Abstract
Background
Although the prevalences of infection with the protozoan parasites Cryptosporidium spp. and Giardia duodenalis in humans appear to be relatively high in the Canadian North, their transmission patterns are poorly understood.
Objective
To determine the detection rate and the molecular characteristics of Cryptosporidium spp. and Giardia duodenalis in diarrhoeic patients in the Qikiqtani (Baffin Island) Region of Nunavut, Canada, in order to better understand the burden of illness and the potential mechanisms of transmission.
Study design/methods
Diarrhoeal stool specimens (n=108) submitted to the Qikiqtani General Hospital for clinical testing were also tested for the presence of Cryptosporidium spp. and Giardia duodenalis using epifluorescence microscopy and polymerase chain reaction (PCR). DNA sequencing and restriction fragment length polymorphism (RFLP) analyses were performed on PCR-positive specimens to determine the species, genotypes and sub-genotypes of the parasites.
Results
Cryptosporidium was detected in 15.7% of the diarrhoeic patients, while Giardia was detected in 4.6%. DNA sequencing of a fragment of the small subunit rRNA gene indicated that all of the Cryptosporidium amplicons had a 100% homology to C. parvum, and a gp60 assay showed that all aligned with C. parvum sub-genotype IIa. Microsatellite analysis revealed 3 cases of sub-genotype IIaA15G2R1, 2 of IIaA15G1R and 1 case each of sub-genotypes IIaA16G1R1 and IIaA15R1. For Giardia, results based on the amplification of both the 16S rRNA gene and the gdh gene were generally in agreement, and both DNA sequencing and RFLP demonstrated the presence of the G. duodenalis Assemblage B genotype.
Conclusions
Both C. parvum and G. duodenalis Assemblage B were present in human diarrhoeal stool specimens from Nunavut, which was suggestive of zoonotic transmission, although human-to-human transmission cannot be ruled out. To fully understand the public health significance of the different Cryptosporidium and Giardia species and genotypes in diarrhoeic patients, it will be imperative to establish the extent of genetic diversity within these parasites through comprehensive studies of the molecular epidemiology of cryptosporidiosis and giardiasis in the Nunavut region.
Cryptosporidium spp. and Giardia duodenalis are enteric protozoan parasites found worldwide in a large number of different hosts, including humans. There are currently more than 20 valid species of Cryptosporidium, and greater than 40 distinct genotypes. However, 90% or more of human infections involve C. hominis, which is found primarily in humans, and C. parvum, which is an important zoonotic species (Citation1). Several other species of Cryptosporidium, as well as several genotypes, have also been reported in humans (Citation2). Of the 6 valid species of Giardia, only G. duodenalis (syn. G. lamblia and G. intestinalis) is of public health concern. G. duodenalis consists of 8 assemblages or genotypes (A-H). The vast majority of human infections involve Assemblages A and B, with only rare reports of other assemblages in humans (Citation3). Both Assemblages A and B are zoonotic, with Assemblage A being commonly found in livestock, companion animals, and wildlife, and Assemblage B being commonly reported in wildlife, and only occasionally in livestock (Citation3).
Cryptosporidiosis is often a self-limiting illness characterized by watery diarrhoea and a variety of other symptoms including cramping, abdominal pain, weight loss, nausea, vomiting, fever, and headache (Citation2). Symptoms can be severe, or even life-threatening, in immunocompromised individuals, and chronic intestinal cryptosporidiosis is an AIDS-defining illness (Citation1). Similarly, giardiasis is generally a self-limiting illness, which may result in diarrhoea, abdominal cramps, bloating, weight loss, and malabsorption (Citation3). There are also emerging data to suggest that the added energetic burden of enteric protozoan infections (including asymptomatic non-diarrhoeal infections which can often be persistent) may lead to an increased risk of environmental enteropathy and undernutrition and, ultimately, to worse neurocognitive outcomes and school performance (Citation4, Citation5). Although initially found in children in resource-limited countries, this phenomenon of environmental enteropathy has also been well described in indigenous children in Australia with Cryptosporidium infections in particular being implicated (Citation6, Citation7). This may also be relevant in Inuit communities where food insecurity has been shown to be associated with shorter stature (Citation8), and where the incidence of acute gastrointestinal illness appears to be much higher than other developed country settings (Citation9).
In the Canadian North, defined in this study as the 3 territories and northern regions of Quebec and Labrador, there are only very limited data on the prevalence and the molecular epidemiology of Cryptosporidium and Giardia infections in humans (Citation10, Citation11). While the prevalence of cryptosporidiosis is thought to be only slightly higher in the north than the Canadian average, the residents of Canada's 3 territories have a considerably higher per capita rate of giardiasis than the Canadian average (Citation10). Several older studies have reported Giardia in humans in northern Canada, with a prevalence ranging from 2 to 29% (Citation10). None of these studies, however, identified the species or genotypes of the parasites. Using nanolitre real-time polymerase chain reaction (PCR), we recently identified Cryptosporidium spp. in 19.8% (17/86) and G. duodenalis in 1.1% (1/86) of diarrhoeal stools collected from patients in Nunavut (Citation11). This Cryptosporidium spp. detection rate was amongst the highest ever reported in non-HIV-infected populations (Citation12).
