Abstract
Background
The American Association for the Study of Liver Diseases (AASLD) recommends semi-annual hepatocellular carcinoma (HCC) screening using ultrasound (US) in persons with chronic hepatitis B (CHB) virus infection at high risk for HCC such as Asian males aged ≥40 years and Asian females aged ≥50 years.
Objective
To analyse the cost-effectiveness of 2 HCC screening methods in the Alaska Native (AN) health system: US-alone, or screening by alpha-fetoprotein (AFP) initially and switching to US for subsequent screenings if AFP >10 ng/mL (AFP→US).
Design
A spreadsheet-based model was developed for accounting the costs of 2 hypothetical HCC screening methods. We used epidemiologic data from a cohort of 839 AN persons with CHB who were offered HCC screening by AFP/US semi-annually during 1983–2012. We assumed that compared with AFP→US, US-alone identifies 33% more tumours at an early stage (defined as a single tumour ≤5 cm or ≤3 tumours ≤3 cm in diameter). Years of life gained (YLG) attributed to screening was estimated by comparing additional years of survival among persons with early- compared with late-stage tumours. Screening costs were calculated using Medicare reimbursement rates in 2012. Future screening costs and YLG were projected over a 30-year time horizon using a 3% discount rate.
Results
The total cost of screening for the cohort by AFP→US would have been approximately $357,000 ($36,000/early-stage tumour detected) compared to $814,000 ($59,000/early-stage tumour detected) by US-alone. The AFP→US method would have yielded an additional 27.8 YLG ($13,000/YLG) compared with 38.9 YLG ($21,000/YLG) for US-alone. Screening by US-alone would incur an additional $114,000 per extra early-tumour detected compared with AFP→US and $41,000 per extra YLG.
Conclusions
Although US-alone HCC screening might have yielded more YLG than AFP→US, the reduced costs of the AFP→US method could expand access to HCC screening in resource constrained settings.
The World Health Organization estimates that 360 million persons live with chronic hepatitis B virus infection (CHB) worldwide (Citation1). Persons with CHB are at increased risk for developing hepatocellular carcinoma (HCC) (Citation2). The American Association for the Study of Liver Diseases (AASLD) guidelines recommend HCC screening when the risk exceeds 0.2% year within a population group, such as among Asian males aged >40 years and Asian females aged >50 years (Citation3). The optimal method for HCC screening remains debatable (Citation4). AASLD guidelines for managing CHB recommend screening of persons at high risk for HCC by ultrasound (US) every 6–12 months, but list serum alpha-fetoprotein (AFP) as an acceptable alternative in areas of the United States where US is not readily available (Citation5). Conversely, separate AASLD guidelines for managing HCC state that AFP lacks sufficient sensitivity/specificity to serve as a useful screening test and, therefore, recommend screening every 6 months by US alone (Citation3).
Alaska Native (AN) persons with CHB have an annual risk of 0.26% for developing HCC and might benefit from semi-annual HCC screening (Citation3, Citation6). Alaska is a large sparsely populated state, and a substantial proportion of AN people live in small villages that are inaccessible by road. In villages without an US facility, the costs associated with transporting persons by air to an US-capable regional health centre are an important barrier to HCC screening by US alone. For that reason, the Alaska Native Tribal Health System has screened all persons with CHB for HCC by semi-annual serum AFP measurements; only persons with an elevated AFP and persons with a family history of HCC or cirrhosis were referred for US.
Between 1982 and 1987, more than 53,000 AN persons were screened for CHB in a mass vaccination campaign (Citation7); 1,535 persons tested positive for hepatitis B surface antigen during and after the campaign. All persons testing positive for hepatitis B surface antigen were offered semi-annual screening for HCC through the Alaska Native Tribal Health System. In total, 1,375 AN persons with CHB provided consent to enroll in a prospective cohort study that followed participants from January 1983 to December 2012. This prospective study was approved by the Institutional Review Boards of the Alaska Native Tribal Health Consortium and the Centres for Disease Control and Prevention. For this present study, we used data from the prospectively studied cohort of AN persons with CHB to compare the cost-effectiveness of HCC screening by 2 methods: screening by US semi-annually (US-alone method) versus semi-annual screening by AFP alone and switching to US alone if an AFP >10 ng/m was detected (AFP→US method). Our analysis can guide public health officials on choosing the optimal HCC screening method for persons with CHB living in resource constrained settings where US is not available, and the cost of transportation to an US-equipped facility can be a barrier.
