°C. A. castellanii was grown at 30°C to a final concentration of 106 cells ml−1 in ATCC medium no. 712 (Karolinska Institutet, Stockholm, Sweden). In order to infect the amoeba, V. cholerae were grown in Luria–Bertani (LB) broth to an absorbance of 0.6 at 600 nm. Co-cultures of each bacterial strain and A. castellanii were incubated in 75 cm2 cell culture flasks (Corning Incorporated Costar) filled with 50 mL of ATCC medium 712 containing an initial concentration of 105 cells mL−1 of A. castellanii and 106 cells mL−1 of each bacterial strain. Control flasks with bacteria cultured in the absence of amoeba were prepared in the same way and with the same initial concentration as those with amoeba. The flasks were incubated statically at 30°C. Samples were withdrawn regularly for microscopy, cell counts, and viable counts.
Gentamycin susceptibility test
Sensitivity of V. cholerae strains to gentamicin was determined by E-test. The test measured the minimal inhibitory concentration of gentamycin (MIC) utilising a plastic strip according to the Swedish Reference Group for Antibiotics (SRGA) (Citation20).
Growth of V. cholerae strains in the absence or presence of amoebae
To estimate the growth and survival of V. cholerae strains in the absence or presence of A. castellanii by viable counts, 1-mL samples from each bacterial control flask and from flasks containing both bacteria and amoebae were withdrawn. The samples were prepared by 10-fold dilution from 10−1 to 10−10 and spread on blood agar plates. All plates were incubated at 37°C overnight. Thereafter, the numbers of colonies were counted.
Growth and survival of V. cholerae strains inside A. castellanii
To examine the growth and survival of V. cholerae strains inside A. castellanii cells by viable count assay, 1 mL of cell suspension from flasks each containing one of the bacterial strains and the amoeba were diluted in 9 mL of PBS, centrifuged for 10 min at 300 g, and washed three times in PBS to minimize extracellular V. cholerae contamination. The pellets were resuspended in 1 mL of PBS and incubated with 500 µg/ml−1 of gentamicin for 1 h at room temperature. The samples were then diluted in 9 mL of PBS and centrifuged for 10 min at 300 g. A 100-µL portion of each supernatant was spread on blood agar plates, and each pellet was diluted two-fold with 0.1% sodium deoxycholate. Series of 10-fold dilution from 10−1 to 10−4 of the sample were prepared and spread on blood agar plates. All plates were incubated at 37°C overnight, and viable counts were performed.
Growth of A. castellanii in the absence or presence of V. cholerae strains
A. castellanii was grown without shaking at 30°C to a final concentration of 106 cells/ mL in ATCC medium. To study the effect of V. cholerae on A. castellanii, growth of A. castellanii in the presence or absence of V. cholerae strains was studied by means of viable amoeba cell counts. The initial concentration of the amoeba in the presence or absence of V. cholerae strains was 2×105 cells mL−1.
Microscopy analysis
A. castellanii cells, in the absence and presence of bacteria were counted in a Bürker chamber (Merck Eurolab, Sweden) under a light microscope (Carl Zeiss, Sweden). Eosin staining was used to detect dead amoeba cells, which were stained red, in contrast to the viable amoeba cells, which remained unstained.
The intracellular localisations of V. cholerae were analysed by electron microscope, for which 5-mL samples from culture flasks containing the amoeba in the presence of bacteria were centrifuged for 10 min at 300 g in a Labofuge GL centrifuge (VWR International). The resulting pellets were washed with PBS. Each pellet of infected amoeba was fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer pH 7.3, with 0.1 M sucrose and 3 mM CaCl2, for 30 min at room temperature. Samples were then washed in sodium cacodylate buffer and post-fixed in 2% osmium tetroxide in the same buffer for 1 h. The samples were centrifuged and the pellets were dehydrated and embedded in epoxy resin, LX-112. The embedded samples were cut into ultra-thin sections, placed on grids, and stained with uranyl acetate and lead citrate. Sections were examined with a transmission electron microscope (SEM, Philips 420).
Statistical analysis
Student's t-test was used for comparison between viable counts of alone and co-cultivated microorganisms. A p value of ≤0.05 was considered statistically significant. Data represent mean ±SD of three independent experiments over the whole course of the experiment (including every day).
