Abstract
Background
Mycoplasma pneumoniae is a common cause of respiratory infections which can cause life-threatening pneumonia and serious extrapulmonary manifestations. Since the year 2000, the emergence of macrolide-resistant M. pneumoniae strains has increased with varying incidences across countries. In China more than 90% of the strains are resistant. M. pneumoniae diagnostics is mostly done with molecular methods, and in Sweden antibiotic resistance surveillance is not routinely performed. The prevalence of macrolide-resistant M. pneumoniae has not previously been studied in Sweden.
Material and methods
A total of 563 M. pneumoniae–positive respiratory samples, collected from four counties in Sweden between 1996 and 2013, were screened for mutations associated with macrolide resistance using a duplex FRET real-time PCR method. The real-time PCR targets the 23S rRNA gene, and differentiation between wild-type and resistant strains was achieved with a melting curve analysis.
Results
Of the 563 samples included, 548 were analyzed for mutations associated with macrolide resistance. No mutations were found. The detection rate of macrolide-resistant M. pneumoniae in this study was 0% [0.00–0.84%].
Conclusion
No macrolide-resistant M. pneumoniae has been detected in Sweden. However, the emergence and spread of macrolide-resistant M. pneumoniae strains in many countries commands continuous epidemiological surveillance.
The increased usage of antibiotics in both humans and in animal production exacerbates the progression of antibiotic resistance, which is a global threat (Citation1, Citation2). Diagnostics together with detection and awareness of antibiotic resistance is important to optimize the treatment of infections so that treatment failure, prolonged illness and suffering, and unnecessary use of antibiotics can be reduced.
Mycoplasma pneumoniae is a common cause of respiratory infections, which are often presented by mild, self-limiting upper respiratory infections but can also cause life-threatening pneumonia and serious extrapulmonary manifestations, such as encephalitis, pericarditis, dermatological disorders, and hemolytic anemia (Citation3). M. pneumoniae is fastidious and slow growing, so cultured isolates are therefore hard to obtain. This contributes to the current limitations of antibiotic resistance surveillance. Laboratory diagnostics is mostly performed by molecular detection of the bacteria or serology, where molecular detection is advantageous since it is a fast and sensitive method for detection of the bacteria in the acute phase of the infection (Citation3, Citation4). In Sweden, clinical laboratories do not routinely perform M. pneumoniae culturing or antibiotic resistance surveillance.
The emergence of macrolide-resistant M. pneumoniae was first observed in the year 2000 in Japan (Citation5). Since then, the incidence of infections caused by resistant M. pneumoniae in Asia has increased dramatically and recent reports from China describe that more than 90% of M. pneumoniae strains are macrolide resistant (Citation6, Citation7). Macrolide resistance is an emerging problem and is detected with varying prevalence from 1 to 30% in countries, such as Australia, Denmark, France, Germany, Israel, and the United States (Citation8–Citation13).
The macrolide resistance in M. pneumoniae is caused by point mutations in the peptidyl transferase loop of domain V of the 23S rRNA gene (Citation5, Citation14). The most common types of mutations are at position A2063G followed by A2064G and A2063C, which all give rise to high levels of macrolide resistance (Citation5–Citation7, Citation14). Other mutations associated with lower level of resistance and which are seldom encountered in clinical findings are A2067G, C2617A, C2617G, and A2063T (Citation5, Citation10) (Citation14, Citation15). Molecular methods for detection of mutations connected to macrolide resistance have been developed, which enable surveillance of macrolide resistance in samples from patients without the need of culturing (Citation16–Citation18).
Only one case of macrolide resistance, which was induced after macrolide treatment, has been described among Swedish M. pneumoniae patient samples (Citation19). Previously, there have been no large studies that aim to investigate if macrolide-resistant strains of M. pneumoniae circulate in the Swedish community. Among the Nordic countries, Denmark has detected 1–2% resistance, and no further studies have been performed in any of the other countries in our region (Citation9).
The aim of this study was to determine the prevalence of macrolide resistance of M. pneumoniae within a large number of patient samples in Sweden. M. pneumoniae–positive samples were analyzed with regard to the presence of macrolide resistance using a PCR method developed by Peuchant et al. (Citation16). This study and future surveillance of macrolide resistance in M. pneumoniae will provide the basis for validation of the currently used treatment guidelines.