Methods
We built a spreadsheet-based model to compare the cost-effectiveness of HCC screening by US-alone and AFP→US methods. For the US-alone method, we assumed patients would have received an US at every screening opportunity. For the AFP→US method, we assumed that all persons would have been screened by AFP initially and switched to US for all subsequent screening opportunities if AFP >10 ng/mL on at least 1 measurement. Based on AASLD guidelines criteria (Citation3), we assumed that HCC screening in the prospectively followed cohort of AN persons with CHB began for all males and females once they attained ages ≥40 and ≥50 years, respectively. Under both screening methods, we assumed that HCC screening continued until patient death or the end of the study period (December 31, 2012).
Epidemiologic data
For this cost-effectiveness analysis, we used data from AN patients with CHB who participated in the prospective cohort study during 1983–2012 (see Appendix Table I for cohort participants’ demographic and clinical characteristics). We reviewed the medical records for cohort participants to determine the: (a) dates and results of all AFP measurements, (b) date of HCC diagnosis, and (c) date of death. For persons who developed HCC, the dates and results of all liver imaging studies were obtained from the medical records. Additionally, we cross-referenced the names of persons in the study cohort with the Alaska Native Tumour Registry, a participant of the National Cancer Institute Surveillance, Epidemiology, and End Results Program, to identify persons with HCC (Citation8). Cohort participants who died or developed HCC <6 months after cohort entry were excluded from our analysis because they likely would not have benefited from either screening method. In total, 564 males attained age ≥40 years and 275 females attained age ≥50 years during 1983–2012 (the age/sex target group for HCC screening) and their data provided epidemiologic input for our model (Table ). Among cohort participants included in our analysis, 21 were diagnosed with HCC ≥6 months after cohort entry.
Table I. Epidemiologic data for a cost analysis from a prospectively followed cohort of AN persons with chronic hepatitis B virus infection, 1983–2012
Tumour stage classification
The HCC tumour size was determined by either US or computed tomography (CT). If the number of tumours or tumour sizes were discrepant between ultrasound and CT for the same patient, we used the CT imaging results. We categorised patients with HCC as having an early-stage tumour as defined by the Milan criteria as follows: single tumour ≤5 cm in diameter or ≤3 tumours each ≤3 cm in diameter, and no vascular or lymph node invasion (Citation9). Tumours not meeting Milan criteria were categorised as late-stage. To be conservative and not overestimate potential benefits of HCC screening, we also categorised as late-stage those tumours of unknown size (for patients who declined additional evaluation).
Estimating the number of early-stage tumours identified by each screening method
Neither the AFP→US method nor the US-alone method was used in practice in the prospectively followed cohort. Therefore, we had to make assumptions to estimate the number of HCC tumours that would have been identified at an early stage by the 2 hypothetical screening methods (AFP→US and US-alone) based on the results of the actual HCC screening method used in the prospectively followed cohort during 1983–2012. For the actual HCC screening method, all AN patients with CHB were offered screening by AFP every 6 months regardless of age or risk factors for developing HCC and only those patients at high risk for HCC or with an elevated AFP were referred for US. Because the hypothetical AFP→US method closely resembles the actual screening method, we assumed the AFP→US method would have identified the same number of early-stage tumours as the actual screening method (Table and II ).
Table II. Base-case model assumptions for estimating the costs for hepatocellular carcinoma screening
In order to determine the number of early-stage tumours detected by the hypothetical US-alone method, we assumed that approximately 33% of the late-stage tumours identified by the actual screening method in the cohort would instead have been identified at an early stage by the US-alone method. This assumption was based on a previous study demonstrating that the sensitivity of AFP >10.9 ng/mL for detecting early-stage HCC was 66%, and on a meta-analysis which reported a pooled sensitivity for ultrasound of 63% for detecting an early-stage HCC and 94% for detecting any tumour before becoming clinically apparent (Citation10, Citation11). Although the sensitivity of US to detect HCC tumours can be lower in certain circumstances, such as in persons with cirrhosis, we opted to use the best-case scenario US sensitivity of 94% when comparing with AFP because guidelines presume that US is superior to AFP for HCC screening (Citation3, Citation5) (Citation12).
