Abstract
Background
Hepatitis B virus (HBV) infection is common in Arctic populations and high alcohol intake has been associated with an increased risk of a number of diseases. Yet, a description of the influence of alcohol intake in persons with HBV infection on liver biochemistry is lacking.
Objective
We aimed to describe the association between reported alcohol intake and liver biochemistry taking into account also HBV infection, ethnicity, Inuit diet, body mass index (BMI), gender and age in an Arctic population.
Design and methods
Population-based investigation of Inuit (n=441) and non-Inuit (94) in Greenland and Inuit living in Denmark (n=136). Participants filled in a questionnaire on alcohol intake and other life style factors. Blood samples were tested for aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), bilirubin, albumin, hepatitis B surface antigen, hepatitis B surface antibody and hepatitis B core antibody. We also performed physical examinations.
Results
Participation rate was 95% in Greenland and 52% in Denmark. An alcohol intake above the recommended level was reported by 12.9% of non-Inuit in Greenland, 9.1% of Inuit in East Greenland, 6.1% of Inuit migrants and 3.4% of Inuit in the capital of Greenland (p=0.035). Alcohol intake was associated with AST (p<0.001) and GGT (p=0.001), and HBV infection was associated with ALP (p=0.001) but not with AST, GGT, bilirubin or albumin in the adjusted analysis. Inuit had higher AST (p<0.001), GGT (p<0.001) and ALP (p=0.001) values than non-Inuit after adjustment for alcohol, diet, BMI and HBV exposure. Ethnic origin modified the association between alcohol and AST, while HBV infection did not modify the associations between alcohol and liver biochemistry.
Conclusions
Non-Inuit in Greenland reported a higher alcohol intake than Inuit. Ethnic origin was more markedly associated with liver biochemistry than was alcohol intake, and Greenlandic ethnicity modified the effect of alcohol intake on AST. HBV infection was slightly associated with ALP but not with other liver biochemistry parameters.
The many effects of alcohol (ethanol) consumption on the human body and mind are well understood. A high consumption of alcohol has been described in societies going through rapid transitions and societies in the circumpolar region are no exception (Citation1–Citation4). A high alcohol consumption has been associated with an increased risk of a number of diseases (Citation5–Citation8), accidents (Citation9), violence (Citation10), sexual assaults (Citation11) and suicides (Citation12), all of which lead to increased mortality (Citation3, Citation6) (Citation9, Citation10) (Citation13–Citation17). However, limited research has focused on alcohol consumption and liver disease in Inuit.
Hospital discharge diagnoses related to liver disease have not been found to differ between Inuit (ethnic Greenlanders) and non-Inuit with high alcohol consumption (Citation18). On the other hand, liver biochemistry has been found to be less affected in Inuit compared to non-Inuit attending alcohol treatment centres in Greenland or Denmark (Citation19). Still, the prevalence of alcohol-induced liver disease parallels the alcohol consumption in other populations (Citation20) and there is a distinct relationship between alcohol consumption and liver cirrhosis (Citation21).
The risk of liver damage induced by alcohol may increase when other liver injuries are present (Citation22), and coexisting viral hepatitis may influence the consequences of alcohol consumption (Citation23). Hepatitis B virus (HBV) infection has been shown to be endemic among the Indigenous peoples of the Arctic with very high prevalence rates among Inuit (Citation24–Citation27). Still, the influence of combined alcohol and HBV infection on liver biochemistry in Inuit remains unsettled.
This led us to investigate Inuit and non-Inuit in Greenland and Inuit in Denmark for the association between alcohol intake and liver biochemistry, also taking into account HBV infection.
Methods
Greenland has a population of around 57,000 people mostly of Inuit (Eskimo) descent. Of the 7,000 people born outside Greenland, most were born in Denmark. Around 18,000 people in Denmark are of Greenlandic descent, and around 5,000 people living in Denmark were born in Greenland (Citation28, Citation29).
The cultural tradition of the people of Greenland is to call themselves Inuit (humans); the term “Eskimo” is considered inappropriate and is seldom used, although other words exist in the native tongue to address the population or parts of the native population according to geography (Tunumiut, Avannaamiut, Kujataamiut, Kitaamiut, Kalaallit and so on). Inuit also refers descendants of the Greenlandic Indigenous people. For the purpose of ethnicity, in our study, we chose to define an Inuit as an individual born in Greenland with at least one parent born in Greenland.
The methods used in the survey in Greenland and Denmark have been described in detail previously (Citation25, Citation26) (Citation30, Citation31). In short, participants’ names and addresses were obtained from the National Civil Registration System in which every person living in Denmark, the Faeroe Islands and Greenland is recorded. We selected a random sample of 25% of men and women aged 50 through 69 years in Nuuk in West Greenland (total population 12,909; in the age group, 1,920; 75% Inuit) and all men and women in that age range in Tasiilaq (total population 1,724; in the selected age group 197, 95% Inuit) and the settlements of Tiniteqilaaq, Sermiligaaq, Kuummiut and Kulusuk (1,093; 161; 98% Inuit) in East Greenland. A letter of invitation was delivered to each subject by the local hospital porter or the nursing station attendant. Non-responders were invited 3 times. The investigation took place at the local hospital or nursing station or, by request, in home visits. Data were collected during late summer and autumn 1998. The participation rate was 95%. The initial selection of participants in Nuuk was based on the hospital registrations system. This lacked updating, and the names and addresses were subsequently validated with the National Civil Registration System.