Results
Growth of V. cholerae strains in the absence or presence of A. castellanii
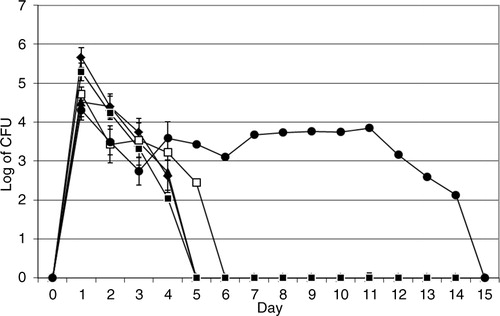
The bacterial strains were cultivated in the absence and presence of A. castellanii to study the interaction between these microorganisms by means of viable count as described in the ‘Methods’ section. In the absence of A. castellanii, viable counts of V. cholerae O3, V. cholerae O4, V. cholerae O5, V. cholerae O11 and V. cholerae O160 increased one log on day one and the bacteria showed different survival rates. V. cholerae O3, O4, O5, O11, and O160 survived 4, 15, 4, 6, and 3 days, respectively ().
Fig. 1 Viable counts of V. cholerae in the absence of A. castellanii. V. cholerae O3 (♦), O4 (▴), O5 (□), O11 (▪) and O160 (●). Data represent means ±SD of three independent experiments.
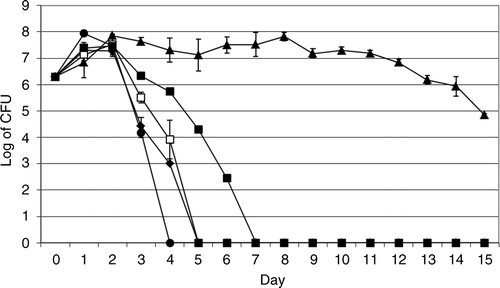
In the presence of A. castellanii the viable counts of V. cholerae O3, O4, O5, O11, and O160 increased one log on day 1 and the bacteria survived 6, 5, 8, 5, and 14 days, respectively ().
Fig. 2 Viable counts of V. cholerae in the presence of A. castellanii. V. cholerae O3 (♦), O4 (▴), O5 (□), O11 (▪), and O160 (●). Data represent means ±SD of three independent experiments.
To compare between the viable counts of the bacterial strains in the absence or presence of A. castellanii. The Student's t-test was used. Viable count of V. cholerae O3, O5, and O11 in the absence or presence of A. castellanii was not significant (p values were>0.05). However, viable count of V. cholerae O4 and V. cholerae O160 in the absence or presence of A. castellanii was significant (p≤0.05).
Growth of intracellular V. cholerae strains
Samples were taken from co-culture flasks and prepared for viable counts of intracellular growth and survival of V. cholerae after gentamycin killing of extracellular bacteria. Sensitivity of V. cholerae to gentamycin was performed by E-test. The MIC value for V. cholerae O3, O4, O5, V. cholerae O11, O160 was 0.25, 1.0, 0.75, 1.0, and 0.75 µg/mL, respectively. These results showed that all V. cholerae examined strains were susceptible to gentamycin since the susceptibility of V. cholerae was (S≤2 µg/mL, R>4 µg/mL).
The intracellular assay showed that after 1 day V. cholerae O3, O4, O5, O11, and O160 grew inside the amoeba cells to 2.7×105, 1.8×105, 5.8×104, 2.1×105, and 2.1×104 cfu/mL, respectively, and survived intracellularly for 5, 5, 6, 5, and 14 days, respectively ().
Intracellular localisation of V. cholerae
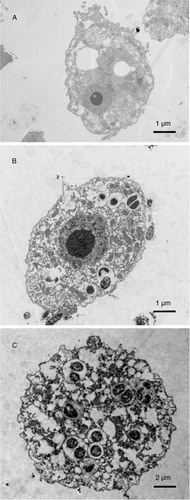
Electron microscopy was used to confirm the intracellular localisation of V. cholerae in A. castellanii. Samples from cultures containing A. castellanii infected with V. cholerae O160 for 2 h and with V. cholerae O4 for 4 h were prepared separately for electron microscopy. The ultramicrography confirmed the intracellular localisation of V. cholerae O160 and V. cholerae O4 () in the cytoplasmic vacuoles of trophozoites of A. castellanii.