Estimating survival by tumour stage
For both hypothetical screening approaches, we calculated the additional years of life gained (YLG) in the cohort of 839 persons with CHB compared with no screening. We assumed that the median (mean) years of survival among persons with late-stage HCC tumours represented survival with no screening. We further assumed that the additional YLG at median (mean) among persons with early-stage tumours were the result of screening (Table ). All persons within a tumour-stage category were assumed to have the same median (mean) survival by either the AFP→US approach or the US-alone approach (Table ); only the number of persons within each tumour-stage category differed between the 2 approaches (Table ). We calculated the additional median YLG attributable to screening as follows:
Additional YLG at median survival=[(# early-stage tumours)×(survival in early-stage tumour)] – [(# late-stage tumours)×(survival in late-stage tumours)].
Total number of screening tests performed
We estimated the number of screening tests that would have been performed in the 839 cohort participants meeting age/sex criteria for HCC screening during the 30-year time horizon of our study by assuming that screening occurred only on dates of when documented AFP tests were actually performed. We further assumed that it would be unlikely that a person failing to have their blood drawn would instead comply with a recommendation to obtain an US. We thus assumed that the total number of documented AFP measurements also represents the potential US screening opportunities. We then determined the total number of AFP and US tests that would have been performed under the 2 hypothetical screening methods.
Assuming that all cohort members received a screening test for HCC every 6 months, there were a total of 21,226 screening opportunities in the cohort during the 30-year time horizon (Table ). However, AFP measurements were documented for only 10,931 (51.4%) screening opportunities (median: 11 HCC screening tests/person). By the AFP→US method, screening would have occurred by measuring serum AFP at 9,378 opportunities and by US at 1,553 opportunities among persons with at least 1 elevated serum AFP (Table ). By the US-alone method, we assume that an US would have been performed at all 10,931 screening opportunities (Table ).
Cost analysis
We estimated direct costs of screening from the payer's perspective, the Alaska Native Health System. We used 2012 Medicare reimbursement rates in Alaska for AFP and US ($26.76 and $109.94 per test, respectively) to calculate the cost of screening at our institution (based on personal communication, Alaska Native Medical Center). For the 60% of patients living in communities without an US facility, we estimated that air transportation to the nearest regional health centre costs on average $200 per person per round trip. We also compared the 2 screening methods in terms of the cost/tumour detected by summing the cost of all AFP and US measurements that would have been performed in the cohort under each screening method and dividing by the number of tumours detected at an early stage in the cohort. We did not account for treatment costs after early- or late-tumour detections because we assumed that treatment costs will be similar irrespective of the methods used to detect tumour.
Cost-effectiveness calculations
We evaluated the cost-effectiveness of the AFP→US and US-alone approaches in terms of the cost/tumour detected and the cost/YLG. The cost/tumour detected was calculated by summing the cost of all AFP and US measurements that would have been performed in the cohort under each screening approach and dividing by the number of early-stage tumours detected in the cohort. The cost/YLG was calculated by summing the cost of all AFP and US measurements that would have been performed in the cohort under each screening approach and dividing by the total number of YLG under each approach, respectively. The cost/YLG was calculated by using both the both the mean and median estimates for YLG. Finally, to account for differences in time between when screening costs may occur and health benefits are obtained, we discounted future costs and benefits (e.g. health outcomes such as YLG) to reference year 2012 at 3% year over a time horizon of 30 years (reflecting the time period our prospectively followed study cohort was followed) (Citation13).
Sensitivity analysis
We performed a sensitivity analysis for the cost/early-stage tumour detected under both hypothetical screening methods by varying the percentage of late-stage tumours identified by AFP→US that would have been identified by US-alone at an early stage from 0% (indicating that the 2 methods had the same sensitivity) to 100% (indicating that US-alone identified all tumours in the cohort at an early stage). The base-case analysis assumed approximately 33% difference between the 2 methods.
We also considered the impact if all persons in our cohort had received HCC screening every 6 months as recommended. Of the 21,226 potential screening opportunities in the cohort, only 10,931 screenings occurred (approximately 51% of potential screenings) (Table ).
Results
To summarise key input data for our cost analysis model (Table and II ), the median survival among persons with early-stage HCC tumours was 3.1 years (minimum [min]=0.3 years; maximum [max]=14.2 years), and for late-stage tumours was 0.2 years (min=0 years; max=2.3 years). Of the 21 tumours that occurred in the prospectively followed cohort, 10 (47%) might have been identified by AFP→US at an early stage. Assuming that approximately 33% of tumours identified at a late stage by AFP→US were instead detected by the US-alone method at an early stage, then 14 (67%) tumours might have been detected at an early stage by US-alone.