For the investigation in Denmark, we invited all Inuit migrants from Greenland to Denmark aged 40 through 69 years recorded and now living in the city of Aarhus or Aalborg. The lowering of the age limit for inclusion from 50 to 40 years in Denmark was due to the limited number of Inuit in the age group that was included in Greenland. A letter of invitation was sent by mail to all 312 subjects identified in Denmark (). As in Greenland, non-responders were invited 3 times. Each of the 220 responders was contacted by telephone for a short telephone interview to clarify whether they had Inuit ancestry. If they were of Inuit origin, they were invited in for interviews and procedures similar to those carried out among the participants in Greenland. The investigation took place at the local hospital in 2006. None of the participants were reimbursed for their time.
Fig. 1. Flow chart for inclusion of Inuit and non-Inuit in Greenland and Inuit in Denmark. The study population comprised 577 Inuit living in Greenland or Denmark and 94 non-Inuit in Greenland aged 40–69 years, 1998 and 2006. *Of Inuit in Denmark 37 were aged 40 through 49 years.
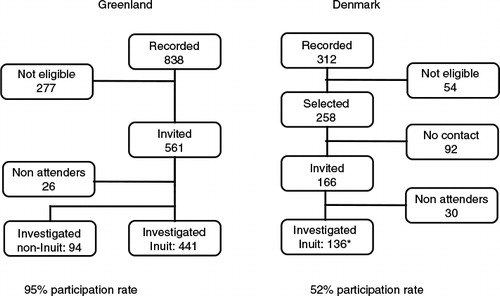
is a flow chart of selection and inclusion of participants. In Denmark, 166 were invited to participate and 30 refrained from attending. Fifty-four did not have parents born in Greenland, had died, moved out of the study area, or were unable to give informed consent for medical reasons. We were unable to determine the eligibility of the 92 subjects in Denmark who did not respond (). In Greenland, 277 had died or moved but were still registered at the address due to late reporting. Twenty-six did not attend the investigation.
The regional ethics committee for Viborg and Nordjylland County approved the study in Denmark (VN-20060038). The Commission for Scientific Research in Greenland approved the study in Greenland (505-99). A letter of invitation was sent to all selected subjects and all participants gave informed written consent in Danish or Greenlandic by participant choice.
Investigational procedures
Information on parents’ birthplace, smoking habits, alcohol intake and dietary habits was obtained by using an interview-based questionnaire in Greenlandic or Danish, as appropriate for the subject. Questions were asked as written in the questionnaires. Twelve grams of alcohol was the equivalent of 1 unit. Average alcohol intake above 2 units per day for women and 3 for men defined an intake above the recommended levels (Citation32). Dietary habits were categorized based on the number of days per week with the main meal of traditional Greenlandic food items (that is, Greenlandic seal, whale, wild fowl, fish, reindeer, musk ox and hare). Participants were classified based on the number of days per week that the main meal consisted of Greenlandic food items. The dietary assessment method has been described in detail previously and validated in Greenland Inuit (Citation31, Citation33). Information on gender and age was obtained from the National Civil Registration System. A physical examination was performed and included recording height and weight in indoor clothing, disabilities, scleral jaundice, spider naevi and signs of hepatic decompensation such as confusion, jaundice, fluid retention and cachexia. Body mass index (BMI) was calculated as weight in kilograms divided by height in metres squared.
A venous blood sample was drawn using minimal tourniquet, separated and stored at −20 C. Blood samples were blinded using an 8-digit code, and analysed in random order. Blood sampling was omitted for 4 participants in Denmark and 1 in Greenland, in compliance with the participants’ choice.
The physical examination and venepuncture were performed by KFR or SA in Denmark and SA or PL in Greenland.
Serology
Testing for hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (anti-HBs) and hepatitis B core antibody (anti-HBc-total) was performed using HBsAg (V2), confirmatory HBsAg, AUSAB®, CORE™ (Abbott Axsym™ System, Abbott Diagnostics a/s, Germany) (Citation25, Citation26). Participants were classified as never exposed if all markers were negative, previously exposed if anti-HBs or anti-HBc-total were positive and as currently infected if HBsAg was positive. This classification was used in the statistical analysis.
Biochemistry
Aspartate aminotransferase (AST), gamma glutamyltransferase (GGT), bilirubin, alkaline phosphatase (ALP) and albumin were measured on a Vitros Chemistry System 950 (Ortho-Clinical Diagnostics, Inc., Raritan, NJ, USA). Alanine aminotransferase and INR were not measured as serum was frozen for transport to the laboratory in Denmark which decreases the reproducibility (Citation34, Citation35). Only data from Inuit and non-Inuit in Greenland were used for comparisons and used in figures and tables, as these serum samples were analysed in the same assay runs in random order.