Transmission generally occurs by means of ingestion of Cryptosporidium oocysts or Giardia cysts through the faecal-oral route, which involves direct contact with human or animal faeces, and is often associated with poor hygiene and sanitation. Transmission may also occur indirectly through faecally contaminated drinking water and is much more common in regions where water treatment is limited. Very few studies have reported on the levels of contamination of Cryptosporidium and Giardia in water sources in the Canadian North. In the Yukon Territory, remote, pristine water samples were found to be contaminated with Giardia cysts in 32% of samples but none were contaminated with Cryptosporidium oocysts (Citation13). These authors also reported Giardia cyst contamination in 17% of drinking water samples in 1 community in the Yukon, and Cryptosporidium oocysts were found to contaminate 5% of samples. With the limited data available, it is not clear whether these water sources may be contaminated with human sewage or with the faeces of infected animals, or both. Zoonotic and foodborne transmission are also of concern in the Canadian North and may occur through direct contact with dogs and wildlife, or indirectly through faecally contaminated foods. As raw meat and organ tissue from marine and terrestrial mammals, fish, and shellfish are regularly consumed in the north, the risk to consumers in these regions is generally higher, due to potential cross-contamination with intestinal contents during butchering. Dried intestines from ringed seals, for example, are commonly consumed, and these animals have been reported to be infected with both Cryptosporidium and Giardia (Citation14–Citation16). A few other northern marine mammals have also been reported as hosts for G. duodenalis or Cryptosporidium spp., including bearded seals and bowhead whales (Citation16, Citation17). There have been several reports of Giardia in terrestrial animals in northern Canada. Giardia cysts have been reported in faeces from dogs and a variety of wild animals including caribou, Dall's sheep, muskoxen, coyote, grizzly bear, wolf, beaver, and muskrat (Citation10). The prevalence of Cryptosporidium spp. in terrestrial animals is, however, thought to be relatively low in the north, and Cryptosporidium oocysts have only been reported in caribou and dogs in these regions (Citation10). Cryptosporidium and Giardia have also been reported in blue mussels harvested in the Nunavik region of Quebec (Citation17).
To date, there is little information available on the species and genotypes of Cryptosporidium and Giardia in wildlife and domestic animals in the Canadian North. C. muris has been reported in ringed seals and blue mussels in Nunavik, Quebec (Citation17, Citation18). This is of some concern to public health as both are important traditional food items, and C. muris has been previously reported in humans in a number of countries (Citation19). With Giardia, only the zoonotic Assemblages A and B have been reported in wildlife and dogs in the Canadian North (Citation16, Citation20) (Citation21), suggesting that zoonotic transmission commonly occurs amongst people, dogs, and wildlife (Citation10).
It is not yet clear to what extent these different sources may contribute to human infections in the Canadian North, and as such there is a need for further molecular epidemiological studies on Cryptosporidium and Giardia in humans and animals in this region. The objectives of the present study, therefore, were to determine the detection rate and the molecular characteristics of Cryptosporidium spp. and G. duodenalis in diarrhoeic patients in the Qikiqtani (Baffin Island) Region of Nunavut, Canada, to better understand the burden of illness and the potential mechanisms of transmission in this region.
Materials and methods
Sample collection
This study was performed on 108 stool samples from diarrhoeic patients originally submitted to the Qikiqtani General Hospital laboratory in Iqaluit, Nunavut, from January 2010 to June 2011. Qikiqtani General Hospital serves the Qikiqtani (Baffin Island) Region of Nunavut (). Some of these stool samples were also submitted to outpatient clinics in communities in this region. All samples were submitted by clinicians for bacterial culture testing as part of routine clinical care. A portion of each stool sample was stored at −20°C and shipped frozen to the Children's Hospital of Eastern Ontario (CHEO) research laboratory in Ottawa, Ontario, for pathogen testing. Eighty-six samples were tested for multiple diarrhoeal pathogens on a nanolitre real-time PCR platform, the results of which have been described previously (Citation11), and 22 additional samples were collected after completion of the initial nanolitre PCR study. Ethics approval for this study was obtained from both the CHEO Research Ethics Committee and the Nunavut Research Institute. Given that this was a laboratory-based surveillance study, and consent was not obtained from individual subjects, the only information collected was the date of stool submission, and there was no information on community of origin, age, gender or clinical outcome.