Our base-case analysis indicates that US-alone alone would have yielded more total YLG compared with AFP→US. Undiscounted YLG in the cohort based on median survival by US-alone was estimated at 41.4 years compared with 29.6 years by AFP→US (Table ). However, the undiscounted total direct cost of the screening program, without including transportation costs, during the study period would have been $1.2 million by US-alone compared to $528,000 by AFP→US. Discounting at 3%/year reduced those costs to $357,000 and $814,000, respectively. The approximate undiscounted cost/YLG without including transportation costs was lower for AFP→US ($18,000) than US-alone ($39,000) (discounted approximately $13,000 and $21,000, respectively). The total cost of the screening program in the cohort, the cost/tumour detected, and the cost/YLG at median/mean survival by AFP→US remained lower than US-alone after accounting for travel expenditure (Table ).
Table III. Comparing the costs of 2 hypothetical screening scenarios for hepatocellular carcinoma (HCC) – Alaska, 1983–2012a
Compared to the AFP→US method, the US-only method had an incremental cost of $100,000 per each additional YLG and $279,000 per each additional early-stage tumour detected (discounted costs including transportation costs). Excluding transportation costs reduced this to $41,000 per additional YLG and $114,000 per each additional early-stage tumour detected (Table ).
Sensitivity analysis
depicts the discounted cost per early-stage tumour detected for the AFP→US and US-alone methods as the percentage of late-stage tumours identified by AFP→US that would potentially have been identified by US-alone at an early stage increases from 0 to 100%. Because we only varied the number of tumours detected by the US-alone method in this analysis, the discounted cost for the AFP→US method remained stable at $36,000/early-stage tumour detected without accounting for travel costs and $59,000 when including travel costs. The discounted cost for US-alone ranged from $38,759 to $81,393/early-stage tumour detected without including travel costs and from $81,064 to $170,235 when including travel costs.
Fig. 1. Sensitivity analysis: Impact of varying the percentage of hepatocellular carcinoma tumours that were identified by AFP→US at a late stage and potentially identified by US-alone at an early stage in hepatitis B virus infected AN persons.*†‡§¶ Abbreviations: AFP, alpha-fetoprotein; US, ultrasound; ↓, indicates base-case assumption *Screening assumed to start for men at age ≥40 years and for women at age ≥50 years. †AFP→US assumes patients received screening for HCC by serum AFP measurements initially and switched to ultrasound if AFP >10 ng/mL; screening method resembles the Alaska Native Health System hepatocellular carcinoma screening program. ‡The number of additional tumours that might have been detected at an early stage (i.e. single tumour ≤5 cm in diameter or ≤3 tumours ≤3 cm in diameter) by an US-alone method is unknown; therefore, sensitivity analysis determined the cost/early-stage tumour detected by assuming US-alone method identified 0–100% of the tumours identified by AFP→US at a late stage. §Direct costs of screening discounted at 3%/year (reference year 2012) over a 30-year time horizon. ¶Assumes 60% of patients lived in a village without ready US access and required transportation to US facility.
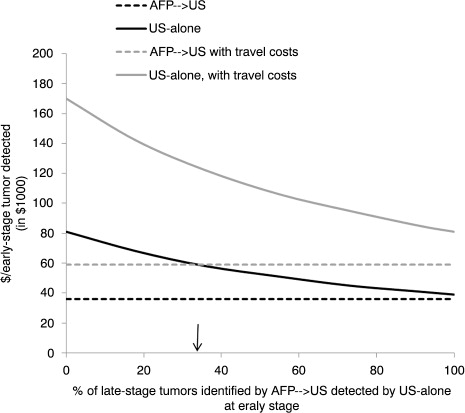
If all persons in our cohort had received HCC screening every 6 months as recommended (i.e. 100% adherence), the total direct discounted costs of the screening program without accounting for travel expenses would have been $556,000 by AFP→US compared with $1.6 million by US-alone (results not shown). Assuming US-alone could have identified all tumours in the cohort at an early stage, full compliance with screening opportunities would have produced a discounted cost without travel expenses of $78,000/early-stage tumour detected.