Statistics
Frequencies are given as mean, with 95% confidence intervals or as median values with 25 and 75 percentiles, as appropriate. Mean values for and were calculated on ln-transformed data that were subsequently transformed back. A chi-square test was used for a comparison of proportions, a Mann-Whitney U-test and Kruskall-Wallis test for a comparison of median values, and Kendall's tau was used to test for trends.
Fig. 2. (a) Aspartate aminotransferase (AST), (b) gamma glutamyltransferase (GGT), (c) alkaline phosphatase (ALP), (d) albumin, and (e) bilirubin in 252 Inuit (left) and 75 non-Inuit (right) men in Greenland by alcohol intake groups. Mean values are shown with 95% confidence intervals. P-values are for trends with increasing alcohol consumption. ns (non-significant) and U/L designate P>0.1 and Units/Litre.
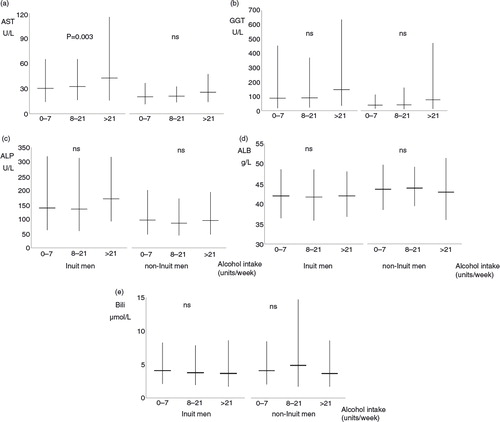
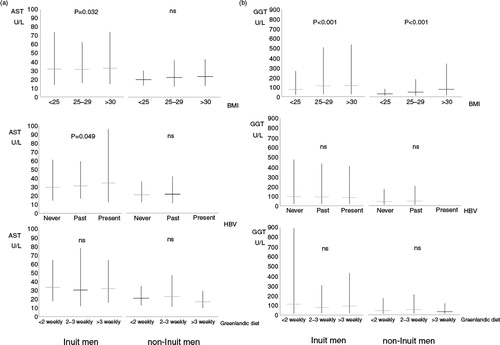
Fig. 3. (a) Aspartate aminotransferase (AST) and (b) gamma glutamyltransferase (GGT) in 252 Inuit (left) and 75 non-Inuit (right) men in Greenland. Levels are given with BMI (upper panel), HBV exposure (middle panel) and frequency of intake of Inuit diet (lower panel). HBV exposure was classified as never exposed if all markers were negative, previously exposed if anti-HBs or anti-HBc were positive, and presently infected if HBsAg was positive. Mean values are shown with 95% confidence intervals. P-values are for trends with increasing BMI, HBV exposure or Greenlandic diet. Ns (non-significant) and U/L designate P>0.1 and Units/Litre.
Liver function tests were entered as dependent variables in multivariate linear regression models after logarithmic transformation. Age (years, included as a continuous variable), gender (men or women), origin (Inuit or non-Inuit), BMI (kg/m2, continuous variable), intake of alcohol (using 5 categories, see Table ), Greenlandic diet (days/week, included as a continuous variable) and HBV infection (HBsAg positivity, yes or no) were entered as explanatory variables. Liver function tests above the upper reference limit were entered as dependent variables in logistic regression models. The effect modification of HBV infection and ethnic origin were tested as interaction terms in the logistic regression models. Data were processed and analysed using Corel Quattro Pro X3 (Corel Corporation, Ottawa, Ontario, Canada) and the Statistical Package for the Social Sciences version 13.0 (SPSS Inc., Chicago, Ill., USA). A 2-sided p-value of less than 0.05 was considered significant in the statistical analyses.
Table I. Characteristics of 634 study participants living in Greenland and Denmark, 1998 and 2006
Results
The participation rates were 95% in Greenland and 52% in Denmark. All participants in Denmark were Inuit according to the inclusion criteria, while 94 participants in Greenland were non-Inuit.
The flow chart for inclusion of participants in both Greenland and Denmark is shown in .
Participant characteristics are listed in Table . Gender distribution differed between participants in Greenland and Denmark (58% men in Greenland; 76% women in Denmark, p<0.001). More men than women had current HBV infection (men 17%, women 10%; p=0.01), and men had a higher alcohol intake than women (p<0.001). Isolated anti-HBs was seen in 39 individuals. This group included 5 non-Inuit.
Table shows the characteristics of participants by alcohol intake groups. An alcohol intake above the recommended level was more frequent in non-Inuit men in Greenland than in any Inuit group (p=0.051). The alcohol intake among Inuit was highest in East Greenland, followed by Inuit in Denmark, with the lowest intake in the capital city in West Greenland. In general, younger, non-Inuit, smoking men had the highest alcohol intake.
Table II. Characteristics by alcohol intake of 540 Inuit in Greenland and Denmark and 94 Non-Inuit in Greenland aged 50–69 years, 1998 and 2006
Table shows measures of body build by alcohol intake groups. Height, weight and BMI did not differ between alcohol intake groups, except for a slight decrease in weight and BMI with increasing alcohol consumption in Inuit men.
