Abstract
Thromboembolic stroke from the left atrial appendage (LAA) is the most feared complication in patients with atrial fibrillation (AF). The cornerstone for the management of chronic non-valvular AF is stroke reduction with oral anticoagulation (OAC). However, poor compliance, maintaining a narrow therapeutic window, and major side effects such as bleeding have severely limited their use, which creates a therapeutic dilemma. As much as 20% of AF patients are not receiving OAC due to contraindications and less than half of AF patients are not on OAC due to reluctance of the prescribing physician and/or patient non-compliance. Fortunately, over the past decade, there have been great interests in providing an alternative strategy unbeknownst to the practicing internist. The introduction of percutaneous approaches for LAA occlusion has added a different dimension to the management of chronic AF in patients with OAC intolerance. Occlusion devices such as the Amplatzer Cardiac Plug and WATCHMAN device are currently being investigated for stroke prophylaxis. More recently, the LARIAT device may provide an alternative means for potential stroke prophylaxis without the need for short-term post-procedural OAC. We aim to review the current literature and bring attention to an alternative strategy for high-risk AF patients intolerant to OAC.
Atrial fibrillation (AF) accounts for 15–20% of all strokes in the United States (Citation1). In one population-based study, it is estimated that 2.3 million US adults have AF and by 2050 it is projected to increase to 5.6 million (Citation1). Other studies have estimated an even larger prevalence of greater than 10 million, highlighting its significance (Citation2). Thromboembolic stroke from the left atrial appendage (LAA) is the most feared complication. The cornerstone for the management of chronic non-valvular AF is stroke reduction with oral anticoagulation (OAC). However poor compliance, maintaining a narrow therapeutic window, and major side effects such as bleeding have severely limited their use, which creates a therapeutic dilemma (Citation3). As much as 20% of AF patients are not receiving OAC due to contraindications and less than half of AF patients are on OAC due to either physician reluctance or patient non-compliance (Citation4, Citation5). Over the past decade, there has been great interest in providing an alternative strategy to anticoagulation. The introduction of percutaneous approaches for LAA occlusion has added a different dimension to the management of chronic AF in patients with OAC intolerance. Several implantation devices such as the percutaneous left atrial appendage transcatheter occlusion (PLAATO®), Amplatzer® Cardiac Plug (ACP), and the WATCHMAN® LAA system have been investigated for stroke prophylaxis (Citation6). In addition to these occlusion devices, a novel pre-tied suture device called the LARIAT® has also been described in the literature (Citation7).
Methods
Literature search strategy
An extensive electronic English language literature search was done using PubMed and Medline, to identify peer-reviewed original and review articles or abstracts using the key words ‘WATCHMAN’, ‘AMPLAZTER’, ‘LARIAT’, and ‘PLAATO’ from 1992 through 2013. In this review, we provide data on these devices. A total of 207 papers were identified and of these papers 9 are discussed. Studies involving non-catheter based procedures, non-prospective, and non-randomized clinical trials were excluded. The mechanism, efficacy, and limitations when available are discussed.
Rationale behind left atrial appendage occlusion
It is widely accepted that thrombus formation in the LAA is the result of stagnant blood flow (Citation8). In patients with non-valvular AF, transesophageal echocardiography (TEE) studies have shown the LAA as the site of more than 90% of thrombi formation and the most likely source of cardioembolic stroke (Citation8). The American College of Cardiology and the American Heart Association have recommended surgical amputation of the LAA during mitral valve surgery to reduce stroke risk (Citation9). Surgical approaches have been attempted to obliterate the LAA; however, it is often restricted to concomitant surgical procedures such as mitral valve surgery. Moreover, surgical ligation during mitral valve surgery is often incomplete (up to 36%), leaving a potential nidus for thrombus formation and risk for an embolic event (Citation10). The limitations of surgery and intolerance to OAC have prompted the need for other approaches.