Appendix Table I. Demographic and clinical characteristics of the cohort participants with history of hepatitis B virus (HBV) infection and those who developed hepatocellular carcinoma (HCC) – Alaska, 1983–2012.a
Discussion
We compared the cost-effectiveness of HCC screening by using the US-alone method versus AFP→US method in AN people with CHB. Because AASLD guidelines recommend HCC screening by US and discourage using AFP as an HCC screening test, we wanted to compare AFP with the best reported sensitivity of US for detecting HCC. Our results indicate that HCC screening by US-alone might have detected more tumours at an early stage but the overall cost of the screening program would have been more than twice the AFP→US method. Despite using sensitivity estimates for detecting HCC tumours in our model that favoured US as the more effective test compared with AFP, the cost/early-stage tumour detected by the AFP→US method remained lower than the US-alone method, even when we assumed US-alone identified all tumours at an early stage in the sensitivity analysis.
The applicability of our study findings to other health systems must be considered within the unique resource and epidemiologic context of each setting. For example, the cost of a screening program is fixed but the cost/tumour detected is enhanced in regions with a higher incidence of HCC because of a greater potential to detect tumours. This concept is demonstrated by an analysis of an HCC screening program that used both US and AFP at a teaching hospital in Australia (Citation14). The incidence of HCC at the hospital was 2.7%/year and the adjusted cost/tumour detected in 2012 US dollars was approximately $12,600. Because HCC incidence was 10 times higher at that teaching hospital in Australia than in the AN population, the cost/tumour detected was 4 to 8 times lower than either of the methods in our unadjusted model. However, in China, which has a very high prevalence of CHB (7.9% among adults in southeast China), annual per capita spending on health in 2002 was $55 and half of Chinese residents in 1 survey stated that they skipped health services because of cost (Citation15–Citation17). In that context, initial screening by AFP (US$0.60/test in 1997) might be more feasible than lifelong screening by US alone (US$1.20/test in 1997) every 6 months (Citation18).
The effectiveness of a screening program in detecting HCC tumours at an early stage depends on patients’ adherence with recommended screenings. Persons in our cohort received the recommended HCC screening only about half of the time during the 30-year study period. By comparison, 60% of patients at 2 gastroenterology clinics in California with CHB had received optimal screening for HCC over a 3-year period (Citation19). In an HCC screening trial among persons with CHB in China, adherence to screening was 58% over a 5-year period (Citation20). The reasons for suboptimal adherence in our study cohort are unclear. An automated system mails a letter to all AN persons in the CHB registry reminding them to have their blood drawn for AFP measurement, thus eliminating the need for a provider to initiate screening. However, many patients (especially those in rural Alaskan villages) with a normal AFP measurement are notified of the result by mail only. The lack of regular contact with a provider combined with the need for lifelong semi-annual phlebotomy could partly account for the suboptimal adherence.
Our study has several limitations. First, our cost-effectiveness analysis did not take into account the treatments costs for early- and late-stage tumours. We were unable to account for treatment costs because the HCC treatment algorithm is complex, and we lacked data on the probability of receiving the various treatments and their associated outcomes (Citation3). However, it is likely that the US-alone screening approach would have remained more costly even after taking into account treatment costs because of the longer survival of the additional patients that would be detected at an early stage and the eligibility of those patients for more expensive treatments such as liver transplantation. In addition, Medicare reimbursement rates might not reflect the true cost of a screening test and it is likely that the cost of those tests might have declined over time because of increases in availability of the test or operators’ productivity. Although we might have underestimated the overall cost of the screening program, our interpretation of the relative costs of both methods should be unaffected because we used the same test cost estimates for both the AFP→US and US-alone method.
Our study also has strengths. Epidemiologic and clinical data for our cost-effectiveness analysis model were obtained from a large population-based cohort of persons at high risk for HCC who were followed prospectively for up to 30 years. As a result, our model accounts for important real-world factors that affect the cost-effectiveness of a screening program such as patient non-adherence. Moreover, it is unlikely that any persons in our cohort with HCC were missed because the Alaska Native Medical Center is the tertiary referral centre for all persons in our cohort and all cohort patients were cross-referenced with the Alaska Native Tumor Registry. Finally, our sensitivity analysis surrounding several model assumptions enhances the generalisability of our results. For example, our assumption regarding the number of HCC tumours that would have been identified at an early stage by US-alone versus AFP→US relied on papers where the study populations were not representative of our cohort (Citation10, Citation21). However, our sensitivity analysis demonstrated that AFP→US remained more cost-effective than US-alone over a broad range of difference in sensitivity between the 2 HCC screening methods.