Table III. Physical findings by alcohol intake among 577 Inuit in Greenland and Denmark and 94 Non-Inuit in Greenland aged 50–69 years (Inuit in Denmark aged 40–69 years), 1998 and 2006
shows liver biochemistry in Inuit and non-Inuit men for 3 alcohol intake categories. AST (panel A), GGT (panel B) and ALP (panel C) were higher in Inuit than in non-Inuit men irrespective of alcohol intake while differences were smaller for albumin (panel D) and bilirubin (panel E). Inuit and non-Inuit men showed similar trends in liver biochemistry with increasing alcohol intake but only the increase in AST with higher alcohol intake in Inuit was statistically significant (p=0.003). Similar patterns were seen for women (not shown in figures).
Alcohol intake questions in Greenland were blank in 12 participants (hereof 3 men, 1 non-Inuit). This group had a median GGT of 135 U/L compared to the overall medians of 55, 72 and 103 U/L in the groups with a weekly alcohol consumption of 7 units or less, 8 through 20 units, and 21 or more units, respectively.
depicts the associations between BMI (top panel), HBV status (central panel) or traditional Inuit diet (lower panel), and AST (a) or GGT (b). Again, GGT and AST were higher in Inuit than in non-Inuit men. GGT increased with higher BMI (p<0.001) but did not differ with HBV status or diet (b). For Inuit men, AST increased slightly with increasing BMI and having been exposed to HBV (a).
Table shows factors important to liver biochemistry as evaluated in multivariate regression models. Alcohol consumption and ethnic origin were associated with AST, GGT and ALP.
Table IV. Factors associated with liver biochemistry in multivariate analyses among 441 Inuit and 94 Non-Inuit in Greenland aged 50–69 years in 1998
Determinants of GGT above the upper reference limit (73 U/L for men and 43 U/L for women) were ethnic origin (p<0.001; odds ratio (OR), 95% confidence interval (CI): 8.8, 3.8–21 (non-Inuit reference)), BMI (p<0.001; OR, 95%-CI; 3.4, 2.0–5.8 (BMI<30 kg/m2 reference)) and alcohol intake above the recommended level (p=0.003; OR, 95%-CI; 3.1, 1.5–6.6 (never users reference) in multivariate logistic regression models. Similarly, AST above 50 U/L for men and 35 U/L for women was independent of HBV status (p=0.57) and diet (p=0.91) but related to alcohol (p=0.038; OR, 95%-CI: 2.6, 1.1–6.2), ethnic origin (p=0.016; OR, 95%-CI: 6.1, 1.4–26) and gender (p=0.007; OR, 95%-CI: 2.3, 1.3–4.2 (women reference)) in the adjusted analysis. ALP, bilirubin and albumin were not associated with any of the parameters in multivariate logistic regression models.
The influence of alcohol on liver biochemistry was not modified by HBV infection when evaluated as an interaction term in regression models. Ethnic origin modified the association between alcohol and AST (interaction term, p=0.024), but not the association with any of the other liver parameters.
Discussion
Reported alcohol consumption was lower among Inuit in Greenland and Denmark than in non-Inuit in Greenland. Alcohol intake was associated with AST, GGT and ALP but not bilirubin, albumin or measures of body build. HBV infection did not modify the association between alcohol intake and liver function tests, while ethnic origin modified the relation between alcohol and AST. Interestingly, Greenland Inuit had higher levels of AST, GGT and ALP than non-Inuit, regardless of alcohol intake.
Transitions in a society contribute to population stress that may herald an excess alcohol intake (Citation36). Major changes in Eastern Europe during a transition of political and economic changes were accompanied by a marked increase in alcohol intake (Citation37). Greater acculturation in Inuit societies has been suggested as a transition that contributes to the higher use of alcohol to cope with stress (Citation38). The transition of society in the capital city Nuuk started more than 50 years ago and hence Inuit in Nuuk may have become more accustomed to changes. This may explain our finding of a lower alcohol intake in the capital city Nuuk, as well as among younger age groups (Citation39). Transition of the society in East Greenland is delayed compared to the capital city Nuuk. This may contribute to the higher alcohol consumption found in rural East Greenland compared to Nuuk in our study.
A high alcohol consumption among Inuit has been reported previously (Citation1, Citation3) (Citation4, Citation40) with lower intake levels in younger individuals (Citation39). In our study, 29% of Inuit reported being abstainers compared to 9.7% of non-Inuit. We found a lower frequency of high alcohol intake among Inuit in Nuuk, which may be related to several campaigns that featured data from the vast research on the adverse health consequences of high alcohol consumption. Also, the capital city, with a population 10 times that of the town Tasiilaq and around 100 times that of a small settlement, provides more employment opportunities, treatment choices, leisure activities and a diversity of people that makes it easier to merge with groups in the society with a lower alcohol intake. This provides opportunities to support reduced alcohol consumption.