Percutaneous devices for left atrial appendage occlusion
Percutaneous left atrial appendage transcatheter occlusion
Percutaneous left atrial appendage transcatheter occlusion (PLAATO®, ev3 Inc., Plymouth, MN) was the first catheter-based approach for LAA occlusion () (Citation11). Multiple observational studies were carried out to evaluate the safety and efficacy of the PLAATO system. Despite these multiple studies showing PLAATO's promising results (see ), there were no plans to proceed with a phase II or III trial due to lack of funding and it was discontinued in 2005 (Citation12–Citation15).
Fig. 1 PLAATO Device (Citation26).
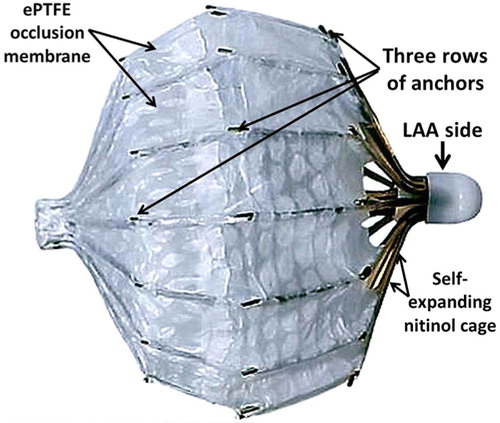
Table 1 Comparison table between devices
Amplatzer cardiac plug (ACP)
The ACP was introduced in 2002 by Meier et al., who adapted the concept of the Amplatzer septal occluder used for atrial septal defects (ASD) and patent foramen ovale (PFO) closure to exclude the LAA () (Citation16). In a retrospective single center study with a 10-year follow-up, Nietlispach et al. evaluated 152 patients who received ACP device implantation (Citation17). The safety endpoints were pericardial effusions, device embolization, procedure-related stroke and major bleeds (Citation17). The efficacy endpoints were cardiovascular and unexplained deaths, neurologic events including ischemic and hemorrhagic stroke or transient ischemic attacks, and systemic embolism (Citation17). In the overall population, no patients reached the efficacy endpoints within 6 months of implantation (Citation17). The major concern was device thrombus and residual leak into the LAA limiting the discontinuation of OAC for at least 3 months (Citation17). Although it was concluded that closure using the ACP device is an alternative to OAC, the benefits, long-term outcomes, and duration of OAC post-implantation still remain unclear (Citation17, Citation18).
Fig. 2 Amplatzer Cardiac Plug (Citation27).
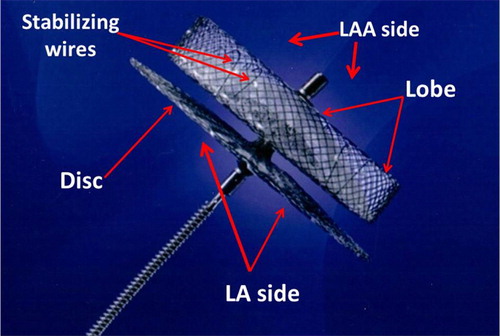
The device is currently unavailable in the United States, though ongoing clinical trials involving a prospective multicenter 2:1 randomization to a device or warfarin are in progress.