Fig. 4 Electron microscope image showing the intracellular localisation of V. cholerae in A. castellanii. A. A. castellanii trophozoite in the absence of bacteria. B. V. cholerae O160 localised in cytoplasmic vacuoles of A. castellanii trophozoite, 2 h after co-cultivation. C. V. cholerae O4 in cytoplasmic vacuoles of A. castellanii trophozoite, 4 h after co-cultivation.
Growth of A. castellanii in the absence or in the presence of V. cholerae
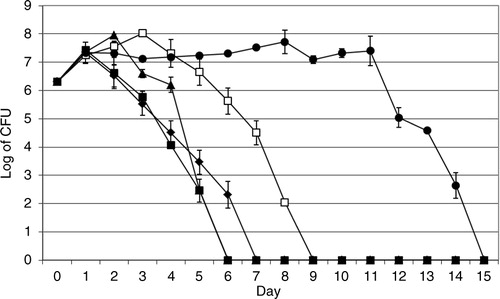
The growth of A. castellanii in the absence or in the presence of V. cholerae was studied by means of viable amoeba cell counts. The viable count of the amoeba in the absence of V. cholerae strains increased from 2×105 cells/mL on day 0 to 1.5×106 cells/mL on day 15 ().
Fig. 5 Growth of A. castellanii. Viable counts of A. castellanii in the absence of V. cholerae strains (⋄), and in the presence of V. cholerae O3 (♦), O4 (▴), O5 (□), O11 (▪), and O160 (●). Data represent mean ±SD of three independent experiments.
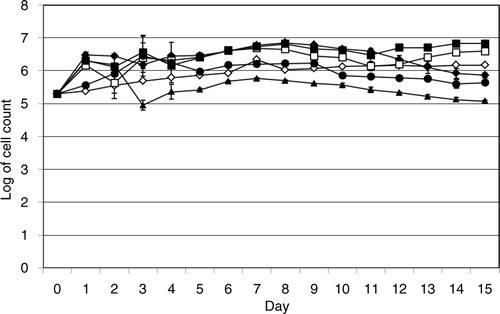
The viable count of A. castellanii in the presence of V. cholerae O3, O5, O11, and O160 increased from 2×105 cells/mL on day 0 to 7.3×105, 3.9×105, 6.7×105, and 4.4×105 cells/mL, respectively. In contrast, the viable count of the amoeba in the presence of V. cholerae O4 decreased to 1.2×105 cells/mL on day 15 ().
The viable count of A. castellanii in the presence of V. cholerae O3, O5, O11, and V. cholerae O160 increased from 2×105 cells/mL on day 0 to 7.3×105, 3.9×105, 6.7×105, 4.4×105 cells/mL, respectively, on day 15 (). However, the viable count of A. castellanii in the presence of V. cholerae O3, O5, O11, O160 and presuming also V. cholerae O4, shows values comparable to those on day 15, already on day 1, that is, the increase was observed much earlier than on day 15. In contrast, the viable count of the amoeba in the presence of V. cholerae O4 decreased to 1.2×105 cells/mL on day 15 ().
The differences in the viable count of A. castellanii in the absence or presence of V. cholerae O3, O4, O5, and O11 strains were statistically significant by student t-test (p<0.05). In contrast, the viable count of A. castellanii in the absence or presence of V. cholerae O160 strain was not significantly different (p>0.05).
Discussion
More than 200 serogroups of V. cholerae are human pathogens causing cholera and vibriosis such as gastroenteritis, open wounds infection, and septicaemia. Recently, interaction of V. cholerae O1, V. cholerae O139, and V. mimicus with Acanthamoeba species has shown that V. cholerae can grow and survive inside A. castellanii (Citation13, Citation14) (Citation21).
The current study examined the ability of the Swedish clinical isolates V. cholerae O3, O4, O5, O11, and O160 to grow and survive in the absence and presence of A. castellanii as well as the growth and survival of bacteria inside A. castellanii to highlight the interaction between the Swedish isolates of V. cholerae with amoebae.
Viable count of the bacteria in the absence of amoeba showed that V. cholerae O3, O5, O11, and O160 died during the first week. This finding is similar to that of previous studies on growth of V. cholerae O1, V. cholerae O139, and V. mimicus (Citation13–Citation15). Surprisingly, it was found that V. cholerae O4 survived (>2 weeks) much longer than did the other serogroups.