Ethnic differences exist in the alcohol-metabolizing enzymes (Citation41), and alcohol metabolism varies across Asian ethnicities (Citation42). Inuit is a distinct ethnic group with Asian ancestry and a difference in alcohol metabolism, as is seen between Asian populations has been suggested (Citation3). It has been suggested that an altered conversion rate of alcohol that leads to the excess build-up of acetaldehyde, and hence a more severe response to alcohol, could reduce heavy alcohol use and related diseases (Citation42). However, Bjerregaard and colleagues did not confirm the presence of the Asian genotype pattern in a study on the genetic variation in alcohol-metabolizing enzymes among Inuit (Citation43). Yet, AST may reflect alcohol consumption (Citation41, Citation44) and we found an increase in AST with increased reported alcohol consumption. Also, we found a higher AST level in Inuit compared to non-Inuit, even though they reported lower alcohol consumption than non-Inuit. This was the case even among abstainers for whom AST was higher in Inuit compared to non-Inuit. Furthermore, ethnic origin modified the association between alcohol and AST. These findings suggest ethnic differences in liver biochemistry irrespective of alcohol consumption. The findings are interesting, although residual confounding cannot be ruled out.
Additional causes for hepatic injury may explain why some people who abuse alcohol are more likely than others to develop liver disease. It has been suggested that both HBV and hepatitis C virus infection influence the course of alcohol-related liver disease (Citation23) and more severe liver disease has been reported in patients with HBV infection compared to those without (Citation45). HBV is endemic in Arctic populations, and we previously reported HBsAg positivity in 29% of East Greenlanders (Citation25, Citation26). Still, we found no association between HBV infection and AST or GGT in Inuit. Nor did HBV infection modify the effect of alcohol on liver biochemistry. This may be attributable to differences in either drinking patterns, the metabolism of alcohol in Inuit or the subtype of HBV (Citation25).
Hepatitis C and D virus infection may influence liver biochemistry, and hepatitis C, particularly, interacts with alcohol (Citation23). However, both were rare in the populations studied by us (Citation26) and did not allow for evaluations.
Migration is a complex process that is associated with a number of health issues. Migration from Greenland to Denmark itself may influence cardiovascular risk factors (Citation46) and cancer (Citation47, Citation48). We found that alcohol consumption differed according to migration, by using methods similar to those used in our comparative studies in Greenland and Denmark. In a previous investigation (Citation4), the general consumption of alcohol was reported to be higher among Inuit in Denmark compared to Inuit in Greenland, although binge drinking was more widespread in Greenland. Although more women participated in our investigation that included other age groups, we also found that high alcohol consumption was more frequent among Inuit in Denmark compared to Inuit in the capital city Nuuk. Still, alcohol consumption in Inuit in Denmark was less frequent than among Inuit in East Greenland (Table ). This suggests that the differences may be caused by both migration and other factors. These could include differences in both the price of alcoholic drinks and income levels among Inuit in Denmark compared to those in Greenland. However, we did not include these factors in our study. Furthermore, drinking habits among Inuit in Denmark may be influenced by the generally more frequent alcohol intake common to non-Inuit (Citation49). Still, the alcohol intake among Inuit in Denmark was markedly lower than that of non-Inuit in Denmark (Citation49).
Alcohol intake is not fully reported by European populations, while Inuit may have the opposite attitude (Citation3). Still, a report from Greenland found some underreporting in all parts of Greenland that did not take origin into account (Citation50), although this reporting bias was reduced when binge drinking was taken into account. The binge drinking pattern is typical among Inuit and the associated alcohol intake was accounted for in our data on overall alcohol consumption even though we were not able to estimate the influence of binge drinking separately. Hence, Inuit in our study are not likely to underreport more prominently than non-Inuit on their alcohol consumption. Still, a limitation of our study is the lack of questions that validate the self-reported alcohol consumption.
Participants in Denmark included a 10-year increase in age range (40-to 69-year-olds) in order to increase the number of participants. This expansion in age range changed the distribution of alcohol consumption and HBV infection status among participants in Denmark by less than 2% (data not shown).
Vaccination against HBV had not been carried out in this age group in Greenland (Citation25). Also, isolated anti-HBs has been discussed previously (Citation25, Citation51). To test for the potential influence of vaccination, we conducted an additional analysis. We categorized individuals that biochemically could have been vaccinated into a separate group (isolated anti-HBs). Repeated analysis, including this group, did not alter the results.
As for liver function tests, isolated elevated ALP was not seen, and any elevation was parallel to GGT elevations.
The participation rates in Denmark and in Greenland have been described previously (Citation25, Citation26) (Citation31). Some Greenland Inuit in Denmark have no permanent address; some non-participants are likely to be individuals with a higher alcohol intake. This may introduce selection bias in relation to alcohol intake. In addition, the categorization into alcohol group may introduce residual confounding.
Misclassification of an Inuit as non-Inuit in the study population consisting of an older age group, in 1998, is highly unlikely as members of this group were born while Greenland was kept secluded.