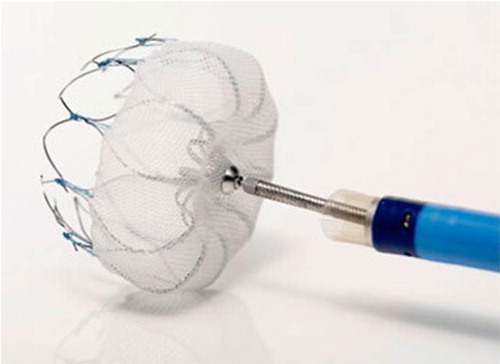
WATCHMAN device
The WATCHMAN LAA system is the best-studied occlusion device (). The largest conducted clinical trial compared the WATCHMAN device to warfarin in the WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) trial. The primary endpoints of efficacy included stroke, death, and systemic embolization (Citation20). Safety endpoints included bleeding and procedure-related complications (Citation20). Successful implantation was achieved in 88% (408/463) of patients (Citation20). 86% of the patients were able to discontinue warfarin at 45-day follow-up and 92% of the patients were able to discontinue warfarin at 6 months (Citation20). The primary efficacy event rate was 3.0 per 100 patient-years (95% credible interval [CrI] 1.9–4.5) with the WATCHMAN device and 4.9 per 100 patient-years (95% CrI: 2.8–7.1) in the warfarin group (Citation20). For successfully implanted patients, the primary efficacy event rate was lower at 1.9 per 100 patient-years (95% CrI: 1.0–3.2) (Citation20). The 21 patients who were assigned to the intervention group died secondary to events unrelated to device closure (Citation20). The cumulative mortality rate at 1 year for the device was 3.0% (95% CI: 1.3–4.6) compared to the warfarin group at 3.1% (95% CI: 0.8–5.4) (Citation20). At 2 years, the morality rate was 5.9% (95% CI: 2.8–8.9) and 9.1% (95% CI: 4.2–14.1) with the device compared to the warfarin group, respectively (Citation20). Primary safety events occurred at a higher rate in the device group (RR 1.69, 95% CrI: 1.01–3.19) than in the control group early on, though primary safety event rates in successfully implanted patients no longer receiving warfarin post-implantation were lower (RR 0.35, 95% CrI: 0.15–0.80) than the control group (Citation20). Most of the complications (55% of the patients) occurred on the day of the procedure, which was attributed to perioperative complications and operator experience (Citation20). Complications included serious pericardial effusions requiring pericardiocentesis (4.8%), major bleeding (3.5%), procedure-related ischemic stroke (1.1%), device embolization (0.6%), and hemorrhagic strokes (0.2%) (Citation20). It was concluded that although the WATCHMAN device met non-inferiority to warfarin therapy, there was a higher rate of adverse safety events due to peri-procedural complications (Citation20).
Fig. 3 WATCHMAN Device (Citation28).
A subsequent analysis of the PROTECT AF trial by Reddy et al. addressed safety by investigating the influence of operator experience with the WATCHMAN device (Citation21). The continued access registry study was a prospective clinical trial that compared the outcomes in patients who received the WATCHMAN device (Continued Access Protocol [CAP]) with the PROTECT AF group (Citation21). The primary safety outcomes included bleeding and procedure related events such as pericardial effusion, stroke, and device embolization (Citation21). The authors showed a significant decline in the rate of procedure or device related safety events within 7 days of the procedure and a decrease in the rate of pericardial effusions in the CAP Registry compared to the PROTECT AF group (Citation21). The authors concluded that there is significant improvement in the safety of the device with operator experience (Citation21).
The confirmatory trial to evaluate improved safety and efficacy were further addressed after the Aspirin and Plavix (ASAP) feasibility study in the PREVAIL trial (Citation22, Citation23). The prospective randomized clinical trial randomized 407 patients from 41 participating US centers (Citation23). The main differences between PROTECT-AF and PREVAIL were the exclusion of patients taking clopidogrel or who had taken clopidogrel within 7 days of enrollment and to include patients with a CHADS2 score ≥ 2 or CHADS2 score = 1 (in patients not eligible for aspirin therapy alone) (Citation23). The first primary endpoint was the occurrence of death, ischemic stroke, systemic embolism and procedure or device-related complications requiring cardiovascular or endovascular intervention within 7 days post-randomization (Citation23). Other endpoints included the comparison of composite stroke, systemic embolism, and cardiovascular/unexplained deaths at 18 months and comparison of ischemic stroke or systemic embolism occurring more than 7 days post-randomization also at 18 months (Citation23). The preliminary data from the study showed that the first endpoint of the occurrence of death, ischemic stroke, systemic embolism and procedure or device related complications requiring major cardiovascular or endovascular intervention pre-specified criterion were met and that there was improved procedural implant success, decreased composite vascular complications, decreased procedural stroke rates, decreased perforations requiring surgical repair, and little difference in outcome of new versus experienced operators (Citation23). The second endpoint through 18 months for composite stroke, systemic embolism, and cardiovascular/unexplained death showed that the warfarin group had lower than expected event rates though pre-specified non-inferiority criterion was not met (Citation23). The third endpoint through 18 months for comparison of ischemic stroke or systemic embolism occurring more than 7 days post-randomization met pre-specified non-inferiority criterion (Citation23). Although promising, no conclusions could be made until further completion of the study (Citation23).