In the presence of the amoebae, survival of V. cholerae O3, O5, and V. cholerae O160 was enhanced up to 6, 8, and 14 days compared with suppressed survival of V. cholerae O4 and V. cholerae O11. The survival of V. cholerae O3, O4, O5, and O11 was not enhanced in the presence of the amoebae compared to previous studies on the survival of V. cholerae O1, V. cholerae O139, and V. mimicus that enhanced from days to weeks (Citation13–Citation15).
Furthermore, the intracellular growth of V. cholerae strains was investigated to examine their ability to grow and survive in A. castellanii. The results showed that V. cholerae O3, O4, O5, O11, and O160 could grow and survive inside the amoeba cells similar to what previously has been shown on the growth and survival of V. cholerae O1, V. cholerae O139, and V. mimicus inside A. castellanii (Citation13–Citation15).
Surprisingly, V. cholerae O4 and V. cholerae O160 cells were seen in the cytoplasmic vacuoles of trophozoites of A. castellanii only. The cysts of A. castellanii containing V. cholerae were not found indicating that intracellular V. cholerae might not suppress the trophozoites to undergo encystation. However, V. cholerae O160 showed more adaption, than other strains in this study, to survive in presence of the amoeba and intracellularly as well ( and ).
The V. cholerae species from the Baltic Sea examined in this study and our previous studies (Citation13–Citation15) showed a similar growth pattern and survival to the facultative intracellular bacteria Francisella tularensis, Shigella sonnei, and S. dysenteriae, which can grow and survive inside A. castellanii (Citation22, Citation23). Huws and Smith 2006 showed that Staphylococcus aureus could grow and survive intracellularly as well as extracellularly in amoebae. The numbers of viable amoebae in the presence or absence of S. aureus were not found to be significantly different (Citation24).
Interaction output of V. cholerae O3, O4, O5, O11, and O160 with A. castellanii was found to be different from that of the extracellular bacteria such as P. aeruginosa and Aeromonas species during interaction with A. castellanii. The presence or absence of the amoebae did not affect growth and survival of P. aeruginosa which instead kills the amoebae (Citation25). Moreover, Aeromonas hydrophila and A. veronii has been shown to inhibit growth of A. castellanii (Citation26). The Swedish isolates of V. cholerae in this study interacted as facultative intracellular bacteria since they grew and survived in cultivation medium, outside and inside the amoebae cells.
A. castellanii in this interaction supported survival of V. cholerae species rather than cholera toxigenic species. The behaviour of V. cholerae O3, O4, O5, O11, and O160 with A. castellanii highlighted the role of the FLA and their intracellular pathogenic microorganisms as risks for water quality.
In summary, interesting differences can be observed regarding the interaction between these strains and A. castellanii, where the amoebae dramatically prolong the survival of strain V. cholerae O160 and in contrast, dramatically reduce the survival of strain O4. This warrants future studies of the mechanisms behind bacterial defence to amoeba predation in these organisms.
Conflict of interest and funding
The authors declare that they have no conflict of interest.
Acknowledgements
The authors gratefully acknowledge Karolinska Institute for supporting this project.
References
- Euzeby JP. List of bacterial names with standing in nomenclature: a folder available on the Internet. Int J Syst Bacteriol. 1997; 47: 590–2. [PubMed Abstract].
- Aydanian A, Tang L, Morris JG, Johnson JA, Stine OC. Genetic diversity of O-antigen biosynthesis regions in Vibrio cholerae. Appl Environ Microbiol. 2011; 77: 2247–53. [PubMed Abstract] [PubMed CentralFull Text].
- Centers for Disease Control and Prevention (CDC). Summary of notifiable diseases – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012; 59: 1–111.
- Brown MR, Barker J. Unexplored reservoirs of pathogenic bacteria: protozoa and biofilms. Trends Microbiol. 1999; 7: 46–50. [PubMed Abstract].
- Backer H. Water disinfection for international and wilderness travelers. Clin Infect Dis. 2002; 34: 355–64. [PubMed Abstract].
- Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004; 17: 413–33. [PubMed Abstract] [PubMed CentralFull Text].
- Page FC. A new key to freshwater and soil Gymnamoebae. 1988; Ambleside, Cumbria, UK: Freshwater Biological Association.
- Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007; 50: 1–26. [PubMed Abstract].
- Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003; 16: 273. [PubMed Abstract] [PubMed CentralFull Text].
- Siddiqui R, Khan NA. Biology and pathogenesis of Acanthamoeba. Parasit Vectors. 2012; 5: 6. [PubMed Abstract] [PubMed CentralFull Text].
- Huq A, West PA, Small EB, Huq MI, Colwell RR. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar 01 associated with live copepods in laboratory microcosms. Appl Environ Microbiol. 1984; 48: 420–4. [PubMed Abstract] [PubMed CentralFull Text].
- Abd H, Shanan S, Saeed A, Sandström G. Survival of Vibrio cholerae inside Acanthamoeba and detection of both microorganisms from natural water samples may point out the amoeba as a Protozoal Host for V. cholerae . J Bacteriol Parasitol. 2011; 1–3.
- Abd H, Saeed A, Weintraub A, Nair GB, Sandstrom G. Vibrio cholerae O1 strains are facultative intracellular bacteria, able to survive and multiply symbiotically inside the aquatic free-living amoeba Acanthamoeba castellanii. FEMS Microbiol Ecol. 2007; 60: 33–9. [PubMed Abstract].
- Abd H, Saeed A, Weintraub A, Sandstrom G. Vibrio cholerae O139 requires neither capsule nor LPS O side chain to grow inside Acanthamoeba castellanii. J Med Microbiol. 2009; 58: 125–31. [PubMed Abstract] [PubMed CentralFull Text].
- Abd H, Valeru SP, Sami SM, Saeed A, Raychaudhuri S, Sandstrom G. Interaction between Vibrio mimicus and Acanthamoeba castellanii . Env Microbiol Rep. 2010; 2: 166–71.
- Smittskyddsinstitutet. Epidemiologisk årsrapport 2004. Solna; 2005. Available from: http://www.smittskyddsinstitutet.se/upload/Publikationer/Epi-arsrapport-050623.pdf [cited 16 November 2015].
- Steen A. Farliga bakterier i träbadkaret. Smittskydd. Contract No.: 5. Folkhälsomyndigheten; 2004.
- Collin B, Rehnstam-Holm AS. Occurrence and potential pathogenesis of Vibrio cholerae, Vibrio parahaemolyticus and Vibrio vulnificus on the South Coast of Sweden. FEMS Microbiol Ecol. 2011; 78: 306–13. [PubMed Abstract].
- Rehnstam-Holm AS, Collin B. [Vibrio species in the waters of Southern Sweden caused bath-wound fever. Increased bacteria frequency according to studies on clams]. Lakartidningen. 2009; 106: 435–8. [PubMed Abstract].
- Antibiotics SRGf. Web-page of the Swedish Reference Group for Antibiotics (SRGA). Available from: www.srga.org/RAFMETOD/etest.htm [cited 11 April 2008].
- Abd H, Weintraub A, Sandstrom G. Intracellular survival and replication of Vibrio cholerae O139 in aquatic free-living amoebae. Environ Microbiol. 2005; 7: 1003–8. [PubMed Abstract].
- Abd H, Johansson T, Golovliov I, Sandstrom G, Forsman M. Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Appl Environ Microbiol. 2003; 69: 600–6. [PubMed Abstract] [PubMed CentralFull Text].
- Saeed A, Abd H, Edvinsson B, Sandstrom G. Acanthamoeba castellanii an environmental host for Shigella dysenteriae and Shigella sonnei. Arch Microbiol. 2009; 191: 83–8. [PubMed Abstract].
- Huws SA, Smith AW, Enright MC, Wood PJ, Brown MR. Amoebae promote persistence of epidemic strains of MRSA. Environ Microbiol. 2006; 8: 1130–3. [PubMed Abstract].
- Abd H, Wretlind B, Saeed A, Idsund E, Hultenby K, Sandstrom G. Pseudomonas aeruginosa utilises its type III secretion system to kill the free-living amoeba Acanthamoeba castellanii. J Eukaryot Microbiol. 2008; 55: 235–43. [PubMed Abstract].
- Rahman M, Abd H, Romling U, Sandstrom G, Mollby R. Aeromonas-Acanthamoeba interaction and early shift to a viable but nonculturable state of Aeromonas by Acanthamoeba. J Appl Microbiol. 2008; 104: 1449–57. [PubMed Abstract].