Material and methods
Sample preparation and extraction
In this study, we included 563 M. pneumoniae–positive respiratory patient samples collected from four counties in Sweden during the period 1996–2013. Four-hundred and twenty-two (75%) of the samples were from the period 2010 to 2013. The samples had previously been diagnosed as M. pneumoniae–positive by PCR methodology at each of the four clinical microbiological laboratories in Falun, Gävle, Karlstad, and Uppsala, and then stored at −70°C until used in this study. More than 95% of the samples consisted of oropharyngeal or nasopharyngeal swab samples and less than 5% of the samples consisted of lower respiratory samples, where sputum or bronchial alveolar samples were the most common types. Before the start of the study, the samples were anonymized and only information about the patient's age, sex, at which county and year it was taken, and if the sample was taken at a policlinic or at a hospital was mapped to the samples.
The majority of the samples were extracted using MagNA pure 96 (Roche Diagnostics, Basel, Switzerland), DNA, and viral NA small volume kits using the program pathogen universal. The starting volume was 200 µL and the elution volume 100 µL, which was then aliquoted into two vials. Samples from one of the counties were extracted with MagNA pure compact (Roche Diagnostics, Basel, Switzerland) using an external lysis program with proteinase K. After verifying the presence of M. pneumoniae by real-time PCR, essentially as described previously (Citation20), the extracted DNA samples were aliquoted and stored at −70°C until macrolide resistance screening PCR was performed.
Since the patient samples were anonymized and the results could not be connected to patient identity, ethical approval was not required for this study.
Control strains
Reference strain ATCC 29342 (M129) was used as wild-type control. Four characterized macrolide-resistant strains derived from clinical samples, kindly received from Professor Cécile Bébéar, University of Bordeaux, France, were used as positive controls. These strains had the following mutations respectively: A2063C, A2063G, A2064G, and C2617G. DNA from each control strain was extracted using MagNA pure 96 (Roche Diagnostics, Basel, Switzerland), as described above. Sterile water was used as a negative control. The controls were included in each run.
Molecular screening of macrolide resistance
Screening of six different mutations of the 23S rRNA, all associated with macrolide resistance, was performed using a duplex FRET real-time PCR method developed by Peuchant et al. (Citation16). A wild-type strain is distinguished from mutant strains, harboring any of the following mutations: A2063C, A2063G, A2064G, A2067G, C2617A, and C2617G, by melting curve analysis showing a difference in melting temperatures (T m) (Citation16). The PCR analysis was performed using Cobas® z480 (Roche Diagnostics, Basel, Switzerland). Primers were obtained from Eurogentec (Liège, Belgium) and the probes from Sigma-Aldrich (St. Louis, USA). PCR mastermix included LightCycler® FastStart DNA Master HybProbe (Roche Diagnostics, Basel, Switzerland) and the composition of PCR mixture and design of PCR program was as described by Peuchant et al. with a small alteration of the melting step (Citation16). The melting step consisted of 95°C for 30 s, 40°C for 40 s followed by a slow rise of the temperature up to 80°C with a rate at 0.1°C/s with continuous acquisition of fluorescence.
Data analysis
Melting curve analysis was performed, and melting temperature (T m) values were determined by the software. Each melting curve and T m was also manually revised and adjusted if necessary in order to ensure that the T m-value represented a true melting peak.
Statistical calculations, using the modified Wald interval, were performed to establish the 95% confidence interval for the rate of macrolide resistance detected (Citation21).
Results
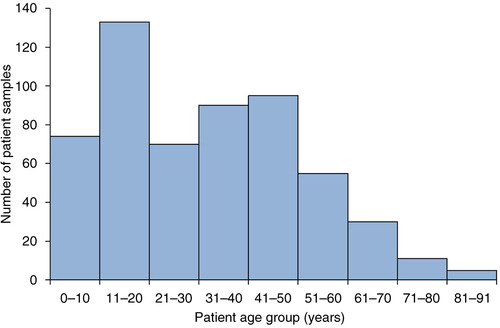
Most of the patients were between 11 and 20 years of age, and the median age was 32 years (range 1–91) (). The age distribution is representative and shows that M. pneumoniae infection can affect all ages, but is more common in adolescents, as described in the literature (Citation3). Two-hundred and ninety-four (52%) of the patients were women and 269 (48%) were men. One-hundred and thirty-eight (24.5%) of the samples were from inpatients, 405 (72%) were from outpatients, and for 20 (3.5%) of the samples information on where the sample had been taken was missing.
Of the 563 samples, 548 could be fully analyzed with the duplex FRET PCR and all samples showed melting curve concordant with the wild-type strain. No samples contained mutations at the positions in the 23S rRNA gene associated with macrolide resistance and thus the detection rate was 0% [0.00–0.84%]. In each run, all control strains showed melting peaks at expected temperatures, confirming the capability of resistance mutation detection (). The mean Ct-value for the 548 samples which could be fully analyzed was 24.2, with a standard deviation of 4.0.