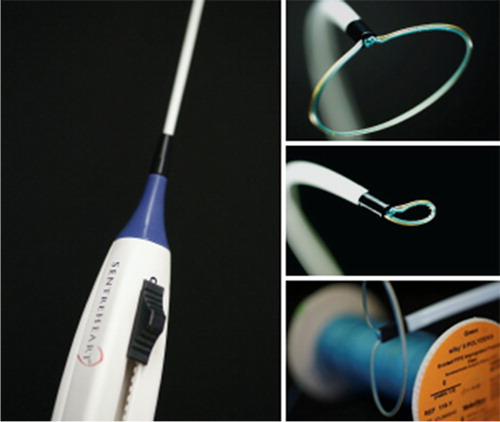
LARIAT device and accessories
Percutaneous LAA closure and exclusion using the LARIAT device is a novel procedure that utilizes a pre-tied suture to ligate the LAA () (Citation7).
Fig. 4 LARIAT Device (Citation29).
Following a feasibility study, a larger prospective study including 89 patients underwent LAA ligation with a 1-year closure follow-up verified by TEE (Citation24). Of the 89 patients, 85 had successful LAA ligation with 81 patients achieving immediate LAA closure (Citation24). Three of the 85 patients had <2 mm residual LAA leak and one with <3 mm residual LAA leak demonstrated by TEE (Citation24). Complications included three access-related (two pericardial and one trans-septal), two with severe pericarditis post-operatively, one late pericardial effusion, two unexplained sudden deaths, and two late strokes thought to be non-embolic (Citation24). Authors of this study concluded that LAA closure using the LARIAT device can be performed effectively with low rates of complications and peri-procedural adverse events (Citation24).
Technical limitations of the procedure include: achieving pericardial access, device limitation with regards to size, morphology, and appendage orientation. Potential complications of PLACE are pericardial effusions, pericarditis, procedure-related chest pain, incomplete ligature of the LAA, right ventricular puncture, LAA puncture, and potential disruption of the epicardial vessels (Citation25). Although the FDA for stroke prevention does not currently approve the device, ongoing prospective clinical trials are currently in progress including the Close Chested Epicardial Ligation of The Left Atrial Appendage in Atrial Fibrillation (EAGLE) registry study. compares and contrasts the different devices that were discussed above.
Conclusion
Little is known about the alternative approaches to the management of non-valvular AF in patients who have intolerance to OAC. The ACP, WATCHMAN, and LARIAT device show promising results; however, further trials are needed to elucidate safety and efficacy for long-term stroke prophylaxis. These devices may provide an alternative to patients who remain intolerant to OAC, are at high risk of bleeding, and to patients who wish to remain off OAC altogether. By doing so, this will give internists more options to provide stroke reduction tailored to each individual patient. Although promising, more randomized clinical trials are needed to establish long-term safety and efficacy before a shift in AF management can be ultimately made.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, etal. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285: 2370–75.
- Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, etal. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006; 114: 119–25.
- Poli D, Antonucci E, Grifoni E, Abbate R, Gensini GF, Prisco D. Bleeding risk during oral anticoagulation in atrial fibrillation patients older than 80 years. J Am Coll Cardiol. 2009; 54: 999–1002.
- Stafford RS, Singer DE. Recent national patterns of warfarin use in atrial fibrillation. Circulation. 1998; 97: 1231–3.
- Gottlieb LK, Salem-Schatz S. Anticoagulation in atrial fibrillation. Does efficacy in clinical trials translate into effectiveness in practice?. Arch Intern Med. 1994; 154(17): 1945–53.
- Cruz-Gonzalez I, Yan BP, Lam Y. Left atrial appendage exclusion: state-of-the-art. Catheter Cardiovasc Interv. 2010; 75: 806–13.
- Morelli R, Khodjaev S. Percutaneous left atrial appendage closure ligation and exclusion as an alternative stroke prevention in patients with nonvalvular atrial fibrillation. EP Lab Dig. 2012; 20–2.
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996; 61(2): 755–9.