Higher alcohol-related mortality rates than those in other populations have previously been reported in aboriginal people (Citation52). The low number of excess drinkers in the older age groups included in our study may be related to a number of factors including such as the costs of alcoholic beverages and spirits. Also, everyday life in the cold climate environment poses specific challenges that are more severe for the old than for the young, and may cause older persons to reduce their alcohol intake. In addition, mortality due to alcohol-related accidents in Greenland is high among at the younger age groups (Citation9, Citation14) (Citation15). These factors may reduce the number of Inuit with excess alcohol consumption included in our study.
Historically, Greenland has been dependent on foreign professionals and skilled workers, especially from Denmark. If the long-standing excess alcohol intake of such foreign professionals compromises their health and ability to work to the point that they can no longer live in Greenland, they may move back to Denmark. This phenomenon may reduce the number of individuals with excess alcohol intake among non-Inuit living in Greenland. Among non-Inuit in Greenland in our study, the fraction of heavy drinkers was smaller than among ethnic Danes in Denmark (Citation49). Still, non-Inuit in Greenland in our study included fewer abstainers and more heavy drinkers compared with Inuit in the same age group. Higher income (Citation53) and cultural differences (Citation49) may explain this, as alcohol in Greenland is expensive (Citation54). Over the past 25 years, the overall alcohol import to Greenland has been halved, and it is still decreasing (Citation55).
Conflict of interest and funding
The authors report no conflict of interest. The study was supported by grants from Greenland Government and Karen Elise Jensen foundation.
Acknowledgements
We gratefully acknowledge Karoline Berglund for her thorough interviewing of Inuit. We are grateful for invaluable support from lægeklinikken in Nuuk, from Hans Chr. Florian Sørensen and the staff at the hospital in Tasiilaq, and from the staff at the nursing stations in Tiniteqilaaq, Sermiligaaq, Kuummiut and Kulusuk.
References
- Wardman D, Quantz D. An exploratory study of binge drinking in the aboriginal population. Am Indian Alsk Native Ment Health Res. 2005; 12: 49–61. [PubMed Abstract].
- Ramisetty-Mikler S, Ebama MS. Alcohol/drug exposure, HIV-related sexual risk among urban American Indian and Alaska Native Youth: evidence from a national survey. J Sch Health. 2011; 81: 671–9. [PubMed Abstract].
- Kozlov A, Vershubsky G, Kozlova M. Indigenous peoples of Northern Russia: anthropology and health. Int J Circumpolar Health. 2007 Suppl 1 1–184. http://www.circumpolarhealthjournal.net/public/journals/32/chs/CHS_2007_1.pdf .
- Madsen MH, Grønbaek M, Bjerregaard P, Becker U. Urbanization, migration and alcohol use in a population of Greenland Inuit. Greenland Population Study. Int J Circumpolar Health. 2005; 64: 234–45. [PubMed Abstract].
- Pedersen OS, Olsen NK, Pedersen M, Andersen JF. Alcohol-induced disease in a Greenland hospital district. Ugeskr Laeg. 1984; 146: 2187–90. [PubMed Abstract].
- Grossman DC, Krieger JW, Sugarman JR, Forquera RA. Health status of Urban American Indians and Alaska Natives. A population-based study. JAMA. 1994; 271: 845–50. [PubMed Abstract].
- Ladefoged K, Rendal T, Skifte T, Andersson M, Søborg B, Koch A. Risk factors for tuberculosis in Greenland: case-control study. Int J Tuberc Lung Dis. 2011; 15: 44–9. [PubMed Abstract].
- Lynge I, Munk-Jørgensen P, Pedersen AL, Mulvad G, Bjerregaard P. Common mental disorders among patients in primary health care in Greenland. Int J Circumpolar Health. 2004; 63(Suppl 2): 377–83. [PubMed Abstract].
- Bjerregaard P. Fatal accidents in Greenland. Arctic Med Res. 1990; 49: 132–41. [PubMed Abstract].
- Bjerregaard P. Disease pattern in Greenland: studies on morbidity in Upernavik 1979–1980 and mortality in Greenland 1968–1985. Arctic Med Res. 1991; 50(Suppl 4): 1–62. [PubMed Abstract].
- Mejlvang P, Boujida V. Sexual assaults in Greenland: characteristics of police reported rapes and attempted rapes. Int J Circumpolar Health. 2007; 66: 257–63. [PubMed Abstract].
- Grove O, Lynge J. Suicide and attempted suicide in Greenland. A controlled study in Nuuk. Acta Psychiatr Scand. 1979; 60: 375–91. [PubMed Abstract].
- Tjepkema M, Wilkins R, Senécal S, Guimond E, Penney C. Mortality of Métis and registered Indian adults in Canada: an 11-year follow-up study. Health Rep. 2009; 20: 31–51. [PubMed Abstract].
- Bjerregaard P. Mortality studies in Greenland. Regional differences in alcohol related deaths and infant mortality. Arctic Med Res. 1985; 40: 69–74. [PubMed Abstract].
- Bjerregaard P. Alcohol related deaths in Greenland. Arctic Med Res. 1988; 47(Suppl 1): 596–7. [PubMed Abstract].