Fig. 2 A representative example of a typical result from one of the channels (645 nm) in the duplex FRET real-time PCR showing distinct T m-differences of patient samples, aligned with the wild-type control strain ATCC 29342 and three control strains, respectively. The blue line without melting point corresponds to a negative sample with no amplification of M. pneumoniae DNA.
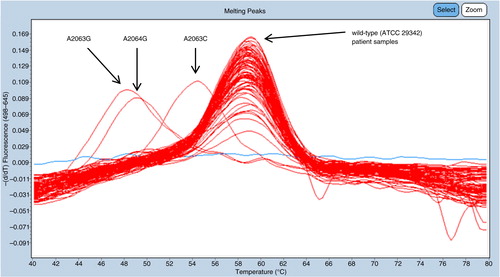
In addition, six samples could be only partially analyzed where results were obtained in only one of the channels in the duplex FRET PCR targeting the 262 base pair sequence, including the nucleotides at positions 2063, 2064, and 2067. A melting signal was obtained, concurrent with the wild-type for these six samples. No results were obtained from the other channel targeting the 495 base pair sequence, including the nucleotide at position 2617, and thus a potential mutation at this position could not be ruled out for these six samples. Nine samples could not be diagnosed, since no amplification or melting curves were acquired from these samples in the duplex FRET PCR. All of the samples that could not be analyzed or only partly analyzed contained low concentration of M. pneumoniae DNA, with Ct-values at the detection level. The mean Ct-value for the 15 samples not fully analyzed was 31.8, with a standard deviation of 1.6.
Discussions
The emergence of macrolide-resistant M. pneumoniae is a serious problem since macrolides are the only type of antibiotics suitable for the treatment of affected children aged <8 years (Citation3). In this study, we investigated the proportion of macrolide-resistant M. pneumoniae in Swedish specimens by screening 563 M. pneumoniae–positive samples for mutations associated with macrolide resistance by using a FRET real-time PCR. Macrolide resistance was not detected in any of the samples.
Previously, only one case of macrolide-resistant M. pneumoniae has been reported in Sweden, which was induced after treatment of erythromycin (Citation19). There have been other reports where M. pneumoniae with macrolide resistance has been induced by treatment (Citation12, Citation22) (Citation23). Treatment status for the patients included in this study is unknown, but the majority of the samples were taken from policlinics and therefore the sampling is assumed to have taken place before antibiotic treatment was initiated. According to Swedish treatment guidelines for respiratory infections within outpatient care, antibiotics should only be used under strict conditions and bronchitis caused by M. pneumoniae is not an indication for antibiotic treatment (Citation24). For adults, doxycycline is the treatment of choice for pneumonia caused by M. pneumoniae at policlinics, whereas doxycycline or erythromycin is the treatment of choice according to guidelines for hospital-treated M. pneumoniae (Citation24, Citation25). Erythromycin is the treatment of choice for children aged <8 years (Citation25). The majority of the samples included in this study was collected around the year of the epidemic peak of 2011, where there was a 25% rise in the prescription of tetracycline and macrolides when compared with the same period in 2009 (Citation26). There was a higher antibiotic burden in the population at that point, but macrolide resistance was not detected in any of the included samples.
Detection of macrolide resistance among specimens within the closely related species M. genitalium, which has the same mechanism of macrolide resistance, was recently performed in samples from two of the four counties included in this study (Citation27). M. genitalium–positive specimens from 2006 to 2011 were analyzed for mutations associated with macrolide resistance, and no mutations were detected from specimens collected in the period 2006–2007. However, afterward, an increase of specimens with mutations was detected each year with up to 21% of the specimens in 2011 (Citation27).
The treatment strategy for M. genitalium differs from that of M. pneumoniae and should, according to Swedish guidelines, always be treated with antibiotics when detected (Citation28). The treatment of choice for M. genitalium is azithromycin, and the previous recommendation of a single-dose treatment with 1 g azithromycin has been associated with the selection of macrolide-resistant M. genitalium strains, which could possibly explain the development of resistance in M. genitalium when compared with M. pneumoniae (Citation27). Further, doxycycline is a common choice of antibiotic for treatment of respiratory tract infections, including infections caused by M. pneumoniae, and thus the selective pressures of macrolides are probably lower for that species (Citation29). Compared with other European countries, Sweden has a generally low consumption of macrolides (Citation30). The use of macrolides and in particular azithromycin is avoided because of the indication that it favors the selection of resistant strains of, for example, Streptococcus pneumoniae, probably due to the long half-life of the substance (Citation31, Citation32).