- ACC/AHA guidelines for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association. Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). J Am Coll Cardiol. 1998; 32(5): 1486–588.
- Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I. Surgical left atrial appendage ligation is frequently incomplete: a tranesophageal echocardiographic study. J Am Coll Cardiol. 2000; 36: 468–71.
- Sievert H, Lesh MD, Trepels T, Omran H, Bartorelli A, Della Bella P, etal. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation. 2002; 105: 1887–9.
- Ostermayer SH, Reisman M, Kramer PH, Matthews RV, Gray WA, Block PC, etal. Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO System) to Prevent Stroke in High-Risk Patients With Non-Rheumatic Atrial Fibrillation. J Am Coll Cardiol. 2005; 46(1): 9–14.
- Block PC, Burstein S, Casale PN, Kramer PH, Teirstein P, Williams DO, etal. Percutaneous Left Atrial Appendage Occlusion for patients in atrial fibrillation suboptimal for warfarin therapy. J Am Coll Cardiol. 2009; 2: 594–600.
- El-Chami MF, Grow P, Eilen D, Lerakis S, Block PC. Clinical outcomes three years after PLAATO implantation. Catheter Cardiovasc Interv. 2007; 69: 704–7.
- Kao J, Shivaraju A. Preventing left atrial appendage emboli. Transcatheter LAA occluders are in clinical trials seeking FDA clearance as a replacement for warfarin therapy. Diagnostic and Interventional Cardiology. Web. 7, Sept 2010.
- Meier B, Palacios I, Windecker S, Rotter M, Cao QL, Keane D, etal. Transcatheter left atrial appendage occlusion with Amplatzer devices to obviate anticoagulation in patients with atrial fibrillation. Catheter Cardiovasc Interv. 2003; 60: 417–22.
- Nietlispach F, Gloekler S, Krause R, Shakir S, Schmid M, Khattab AA, etal. Amplatzer left atrial appendage occlusion: single center 10-year experience. Catheter Cardiovasc Interv. 2013; 82: 283–9.
- Lam L, Yip GW, Yu CM, Chan WW, Cheng BC, Yan BP, etal. Left atrial appendage closure with Amplatzer Cardiac Plug for stroke prevention in atrial fibrillation: initial Asia-Pacific experience. Catheter Cardiovasc Interv. 2012; 79: 794–800.
- Sick PB, Schuler G, Hauptmann KE, Grube E, Yakubov S, Turi ZG, etal. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007; 49: 1490–5.
- Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, etal. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomized non-inferiority trial. Lancet. 2009; 374: 534–42.
- Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the watchman left atrial appendage system for embolic protection in patients with AF (PROTECT AF) clinical trial and the continued access registry. Circulation. 2011; 123: 417–24.
- Reddy VY, Neuzil P, Miller MA, Schuler G, Mobius-Winkler SS, Wiebe J, etal. LB02-2- First Formal Analysis of The ‘ASA Plavix Registry’ (ASAP): watchman left atrial appendage closure in atrial fibrillation patients with contraindication to oral anticoagulation. Breaking abstract session presented at Heart Rhythm Scientific Sessions. 2012. Friday May 11.
- PREVAIL Yanked from ACC Program; Watchman device meets safety end point. Medscape. 2013. Mar 9.
- Bartus K, Bednarek J, Myc J, Kapelak B, Sadowski J, Lelakowski J, etal. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm. 2011; 8: 188–93.
- Bartus K, Han FT, Bednarek J, Myc J, Kapelak B, Sadowski J, etal. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation. J Am Coll Cardiol. 2013; 62(2): 108–18.
- Chiam PT, Ruiz CE. Percutaneous transcatheter left atrial appendage exclusion in atrial fibrillation. J Invasisve Cardiol. 2008; 20(4): E109–13.
- Nicholson W. Left Atrial Appendage Occlusion with the Amplazter Cardiac Plug. EP Lab Digest. 2010; 10(11): 22–23.
- Boston Scientific. WATCHMAN Left Atrial Appendage Closure Device. Available from: www.bostonscientific.com/watchman-eu/.
- SentreHeart. Lariat Suture Delivery Device. Available from: www.sentreheart.com/us/products/lariat.