Fig. 1. Map of Qikiqtani Region of Nunavut, Canada, © Government of Canada, Source: Library and Archives Canada's website (www.collectionscanada.ca).
Microscopy
Faecal samples were examined microscopically for the presence of Cryptosporidium oocysts and for Giardia cysts prior to molecular testing. For each sample, 150 µl of faecal suspension in phosphate-buffered saline (PBS), pH 7.4, was transferred to a microcentrifuge tube. Fluorescein isothiocyanate-labelled monoclonal antibody solution (50 µl each of Crypt-a-Glo and Giardi-a-Glo; Waterborne, Inc., New Orleans, LA) was added to the tube, which was then vortexed. The tube was then incubated at room temperature for 45 min in the dark. After incubation, the excess antibody was removed by adding 1 ml of PBS, vortexing, and centrifuging at 10,000×g for 10 min. The supernatant was then pipetted off, leaving 100 µl, which was then used to re-suspend the pellet. Twenty microlitres was added to a microscope slide and examined at 200× on a Nikon Eclipse E600 epi-fluorescence microscope (Nikon Canada, Inc. Instruments, Mississauga, ON) to confirm the presence of Cryptosporidium oocysts and Giardia cysts.
Concentration of cysts/oocysts from stool samples using immunomagnetic separation
A total of 108 stool samples were used for molecular characterization. The samples were purified by immunomagnetic separation (IMS) according to manufacturer's instructions in the Dynal IMS Kit from Dynabeads GC-Combo kit (Cat. no. 730.12, Invitrogen Dynal AS, Oslo, Norway). Briefly, 10 ml of sample suspension was transferred into a tube containing 1 ml of 10% SL buffer A and buffer B, and 100 µl of Dynabeads anti-Cryptosporidium and anti-Giardia. The tube was affixed to a rotating mixer and was rotated at approximately 18 rpm for 1 h at room temperature. The sample tube was removed from the mixer and placed in the magnetic particle concentrator (MPC-1). The sample was then rocked gently for 2 min, and all supernatant was decanted from the tube. The capture tube was then removed from the MPC-1, and the sample was resuspended in 1 ml of 1% SL Buffer A. The mixture was then transferred into a 1.5 ml microcentrifuge tube. The microcentrifuge tube was placed into a second magnetic particle concentrator (MPC-S) with a magnetic strip in place. The tube was gently rocked/rolled for 1 min. Without removing the tube from the MPC-S, the supernatant was gently aspirated from the tube. The oocysts/cysts beads complex was stored at −20°C until further use.
DNA extraction
Genomic DNA was extracted from the IMS-concentrated oocysts/cysts using QIAgen DNA Mini Kit (Qiagen, Inc., Mississauga, ON) with a slightly modified protocol. Briefly, IMS-concentrated oocysts/cysts were resuspended in lysis buffer and subjected to 5 consecutive cycles of freezing in liquid nitrogen for 1 min and thawing at 56°C for 2 min, with vortexing for 30 sec for every cycle, to rupture the Cryptosporidium oocysts and Giardia cysts. DNA was extracted with proteinase K (20 mg/ml) and then purified using the DNA Mini Kit. DNA was eluted in 50 µl of elution buffer (Qiagen) and stored at −20°C until further use.
Cryptosporidium PCR
For Cryptosporidium detection and characterization, a nested-PCR procedure was performed to amplify a ~435 bp polymorphic fragment of the small subunit (SSU) rRNA gene (Citation22). For further genotyping and sub-genotyping, a 450 bp fragment of the 60 kDa glycoprotein (gp60) gene was amplified according to the protocol described by Iqbal et al. (Citation23).
Positive control (extracted DNA of C. parvum oocysts purchased from Waterborne, Inc., New Orleans, LA) and negative control (DNase-free water instead of DNA template) were included in each amplification and run along with samples on each agarose gel. The quality and banding intensity of individual amplicons were examined on 1.2% agarose gels containing GelRed (5 ml/100 ml) (Biotium, Inc., Hayward, CA).
Giardia PCR
For Giardia, a nested procedure was performed to amplify a 292 bp fragment of the 16S rRNA gene as described by Coklin et al. (Citation24). A 432 bp fragment of glutamate dehydrogenase (gdh) gene was amplified according to Read et al. (Citation25).
Positive control (extracted DNA of G. duodenalis cysts purchased from Waterborne, Inc., New Orleans, LA) and negative control (DNase-free water instead of DNA template) were included in each amplification and run along with samples on each agarose gel. The quality and banding intensity of individual amplicons were examined on 1.2% agarose gels containing GelRed (5 ml/100 ml; Biotium, Inc., Hayward, CA).