Undetected macrolide-resistant M. pneumoniae could lead to exposure to prolonged illness for the patient. Studies have shown that hospitalized children infected with macrolide-resistant, as compared with macrolide sensitive, M. pneumoniae strains have a longer duration of fever, cough, and hospital stays (Citation33, Citation34). Zhou et al. also saw a higher prevalence of extrapulmonary complications in children infected with macrolide-resistant M. pneumoniae (Citation34). The high incidence of macrolide-resistant M. pneumoniae could result in the need to change treatment guidelines.
The method of resistance detection applied in this study enables antibiotic resistance surveillance screening with a high sensitivity, where 97% of the samples were successfully analyzed. This study showed no detection of macrolide-resistant M. pneumoniae in Sweden; whether this reflects a prudent use of macrolides remains to be investigated. Despite the situation in Sweden, the progress of antibiotic resistance in many countries and the dramatic spread of macrolide-resistant M. pneumoniae strains in Asia is a threat of great concern. This commands continuous epidemiological surveillance of macrolide resistance, and global strategies to minimize overuse of antibiotics must be a priority.
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study. This study was supported by grants received by Uppsala-Örebro Regional-Research Council and Uppsala County Council.
Acknowledgements
We thank Professor Cécile Bébéar, Associated Professor Sabine Pereyre and co-workers at the University of Bordeaux, France, for sharing knowledge about the screening method and providing control strains. We also thank Andreas Edberg, Department of Clinical Microbiology in Karlstad, as well as Britta Loré and Karin Elfving, Department of Clinical Microbiology in Falun, Sweden, for taking part in the study and providing patient samples from their counties.
References
- Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, etal. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014; 14: 742–50.
- Roca I, Akova M, Baquero F, Carlet J, Cavaleri M, Coenen S, etal. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 2015; 6: 22–9.
- Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004; 17: 697–728.
- Nilsson AC, Bjorkman P, Persson K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol. 2008; 8: 93.
- Matsuoka M, Narita M, Okazaki N, Ohya H, Yamazaki T, Ouchi K, etal. Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob Agents Chemother. 2004; 48: 4624–30.
- Zhou Z, Li X, Chen X, Luo F, Pan C, Zheng X, etal. Macrolide-resistant Mycoplasma pneumoniae in adults in Zhejiang, China. Antimicrob Agents Chemother. 2015; 59: 1048–51.
- Liu X, Jiang Y, Chen X, Li J, Shi D, Xin D. Drug resistance mechanisms of Mycoplasma pneumoniae to macrolide antibiotics. Biomed Res Int. 2014; 2014: 320801. [PubMed Abstract] [PubMed CentralFull Text].
- Xue G, Wang Q, Yan C, Jeoffreys N, Wang L, Li S, etal. Molecular characterizations of PCR-positive Mycoplasma pneumoniae specimens collected from Australia and China. J Clin Microbiol. 2014; 52: 1478–82.
- Rasmussen JN, Voldstedlund M, Andersen RL, Ellermann-Eriksen S, Jensen TG, Johansen HK, etal. Increased incidence of Mycoplasma pneumoniae infections detected by laboratory-based surveillance in Denmark in 2010. Euro Surveill. 2010; 15 pii=19708..
- Pereyre S, Touati A, Petitjean-Lecherbonnier J, Charron A, Vabret A, Bebear C. The increased incidence of Mycoplasma pneumoniae in France in 2011 was polyclonal, mainly involving M. pneumoniae type 1 strains. Clin Microbiol Infect. 2013; 19: E212–17.
- Dumke R, Luck C, Jacobs E. Low rate of macrolide resistance in Mycoplasma pneumoniae strains in Germany between 2009 and 2012. J Antimicrob Chemother. 2013; 57: 3460.
- Averbuch D, Hidalgo-Grass C, Moses AE, Engelhard D, Nir-Paz R. Macrolide resistance in Mycoplasma pneumoniae, Israel, 2010. Emerg Infect Dis. 2011; 17: 1079–82.
- Zheng X, Lee S, Selvarangan R, Qin X, Tang YW, Stiles J, etal. Macrolide-resistant Mycoplasma pneumoniae, United States. Emerg Infect Dis. 2015; 21: 1470–2.
- Bebear C, Pereyre S, Peuchant O. Mycoplasma pneumoniae: susceptibility and resistance to antibiotics. Future Microbiol. 2011; 6: 423–31.