- Bjerregaard P, Juel K. Avoidable deaths in Greenland 1968–1985: variations by region and period. Arctic Med Res. 1990; 49: 119–27. [PubMed Abstract].
- Bjerregaard P. Child survival in Greenland. Arctic Med Res. 1995; 54(Suppl 1): 11–4. [PubMed Abstract].
- Rokkjaer MS, Jensen MT, Grønbaek H, Jepsen P, Pedersen HS, Vilstrup H. Discharge diagnoses of liver diseases in Nuuk Greenland compared to a Danish county hospital. Int J Circumpolar Health. 2006; 65: 162–8. [PubMed Abstract].
- Lavik B, Holmegaard C, Becker U. Drinking patterns and biochemical signs of alcoholic liver disease in Danish and Greenlandic patients with alcohol addiction. Int J Circumpolar Health. 2006; 65: 219–27. [PubMed Abstract].
- Gunnarsdottir SA, Olsson R, Olafsson S, Cariglia N, Westin J, Thjódleifsson B, etal. Liver cirrhosis in Iceland and Sweden: incidence, aetiology and outcomes. Scand J Gastroenterol. 2009; 44: 984–93. [PubMed Abstract].
- Dufour M, Stinson FS, FeCaces M. Trends in cirrhosis morbidity and mortality: United States 1979–1988. Semin Liver Dis. 1993; 13: 109–16. [PubMed Abstract].
- Oshita M, Hayashi N, Kasahara A, Hagiwara H, Mita E, Naito M, etal. Increased serum hepatitis C virus RNA levels among alcoholic patients with chronic hepatitis C. Hepatology. 1994; 20: 1115–20. [PubMed Abstract].
- Shiomi S, Kuroki T, Minamitani S, Ueda T, Nishiguchi S, Nakajima S, etal. Effect of drinking on the outcome of cirrhosis in patients with hepatitis B or C. J Gastroenterol Hepatol. 1992; 7: 274–6. [PubMed Abstract].
- McMahon BJ. Viral hepatitis in the Arctic. Int J Circumpolar Health. 2004; 63(Suppl 2): 41–8. [PubMed Abstract].
- Krarup HB, Andersen S, Madsen PH, Okkels H, Hvingel BH, Laurberg P. Benign course of long-standing hepatitis B virus infection among Greenland Inuit?. Scand J Gastroenterol. 2008; 43: 334–43. [PubMed Abstract].
- Rex KF, Krarup HB, Laurberg P, Andersen S. Population-based comparative epidemiological survey of hepatitis B, D, and C among Inuit migrated to Denmark and in high endemic Greenland. Scand J Gastroenterol. 2012; 47: 692–701. [PubMed Abstract].
- Rex KF, Andersen S, Krarup HB. Hepatitis B among Inuit. A review with focus on Greenland Inuit. World J Hepatol. 2015; 7: 1265–71. doi: http://dx.doi.org/10.4254/wjh.v7.i9.1265 [PubMed Abstract] [PubMed CentralFull Text].
- Report by The North Atlantic Group in the Danish Parliament. Greenlanders living in Denmark. 2007 [cited 2015 Jun 29]. 2007. Available from: http://www.udsattegroenlaendere.dk/wp-content/uploads/dnag-groenlaendere-20i-20danmark-201-1.pdf. [in Danish, Greenlandic].
- Statistics Greenland. [cited 2015 Jun 29]. Available from: http://www.stat.gl/dialog/topmain.asp?lang=en&subject=Befolkning&sc=BE .
- Andersen S, Mulvad G, Pedersen HS, Laurberg P. Gender diversity in developing overweight over 35 years of Westernisation in an Inuit hunter cohort and ethno-specific body mass index for evaluation of body-weight abnormalities. Eur J Endocrinol. 2004; 151: 735–40. [PubMed Abstract].
- Andersen S, Hvingel B, Kleinschmidt K, Jørgensen T, Laurberg P. Changes in iodine excretion in 50–69-y-old denizens of an Arctic society in transition and iodine excretion as a biomarker of the frequency of consumption of traditional Inuit foods. Am J Clin Nutr. 2005; 81: 656–63. [PubMed Abstract].
- Danish Health and Medicines Authority. Copenhagen, Denmark. [cited 2015 Mar 20]. Available from: http://sundhedsstyrelsen.dk/da/sundhed/alkohol/~/media/BC7F94D1D0124D3BAED38D0D0E8C0DAC.ashx. [in Danish].
- Andersen S, Laurberg P, Hvingel B, Kleinschmidt K, Heickendorff L, Mosekilde L. Vitamin D status in Greenland is influenced by diet and ethnicity: a population-based survey in an Arctic society in transition. Br J Nutr. 2013; 109: 928–35. [PubMed Abstract].
- Cuhadar S, Koseoglu M, Atay A, Dirican A. The effect of storage time and freeze-thaw cycles on the stability of serum samples. Biochem Med. 2012; 2: 70–7.