Restriction fragment length polymorphism of the Giardia gdh gene
Restriction fragment length polymorphism (RFLP) map analysis was carried out, and the restriction profiles for the enzymes Nla IV (capable of discriminating amongst all the major assemblages, and between subgroups AI and AII) and Rsa I (capable of discriminating between subgroups BIII and BIV) were determined for Giardia gdh PCR-positive samples (Citation25). Restriction digests were carried out directly on PCR products in 20 µl reactions. Ten microlitres of PCR product was added to 1% reaction buffer, 2U of Nla IV and 2U of Rsa I (New England Biolabs, Ipswich, MA). Digestion took place at 37°C for 2 h. Profiles were visualized on 2% agarose gels containing GelRed.
Purification of PCR products
PCR products were purified using a Mini Elute PCR purification kit (Cat. no. 28004, Qiagen) according to the manufacturer's protocol. A minimal volume of elution buffer was added to the column so as not to decrease the concentration of the eluted DNA.
DNA sequence analysis
The PCR targets used in the present study (SSU rRNA and gp60 genes for Cryptosporidium and the 16S rRNA gene for Giardia) were subjected to bidirectional, automated sequencing (ABI PRISM BigDye Terminator v3.1 Cycle Sequencing Kit, Applied Biosystems, CA, USA) using the same primers as employed in the secondary PCR.
The DNA sequences derived from the SSU amplicons were compared with available target sequences in GenBank, representing C. parvum (accession nos. AF093493 and EU553557) (Citation26, Citation27). The DNA sequence of the Giardia 16S rRNA gene fragment was compared with G. duodenalis Assemblage B (accession nos. HQ616612 and HQ179642) (Citation28, Citation29).
DNA sequences for 7 gp60-positive amplicons of Cryptosporidium were obtained directly from the nested-PCR amplicons and sequenced in forward and reverse directions. All 7 pairs of sequences were analysed using BioEdit v7.2.2 (Citation30). Multiple alignments were performed using Clustal W (Citation31), and neighbour-joining trees were constructed from the aligned sequences using MEGA5 software (Citation32). Gp60 sequences were compared with reference sequences of C. parvum IIa (accession nos. DQ192504, FJ917373 and JF727774) (Citation33–Citation35), and with other C. parvum genotype families (i.e. IIa to IIj inclusive), with C. hominis (accession no. AY738187) (Citation36) being used as an out-group.
Microsatellite analysis of Cryptosporidium gp60 sub-genotypes
All 7 Cryptosporidium gp60 DNA sequences were analysed for “TCA” microsatellite region. The gp60 analyses displayed high mutation rates, in particular, a “hyper-variable” microsatellite region. The gp60 sub-genotype “TCA” microsatellite region, showed triplet codons that were categorized according to the number of trinucleotide repeats coding for the amino acid serine. Cryptosporidium gp60 sub-genotypes consist of a variable number of “A” (TCA), “G” (TCG), “T” (TCT) and “R” (ACATCA) as described by Jex and Gasser (Citation37).
Results
Examination of faecal samples for the presence of Cryptosporidium and Giardia by microscopy and PCR revealed a total of 22 positives (20.3%) amongst the 108 diarrhoeic patients. Of the 22 positive samples, Cryptosporidium was detected in 17 (15.7%) and Giardia was detected in 5 (4.6%) (Table ). None of the positive samples showed mixed infections with both Cryptosporidium and Giardia. Detection rate was based on the total number of positive stool samples detected by either microscopy or PCR.
Table I. Detection of Cryptosporidium and Giardia infections in diarrhoeic patients from Nunavut (n=108) by polymerase chain reaction (PCR) and microscopy
For Cryptosporidium, 17 (15.7%) samples were PCR-positive. Of these PCR-positives, 8 were confirmed by immunofluorescence microscopy. Following nested-PCR targeting the Cryptosporidium SSU rRNA gene, amplicons were obtained from 15 of 17 (88.2%) Cryptosporidium-positive diarrhoeic patients. To determine the species of Cryptosporidium, all positive amplicons were successfully sequenced. BLAST results of the 15 sequences showed 100% homology with C. parvum (accession nos. AF093493 and EU553557).