- Liu Y, Ye X, Zhang H, Xu X, Li W, Zhu D, etal. Characterization of macrolide resistance in Mycoplasma pneumoniae isolated from children in Shanghai, China. Diagn Microbiol Infect Dis. 2010; 67: 355–8.
- Peuchant O, Menard A, Renaudin H, Morozumi M, Ubukata K, Bebear CM, etal. Increased macrolide resistance of Mycoplasma pneumoniae in France directly detected in clinical specimens by real-time PCR and melting curve analysis. J Antimicrob Chemother. 2009; 64: 52–8.
- Spuesens EB, Hoogenboezem T, Sluijter M, Hartwig NG, van Rossum AM, Vink C. Macrolide resistance determination and molecular typing of Mycoplasma pneumoniae by pyrosequencing. J Microbiol Methods. 2010; 82: 214–22.
- Wolff BJ, Thacker WL, Schwartz SB, Winchell JM. Detection of macrolide resistance in Mycoplasma pneumoniae by real-time PCR and high-resolution melt analysis. Antimicrob Agents Chemother. 2008; 52: 3542–9.
- Nilsson AC, Jensen JS, Bjorkman P, Persson K. Development of macrolide resistance in Mycoplasma pneumoniae-infected Swedish patients treated with macrolides. Scand J Infect Dis. 2014; 46: 315–19.
- Gullsby K, Storm M, Bondeson K. Simultaneous detection of Chlamydophila pneumoniae and Mycoplasma pneumoniae by use of molecular beacons in a duplex real-time PCR. J Clin Microbiol. 2008; 46: 727–31.
- Agresti A, Coull B. Approximate is better than ‘Exact’ for interval estimation of binomial proportions. Am Stat. 1998; 52: 119–29.
- Dumke R, Stolz S, Jacobs E, Juretzek T. Molecular characterization of macrolide resistance of a Mycoplasma pneumoniae strain that developed during therapy of a patient with pneumonia. Int J Infect Dis. 2014; 29: 197–9.
- Chironna M, Sallustio A, Esposito S, Perulli M, Chinellato I, Di Bari C, etal. Emergence of macrolide-resistant strains during an outbreak of Mycoplasma pneumoniae infections in children. J Antimicrob Chemother. 2011; 66: 734–7.
- Läkemedelsverket. Farmakologisk behandling av nedre luftvägsinfektioner i öppen vård. 2008; Swedish: Läkemedelsverket. 3.
- Svenska Infektionsläkarföreningen. Vårdprogram för samhällsförvärvad pneumoni. 2011; Swedish: Svenska Infektionsläkarföreningen.
- Linde A, Ternhag A, Torner A, Claesson B. Antibiotic prescriptions and laboratory-confirmed cases of Mycoplasma pneumoniae during the epidemic in Sweden in 2011. Euro Surveill. 2012; 17 pii=20082..
- Anagrius C, Lore B, Jensen JS. Treatment of Mycoplasma genitalium. Observations from a Swedish STD clinic. PLoS One. 2013; 8: e61481.
- Läkemedelsverket. Sexuellt överförbara bakteriella infektioner – behandlingsrekommendation: information från Läkemedelsverket. 2015; Läkemedelsverket: Swedish. 26.
- Swedres-Svarm. Public health agency of Sweden and National veterinary institute. Consumption of antibiotics and occurrence of antibiotic resistance in Sweden. 2014. Available from: https://www.folkhalsomyndigheten.se/pagefiles/20281/Swedres-Svarm-2014-14027.pdf [cited 19 April 2016]..
- European Centre for Disease prevention and Control. Antimicrobial consumption interactive database: ESAC-Net. Antimicrobial consumption rates by country. Available from: esac-net-database/Pages/Antimicrobial-consumption-rates-by-country.aspx [cited 19 April 2016]..
- Bergman M, Huikko S, Huovinen P, Paakkari P, Seppala H, Finnish Study Group for Antimicrobial R. Macrolide and azithromycin use are linked to increased macrolide resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2006; 50: 3646–50.
- Barkai G, Greenberg D, Givon-Lavi N, Dreifuss E, Vardy D, Dagan R. Community prescribing and resistant Streptococcus pneumoniae. Emerg Infect Dis. 2005; 11: 829–37.
- Cardinale F, Chironna M, Chinellato I, Principi N, Esposito S. Clinical relevance of Mycoplasma pneumoniae macrolide resistance in children. J Clin Microbiol. 2013; 51: 723–4.
- Zhou Y, Zhang Y, Sheng Y, Zhang L, Shen Z, Chen Z. More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother. 2014; 58: 1034–8.