Decisions surrounding the optimal method to screen for HCC must balance the cost-effectiveness, as presented here, with other factors not included in this analysis, such as the availability and quality of the screening test (e.g. sensitivity/specificity) (Citation22). The efficacy of AFP as a screening tool for HCC has long been criticised for having a lower sensitivity and specificity than imaging modalities (Citation23, Citation24). But for many of patients in rural Alaska, AFP is the only locally available option for HCC screening, and it could potentially identify patients with CHB at high risk for HCC who could benefit from referral for a liver ultrasound or CT. Thus, public health officials should evaluate the cost-effectiveness of AFP→US to increase access to HCC screening for persons living in remote communities without access to US.
Conflict of interest and funding
None of the authors have any relevant conflicts of interest to disclose. This work was supported by the Centers for Disease Control and Prevention, NCHHSTP, Division of Viral Hepatitis (CA# 1U01PS004113).
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Acknowledgements
We thank Chriss Homan and Susan Negus for their assistance in preparing the data for analysis.
References
- World Health Organization. Hepatitis B vaccines. WHO position paper. 2009; Geneva, Switzerland: World Health Organization. 405–20.
- Simonetti J, Bulkow L, McMahon BJ, Homan C, Snowball M, Negus S, etal. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology. 2010; 51: 1531–7. [PubMed Abstract].
- Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53: 1020–2. [PubMed Abstract] [PubMed CentralFull Text].
- Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012; 156: 387–9. [PubMed Abstract].
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009; 50: 661–2. [PubMed Abstract].
- McMahon BJ, Alberts SR, Wainwright RB, Bulkow L, Lanier AP. Hepatitis B-related sequelae. Prospective study in 1400 hepatitis B surface antigen-positive Alaska native carriers. Arch Intern Med. 1990; 150: 1051–4. [PubMed Abstract].
- McMahon BJ, Rhoades ER, Heyward WL, Tower E, Ritter D, Lanier AP, etal. A comprehensive programme to reduce the incidence of hepatitis B virus infection and its sequelae in Alaskan natives. Lancet. 1987; 2: 1134–6. [PubMed Abstract].
- Kelly J, Schade T, Starkey B, White S, Ashokkumar R, Lanier A 2012; Anchorage, AK: Alaska Native Tribal Health Consortium, Alaska Native Epidemiology Center. Cancer in Alaska. 1969–2008 40-year report.
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, etal. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334: 693–9. [PubMed Abstract].
- Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, etal. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009; 137: 110–8. [PubMed Abstract] [PubMed CentralFull Text].
- Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, etal. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009; 30: 37–47. [PubMed Abstract].
- Singal AG, Conjeevaram HS, Volk ML, Fu S, Fontana RJ, Askari F, etal. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol Biomarkers Prev. 2012; 21: 793–9. [PubMed Abstract].
- Haddix AC, Teutsch SM, Corso PS. Prevention effectiveness: a guide to decision analysis and economic evaluation. 2003; Oxford: Oxford University Press. 264. 2nd ed.
- Qian MY, Yuwei JR, Angus P, Schelleman T, Johnson L, Gow P. Efficacy and cost of a hepatocellular carcinoma screening program at an Australian teaching hospital. J Gastroenterol Hepatol. 2010; 25: 951–6. [PubMed Abstract].
- Blumenthal D, Hsiao W. Privatization and its discontents – the evolving Chinese health care system. N Engl J Med. 2005; 353: 1165–70. [PubMed Abstract].
- Lim MK, Yang H, Zhang T, Feng W, Zhou Z. Public perceptions of private health care in socialist China. Health Aff. 2004; 23: 222–34.
- Luo Z, Xie Y, Deng M, Zhou X, Ruan B. Prevalence of hepatitis B in the southeast of China: a population-based study with a large sample size. Eur J Gastroenterol Hepatol. 2011; 23: 695–700. [PubMed Abstract].
- Zhang B, Yang B. Combined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999; 6: 108–10. [PubMed Abstract].
- Wong CR, Garcia RT, Trinh HN, Lam KD, Ha NB, Nguyen HA, etal. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009; 54: 2712–21. [PubMed Abstract].
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004; 130: 417–22. [PubMed Abstract].
- Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, etal. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001; 48: 251–9. [PubMed Abstract] [PubMed CentralFull Text].
- Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008; 8: 165–78. [PubMed Abstract].
- Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, etal. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001; 34: 570–5. [PubMed Abstract].
- Sherman M. Alphafetoprotein: an obituary. J Hepatol. 2001; 34: 603–5. [PubMed Abstract].