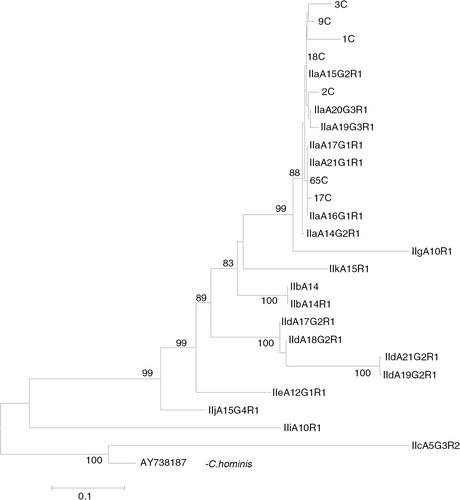
Nested PCR targeting the Cryptosporidium 60 kDa glycoprotein (gp60) gene resulted in a distinct band at 450 bp in 7 of 17 (41.1%) samples. BLAST results of these gp60-positive samples showed that all aligned with C. parvum sub-genotype IIa. Phylogenetic analysis of the sequence data of gp60 C. parvum genotype IIa was conducted using the neighbour-joining method (). The gp60 Cryptosporidium-positive amplicons and the reference sequences of C. parvum were identified as genotype IIa. C. parvum genotype IIa from the present study clustered with reference sequences of C. parvum genotypes IIa (accession nos. A14G2R1, A17G1R1, A19G3R1, A20G3R1, A22G3R1 and A23G3R1).The evolutionary distances were computed using the Kimura 2-parameter method, using C. hominis as an out-group.
Fig. 2. Phylogenetic analysis of Cryptosporidium parvum gp60 sequence data using neighbour-joining analysis. Sequences from the present study (3C, 9C, 1C, 18C, 2C, 65C and 17C) as well as reference sequences representing C. parvum sub-genotypes (acquired from GenBank) are indicated. Evolutionary distances were computed using the Kimura 2-parameter method. Bootstrap values>75% from 1,000 replicates are shown.
Microsatellite analysis revealed that, in the present study, C. parvum infecting these diarrhoeic patients comprised genotype IIa, including 3 cases of sub-genotype IIaA15G2R1, 2 of IIaA15G1R and 1 case each of sub-genotypes IIaA16G1R1 and IIaA15R1 (Table ).
Table II. Microsatellite analysis of Cryptosporidium gp60 subtypes in diarrhoeic patients from Nunavut (n=7)
For Giardia, 5 samples (4.6%) were PCR-positive and 3 of these were confirmed by immunofluorescence microscopy. PCR amplification of the Giardia 16S rRNA gene produced targeted amplicons in all 5 Giardia-positive samples, which were successfully sequenced. The Giardia amplicons that were sequenced showed a 100% homology to G. duodenalis Assemblage B (accession nos. HQ179642 and HQ616612). Results based on the amplification of both the 16S rRNA gene and the gdh gene were generally in agreement, although the latter occasionally picked up positives that tested negative by 16S rRNA, justifying the use of multiple genes in studies such as this. Four of 5 (80%) samples were positive by PCR using the gdh gene, which is a species-specific marker. PCR products of Giardia-positive samples were sequenced, prior to RFLP analysis, to compare the genotyping results obtained by DNA sequencing and by RFLP of the gdh gene. Amplicons that exhibited Assemblage B profiles were digested with Rsa I, and 2 profiles were identified as subgroups BIII and BIV based on banding patterns. There was a 100% agreement between the genotyping results obtained by sequencing and by RFLP.
Discussion
In the present study, both C. parvum and G. duodenalis were identified in faecal samples obtained from diarrhoeic patients in the Qikiqtani Region of Nunavut, Canada. This study represents one of very few such studies on the detection of Cryptosporidium spp. and G. duodenalis in humans in the Canadian North, and is the first to report on the molecular characteristics of these parasites. Interestingly, the number of cases of Cryptosporidium infection (15.7%) was higher in these patients than that of Giardia (4.6%), which was unexpected given that Giardia infections are thought to be much more common in the north than Cryptosporidium (Citation10). Co-infections were not identified in the present study, possibly due to the relatively small sample size. However, a limitation of this study was that we only tested samples that were submitted by clinicians for bacterial culture as part of clinical care. Samples submitted for parasitology (microscopy) were in fixative and, therefore, could not be tested with PCR. A prospective study would be required to determine the true incidence of infection with Cryptosporidium and Giardia in people with and without diarrhoea.
Recently, advances in PCR-based molecular techniques have increased the sensitivity of detection of both Cryptosporidium and Giardia in faecal samples, as well as allowing for high-resolution molecular characterization. In the present study, nested PCR targeting the gp60 gene demonstrated that all of the Cryptosporidium infections were of the C. parvum genotype IIa. All sequences were identical in the non-repeat region (i.e. they all had 1 copy of sequence ACATCA immediately after the trinucleotide repeats), while the trinucleotide repeat region contained 1 TCG and 2 copies of the TCG repeat, whereas there were 15 and 16 copies of TCA. Of the 7 gp60-positive amplicons, 3 were further identified as being of the sub-genotype IIaA15G2R1, while 2 were sub-genotype IIaA15G1R1, and there was 1 each of sub-genotype IIaA16G1R1 and IIaA15R1.