- van den Besselaar AM, Witteveen E, van der Meer FJ. Long-term stability of frozen pooled plasmas stored at 70°C, 40°C, and 20°C for prothrombin time and international normalized ratio (INR) assessment. Thromb Res. 2013; 131: 349–51. [PubMed Abstract].
- Curtis T, Kvernmo S, Bjerregaard P. Changing living conditions, life style and health. Int J Circumpolar Health. 2005; 64: 442–50. [PubMed Abstract].
- Moskalewicz J, Simpura J. Alcohol and alcohol policy in eastern European transitions 2000. J Subst Use. 2005; 5: 30–8.
- Wolsko C, Lardon C, Mohatt GV, Orr E. Stress, coping, and well-being among the Yup'ik of the Yukon-Kuskokwim Delta: the role of enculturation and acculturation. Int J Circumpolar Health. 2007; 66: 51–61. [PubMed Abstract].
- Kuntsche E, Kuntsche S, Knibbe R, Simons-Morton B, Farhat T, Hublet A, etal. Cultural and gender convergence in adolescent drunkenness. Evidence from 23 European and North American Countries. Arch Pediatr Adolesc Med. 2011; 165: 152–8. [PubMed Abstract] [PubMed CentralFull Text].
- Seale JP, Shellenberger S, Spence J. Alcohol problems in Alaska Natives: lessons from the Inuit. Am Indian Alsk Native Ment Health Res. 2006; 13: 1–31. [PubMed Abstract].
- Feldman M, Friedman LS, Sleisenger MH. Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology/diagnosis/management, Vol. 2. 7th ed. 2002; Philadelphia, PA: Saunders. 2305. 2305.
- Eng MY, Luczak SE, Wall TL. ALDH2, ADH1B, and ADH1C genotypes in Asians: a literature review. Alcohol Res Health. 2007; 30: 22–7. [PubMed Abstract] [PubMed CentralFull Text].
- Bjerregaard P, Mikkelsen SS, Becker U, Hansen T, Tolstrup JS. Genetic variation in alcohol metabolizing enzymes among Inuit and its relation to drinking patterns. Drug Alcohol Depend. 2014; 144: 239–44. [PubMed Abstract].
- Mendenhall CL. Alcoholic hepatitis. Clin Gastroenterol. 1981; 10: 417–41. [PubMed Abstract].
- Mota A, Guedes F, Areias J, Pinho L, Cardoso MF. Alcohol consumption among patients with hepatitis B infection in northern Portugal considering gender and hepatitis B virus genotype differences. Alcohol. 2010; 44: 149–56. [PubMed Abstract].
- Bjerregaard P, Jørgensen ME, Lumholt P, Mosgaard L, Borch-Johnsen K. Greenland population study. Higher blood pressure among Inuit migrants in Denmark than among the Inuit in Greenland. J Epidemiol Community Health. 2002; 56: 279–84. [PubMed Abstract] [PubMed CentralFull Text].
- Prener A, Nielsen NH, Hansen JP, Jensen OM. Cancer pattern among Greenlandic Inuit migrants in Denmark, 1968–1982. Br J Cancer. 1987; 56: 679–84. [PubMed Abstract] [PubMed CentralFull Text].
- Boysen T, Friborg J, Andersen A, Poulsen GN, Wohlfahrt J, Melbye M. The Inuit cancer pattern–the influence of migration. Int J Cancer. 2008; 122: 2568–72. [PubMed Abstract].
- National Health Interview Surveys. [Sundheds- og Sygelighedsundersøgelsen 2005]. Copenhagen: National Institute of Public Health; 2005 [cited 2015 Jun 6]. 2005. Available from: http://www.si-folkesundhed.dk/upload/hele_rapporten_2005_001.pdf. [in Danish].
- Bjerregaard P, Becker U. Validation of survey information on smoking and alcohol consumption against import statistics, Greenland 1993–2010. Int J Circumpolar Health. 2013; 72 20314, doi: http://dx.doi.org/10.3402/ijch.v72i0.20314 .
- Hønge BL, Jespersen S, Medina C, Té Dda S, da Silva ZJ, Lewin S. Hepatitis B and Delta virus are prevalent but often subclinical co-infections among HIV infected patients in Guinea Bissau, West Africa: a cross-sectional study. Bissau HIV cohort study group. PLoS One. 2014; 9 e99971. doi: http://dx.doi.org/10.1371/journal.pone.0099971 .
- Li G, Smith GS, Baker SP. Drinking behavior in relation to cause of death among US adults. Am J Publ Health. 1994; 84: 1402–6.
- Statistics Greenland. [cited 2015 Jun 29]. Available from http://www.stat.gl/dialog/main.asp?lang=en&version=201013&sc=SA&colcode=t .
- Statistics Greenland. [cited 2015 Jun 29]. Available from http://www.stat.gl/dialog/main.asp?lang=en&sc=SA&subthemecode=o8&colcode = o & version = 201013 .
- Statistics Greenland. [cited 2015 Jun 29]. Available from: http://www.stat.gl/dialog/topmain.asp?lang=da&subject=Tobakogalkohol&sc=AL. [in Greenlandic, Danish].