Recent genotyping studies have shown that some C. parvum genotype families (IIb, IIc, IIe) are only present in humans, whereas C. parvum genotype families IIa and IId have been reported in both humans and animals (Citation33, Citation36) (Citation38). Human infections with C. parvum genotype family IIa are commonly seen in areas with intensive animal production, such as the UK, Portugal, Slovenia, southeastern Australia and rural areas of the North America (Citation38–Citation43). The relatively high occurrence of zoonotic infections in these areas is likely because cattle and sheep are commonly infected with C. parvum of the genotype family IIa. Furthermore, some of the sub-genotypes within this family have been reported in both humans and animals in the same area (Citation40, Citation41). The most common C. parvum IIa sub-genotype in the present study (IIaA15G2R1) is a common sub-genotype and has been widely reported from various regions in the UK and in the United States, as well as in Canada (Ontario) (Citation31, Citation33) (Citation38–Citation40, Citation42) (Citation44–Citation46). The high occurrence of this sub-genotype in Nunavut is interesting as the typical livestock reservoirs are absent. Further molecular epidemiological work will be required to determine if this sub-genotype is transmitted human-to-human or if wildlife reservoirs may exist in this region.
In the present study, all of the G. duodenalis infections in the diarrhoeic patients were found to belong to the zoonotic genotype, Assemblage B, which is commonly reported in humans and in wildlife worldwide, including some northern mammals. The exclusive presence of G. duodenalis Assemblage B in diarrhoeic patients in this study, therefore, is suggestive of zoonotic or foodborne transmission. However, since RFLP analysis demonstrated the presence of the subgroups BIII and BIV, which are generally associated with human infections (Citation25), the source of infection is unclear and, as with Cryptosporidium, further work is required to fully understand the transmission patterns of Giardia in this region.
Conclusions
Cryptosporidium parvum oocysts, and to a lesser degree Giardia duodenalis cysts, were present in the faeces of diarrhoeic patients in the Qikiqtani Region of Nunavut in northern Canada. The present study represents the first molecular characterization of these parasites in humans in the Canadian North and provides some evidence for possible zoonotic or foodborne transmission. To fully understand the public health significance of the different Cryptosporidium and Giardia species and genotypes in diarrhoeic patients, it will be imperative to determine the extent of genetic diversity within these parasites through comprehensive studies of the burden and molecular epidemiology of cryptosporidiosis and giardiasis in Nunavut and other northern regions.
Conflict of interest and funding
This project was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Visiting Fellowships in Canadian Government Laboratories Program (A.I.).
Acknowledgements
The authors thank Dr. Maureen Baikie, Nunavut Department of Health, for facilitating the study. They also thank Ioana Moldovan for assembling the samples used in this study. They also extend their appreciation to the patients from the Qikiqtani Region of Nunavut who participated in this study, and staff at the Qikiqtani General Hospital who assisted the project.
References
- Bouzid M, Hunter PR, Chalmers RM, Tyler KM. Cryptosporidium pathogenicity and virulence. Clin Microbiol Rev. 2013; 26: 115–34. [PubMed Abstract] [PubMed CentralFull Text].
- Chalmers RM, Davies AP. Minireview: clinical cryptosporidiosis. Exp Parasitol. 2010; 124: 138–46. [PubMed Abstract].
- Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. 2011; 24: 110–40. [PubMed Abstract] [PubMed CentralFull Text].
- Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002; 359: 564–71. [PubMed Abstract].
- Bartelt LA, Lima AA, Kosek M, Peñataro Yori P, Lee G, Guerrant RL. “Barriers” to child development and human potential: the case for including the “neglected enteric protozoa” (NEP) and other enteropathy-associated pathogens in the NTDs. PLOS Negl Trop Dis. 2013; 7: e2125. [PubMed Abstract] [PubMed CentralFull Text].
- Kukuruzovic R, Brewster DR, Gray E, Anstey NM. Increased nitric oxide production in acute diarrhoea is associated with abnormal gut permeability, hypokalaemia and malnutrition in tropical Australian aboriginal children. Trans R Soc Trop Med Hyg. 2003; 97: 115–20. [PubMed Abstract].
- Kukuruzovic R, Robins-Browne RM, Anstey NM, Brewster DR. Enteric pathogens, intestinal permeability and nitric oxide production in acute gastroenteritis. Pediatr Infect Dis J. 2002; 21: 730–9. [PubMed Abstract].
- Pirkle CM, Lucas M, Dallaire R, Ayotte P, Jacobson JL, Jacobson SW, etal. Food insecurity and nutritional biomarkers in relation to stature in Inuit children from Nunavik. Can J Public Health. 2014; 105: e233–8. [PubMed Abstract].
- Harper SL, Edge VL, Ford J, Thomas MK, Pearl DL, Shirley J. Acute gastrointestinal illness in two Inuit communities: burden of illness in Rigolet and Iqaluit, Canada. Epidemiol Infect. 2015; 1–16.
- Jenkins EJ, Castrodale LJ, de Rosemond S, Dixon B, Elmore SA, Gesy KM, etal. Tradition and transition: parasitic zoonoses of people and animals in Alaska, Northern Canada, and Greenland. Adv Parasitol. 2013; 82: 33–204. [PubMed Abstract].
- Goldfarb D, Dixon B, Moldovan L, Barrowman N, Mattison K, Zentner C. Nanolitre real-time PCR detection of bacterial, parasitic, and viral agents from patients with diarrhoea in Nunavut, Canada. Int J Circumpolar Health. 2013; 72: 19903. doi: http://dx.doi.org/10.3402/ijch.v72i0.19903 [PubMed Abstract].
- Shirley DA, Moonah SN, Kotloff KL. Burden of disease from cryptosporidiosis. Curr Opin Infect Dis. 2012; 25: 555–63. [PubMed Abstract] [PubMed CentralFull Text].
- Roach PD, Olson ME, Whitley G, Wallis PM. Waterborne Giardia cysts and Cryptosporidium oocysts in the Yukon, Canada. Appl Environ Microbiol. 1993; 59: 67–73. [PubMed Abstract] [PubMed CentralFull Text].
- Olson ME, Roach PD, Stabler M, Chan W. Giardiasis in ringed seals from the western Arctic. J Wildl Dis. 1997; 33: 646–48. [PubMed Abstract].
- Hughes-Hanks JM, Rickard LG, Panuska C, Saucier JR, O'Hara TM, Dehn L, etal. Prevalence of Cryptosporidium spp. and Giardia spp. in five marine mammals species. J Parasitol. 2005; 91: 1225–8. [PubMed Abstract].
- Dixon BR, Parrington LJ, Partenteau M, Leclair D, Santin M, Fayer R. Giardia duodenalis and Cryptosporidium spp. in the intestinal contents of ringed seals (Phoca hispida) and bearded seals (Erignathus barbatus) in Nunavik, Quebec, Canada. J Parasitol. 2008; 94: 1161–3. [PubMed Abstract].
- Lévesque B, Barthe C, Dixon BR, Parrington LJ, Martin D, Doidge B, etal. Microbiological quality of blue mussels (Mytilus edulis) in Nunavik, Quebec: a pilot study. Can J Microbiol. 2010; 56: 968–77.
- Santín M, Dixon BR, Fayer R. Genetic characterization of Cryptosporidium isolates from ringed seals (Phoca hispida) in Northern Quebec, Canada. J Parasitol. 2005; 91: 712–16.
- Fayer R. Taxonomy and species delimitation in Cryptosporidium. Exp Parasitol. 2010; 124: 90–7. [PubMed Abstract].
- Kutz SJ, Thompson RCA, Polley L, Kandola K, Nagy J, Wielinga CM. Giardia assemblage A: human genotype in muskoxen in the Canadian Arctic. Parasit Vectors. 2008; 1: 32. [PubMed Abstract] [PubMed CentralFull Text].
- Salb AL, Barkema HW, Elkin BT, Thompson RCA, Whiteside DP, Black SR, etal. Dogs as sources and sentinels of parasites in humans and wildlife, northern Canada. Emerg Infect Dis. 2008; 14: 60–3. [PubMed Abstract] [PubMed CentralFull Text].
- Nichols RAB, Campbell BM, Smith HV. Identification of Cryptosporidium spp. oocysts in United Kingdom noncarbonated natural mineral waters and drinking waters by using a modified nested PCR-restriction fragment length polymorphism assay. Appl Environ Microbiol. 2003; 69: 4183–9. [PubMed Abstract] [PubMed CentralFull Text].
- Iqbal A, Lim YA, Surin J, Sim BL. High diversity of Cryptosporidium subgenotypes identified in Malaysian HIV/AIDS individuals targeting gp60 gene. PLoS One. 2012; 7: e31139. [PubMed Abstract] [PubMed CentralFull Text].
- Coklin T, Farber J, Parrington L, Dixon B. Prevalence and molecular characterization of Giardia duodenalis and Cryptosporidium spp. in dairy cattle in Ontario, Canada. Vet Parasitol. 2007; 50: 297–305.
- Read MC, Monis PT, Thompson RCA. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infect Genet Evol. 2004; 4: 125–30. [PubMed Abstract].
- Xiao L, Escalante L, Yang C, Sulaiman I, Escalante AA, Montali RJ, etal. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl Environ Microbiol. 1999; 65: 1578–83. [PubMed Abstract] [PubMed CentralFull Text].
- Pedraza-Diaz S, Ortega-Mora LM, Carrion BA, Navarro V, Gomez-Bautista M. Molecular characterisation of Cryptosporidium isolates from pet reptiles. Vet Parasitol. 2009; 160: 204–10. [PubMed Abstract].
- Martínez-Díaz RA, Sansano-Maestre J, Martínez-Herrero Mdel C, Ponce-Gordo F, Gómez-Muñoz MT. Occurrence and genetic characterization of Giardia duodenalis from captive nonhuman primates by multi-locus sequence analysis. Parasitol Res. 2011; 109: 539–44.
- Wielinga C, Ryan U, Thompson RCA, Monis P. Multi-locus analysis of Giardia duodenalis intra-Assemblage B substitution patterns in cloned culture isolates suggests sub-Assemblage B analyses will require multi-locus genotyping with conserved and variable genes. Int J Parasitol. 2011; 41: 495–503. [PubMed Abstract].
- Hall BG. Phylogenetic trees made easy: a how-to manual. 2004; Sunderland, MA: Sinauer Associates.
- Thompson HP, Dooley JS, Kenny J, McCoy M, Lowery CJ, Moore JE, etal. Genotypes and subtypes of Cryptosporidium spp. in neonatal calves in Northern Ireland. Parasitol Res. 2007; 100: 619–24. [PubMed Abstract].
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28: 2731–9. [PubMed Abstract] [PubMed CentralFull Text].
- Trotz-Williams LA, Martin DS, Gatei W, Cama V, Peregrine AS, Martin SW, etal. Genotype and subtype analyses of Cryptosporidium isolates from dairy calves and humans in Ontario. Parasitol Res. 2006; 99: 346–52. [PubMed Abstract].
- Silverlås C, Näslund K, Björkman C, Mattsson JG. Molecular characterisation of Cryptosporidium isolates from Swedish dairy cattle in relation to age, diarrhoea and region. Vet Parasitol. 2010; 169: 289–95.
- Waldron LS, Power ML. Fluorescence analysis detects gp60 subtype diversity in Cryptosporidium infections. Infect Genet Evol. 2011; 11: 1388–95. [PubMed Abstract].
- Sulaiman IM, Hira PR, Zhou L, Al-Ali FM, Al-Shelahi FA, Shweiki HM, etal. Unique endemicity of cryptosporidiosis in children in Kuwait. J Clin Microbiol. 2005; 43: 2805–9. [PubMed Abstract] [PubMed CentralFull Text].
- Jex AR, Gasser RB. Genetic richness and diversity in Cryptosporidium hominis and C. parvum reveals major knowledge gaps and a need for the application of “next generation” technologies-research review. Biotechnol Adv. 2010; 28: 17–26. [PubMed Abstract].
- Alves M, Matos O, Antunes F. Microsatellite analysis of Cryptosporidium hominis and C. parvum in Portugal: a preliminary study. J Eukaryot Microbiol. 2003; 50: 529–30. [PubMed Abstract].
- Glaberman S, Moore JE, Lowery CJ, Chalmers RM, Sulaiman L, Elwin K, etal. Three drinking-water-associated cryptosporidiosis outbreaks, Northern Ireland. Emerg Infect Dis. 2002; 8: 631–3. [PubMed Abstract] [PubMed CentralFull Text].
- Peng MM, Wilson ML, Holland RE, Meshnick SR, Lal AA, Xiao L. Genetic diversity of Cryptosporidium spp. in cattle in Michigan: implications for understanding the transmission dynamics. Parasitol Res. 2003; 90: 175–80. [PubMed Abstract].
- Stantic-Pavlinic M, Xiao L, Glaberman S, Lal AA, Orazen T, Rataj-Verglez A, etal. Cryptosporidiosis associated with animal contacts. Wien Klin Wochenschr. 2003; 115: 125–7. [PubMed Abstract].
- Chalmers RM, Ferguson C, Caccio S, Gasser RB, Abs El-Osta YG, Heijnen L, etal. Direct comparison of selected methods for genetic categorisation of Cryptosporidium parvum and Cryptosporidium hominis. Int J Parasitol. 2005; 35: 397–410. [PubMed Abstract].
- Feltus DC, Giddings CW, Schneck BL, Monson T, Warshauer D, McEvoy JM. Evidence supporting zoonotic transmission of Cryptosporidium spp. in Wisconsin. J Clin Microbiol. 2006; 44: 4303–8. [PubMed Abstract] [PubMed CentralFull Text].
- Alves M, Xiao L, Antunes F, Matos O. Distribution of Cryptosporidium subtypes in humans and domestic and wild ruminants in Portugal. Parasitol Res. 2006; 99: 287–92. [PubMed Abstract].
- Xiao L, Ryan UM. Cryptosporidiosis: an update in molecular epidemiology. Curr Opin Infect Dis. 2004; 17: 483–90. [PubMed Abstract].
- Xiao L, Zhou L, Santín M, Yang W, Fayer R. Distribution of Cryptosporidium parvum subtypes in calves in eastern United States. Parasitol Res. 2007; 100: 701–6. [PubMed Abstract].
