Abstract
Background
Chorea can be caused by a variety of diseases, including neurodegenerative disorders, vascular events, toxic-metabolic states, and immunologic and infectious diseases. We describe a patient who presented with hemichorea as the initial manifestation of Diabetes Mellitus (DM) and responded partially to the glycemic control.
Case report
A 63-year-old, healthy Hispanic man with no prior history of medical illness presented with subacute onset, gradually progressive hemichorea of 6 weeks’ duration. On evaluation, he was found to have non-ketotic hyperglycemia with high serum glucose (328 mg/dL), elevated hemoglobin A1C (9.9%), and absent ketones. Magnetic Resonance Imaging of the brain demonstrated hyper intense signals in bilateral basal ganglia on T1W images. He was diagnosed to have DM. Despite optimal glycemic control with insulin, the patient continued to have hemichorea at 3 months follow-up and required haloperidol for control of the involuntary movements.
Significance
Involuntary movements, particularly hemichorea, can be a manifestation and rarely be a presenting sign of DM.
Chorea is a symptom of neurological dysfunction which can be caused by a variety of diseases, including neurodegenerative disorders, vascular events, toxic-metabolic states, and immunologic and infectious diseases. Manifestation of hemichorea (HC) as the initial presentation of non-ketotic hyperglycemia (NKH) is rare in patients with diabetes mellitus (DM). We describe HC as presenting sign of diabetes in a Hispanic patient.
Case
A 63-year-old, right-handed Hispanic man was brought to the emergency room with the complaint of abnormal movements of right arm and right leg for about 6 weeks. The patient initially developed intermittent twitches in the right shoulder and arm as noticed by his daughter, which the patient was unaware of. Two weeks later, he was briefly admitted to an outside hospital for dizziness, dehydration, and high serum glucose levels up to 1,100 mg/dL. He was diagnosed to have DM and was started on subcutaneous insulin regimen. After 6 days of discharge from the hospital, he experienced worsening of involuntary movements which now involved whole right upper extremity and progressed in 2 weeks to right leg and foot. These movements were interfering with his daily activities prompting him to come to our hospital. He did not have abnormal behavior, headache, and weakness on any side or sensory disturbances. Patient was never treated with antipsychotic, antiemetic, or herbal medications. Family history was unremarkable. He was a reformed smoker and alcoholic.
On examination, he was alert, awake, and oriented. Blood pressure was 148/60 mmHg, and heart rate was 64/min. Cranial nerve examination was normal. Motor examination revealed normal strength, but hypotonia, hyporeflexia, and rapid, jerky, continuous non-stereotyped involuntary choreiform movements involving right upper and lower extremities. Plantar reflex was flexor bilaterally. The sensations were intact. He could not walk without support due to abnormal movements in right leg and foot.
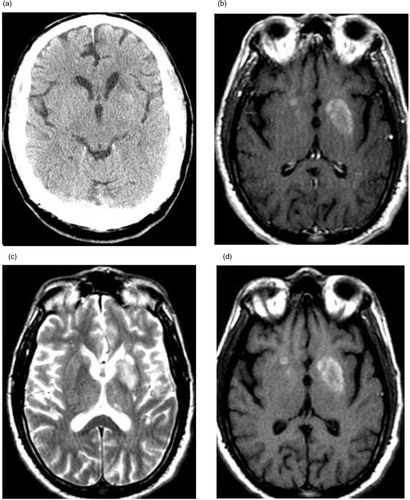
Complete blood count, liver, and renal function tests were normal. His serum glucose was 328 mg/dL, HbA1C level 9.9%, and serum ketones were negative. Urine toxicology, serum alcohol, as well as TSH and HIV were unremarkable. CT brain (a) revealed a 1×1.7 cm area of subtle increased density in left lentiform nucleus with surrounding decreased attenuation. Magnetic resonance imaging (MRI) brain with gadolinium contrast showed non-enhancing hyper intense lesions in bilateral lentiform and caudate nuclei on T1W images and old stable infarcts (b–d).
Fig. 1 (a) Non-contrast CT scan showing ill-defined hyperdensity in left putamen. (b) Axial T1-W without contrast showing hyperintensities in bilateral putamen and caudate. (c) Axial T2-W without contrast showing corresponding hypointensities in putamen and caudate. (d) Axial T1-W with contrast showing no enhancement of abnormal signals after gadolinium.
Despite adequate glycemic control using insulin, he continued to have HC and responded partially to haloperidol. At 3-month follow-up, the patient had persistent but mild choreiform movements.
Discussion
Polyphagia, polydipsia, weight loss, fatigue, and weakness are common presenting symptoms in patients with DM. Chorea is a rare manifestation of NKH in DM and was described first in 1960 (Citation1). It has rarely been described as the presenting sign of new onset diabetes (Citation2, Citation3). Hemichorea has been more commonly described to be associated with NKH than generalized chorea. In a meta-analysis of 53 patients by Oh et al., mean age was 71 years and male: female ratio was 1:1.8 (Citation4). In a study of 35 patients, 86% were Asians suggesting genetic predisposition (Citation5). Patients typically present with subacute onset, gradually progressive involuntary movements over days to weeks associated with high glucose levels, absent ketones, and high serum osmolality. However, chorea starting few days after the hyperglycemic crisis and even after adequate sugar control has been reported (Citation6). The movement disorder in NKH range from mild choreoathetoid movements to hemiballism with violent flinging movements. Associated features such as personality changes, seizures, painful sensory symptoms, and weakness have been described as well (Citation7, Citation8). Usually, movements resolve within 24–48 h of aggressive sugar control (Citation9, Citation10). However, prolonged HC and relapse after the initial response has also been described (Citation11, Citation12).
Ahlskog et al. reported five female patients who developed chorea concurrently or shortly after hyperglycemic episode and all patients continued to have persistent chorea despite sugar control for 6 months to 5 years of follow-up (Citation6). Patients with HC with incomplete recovery from glycemic control may respond well to the conventional neuroleptics, such as haloperidol, perphenazine, and chlorpromazine. Also, risperidone and an anticonvulsant such as topiramate may be useful (Citation11–(Citation13)). The prognosis of chorea associated with NKH has been reported as good (Citation10).
Exact pathogenesis of chorea in NKH is unclear. Striatum may be directly susceptible to alterations of blood glucose because even patients with hypoglycemia can develop choreoathetoid movements (Citation14). Ischemia secondary to hyperviscosity or neural injury due to hyperglycemia could be the possible cause for abnormal movements (Citation15). Another theory is putaminal petechial hemorrhage causing dyskinesia (Citation16). Broderick et al. reported two cases of DM and chorea where hemorrhagic infarction was caused by diapedesis from damaged but not ruptured capillaries (Citation17). Biopsy and autopsy studies have showed striatal neuronal loss and astrocytosis (Citation18). It is also possible that chorea in NKH is due to a functional abnormality rather than structural disease. Putamen may have inhibitory influence on globus pallidus and lesions of putamen cause uninhibited activity of globus pallidus leading to choreiform movements. Neurotransmitter dysfunction mainly hyperactivity of dopaminergic neurons is thought to be a prominent cause. In NKH, brain metabolism shifts to anaerobic pathway and brain utilizes GABA as energy source. Unlike in diabetic ketoacidosis, GABA is not resynthesized and rapid depletion of GABA leads to decreased acetyl choline synthesis. Reduced GABA, acetyl choline, decreased energy, and regional metabolic failure may cause basal ganglia dysfunction leading to chorea (Citation4).
HC in NKH presents with characteristic though not specific neuroimaging findings. Majority have hyperintense signals in contralateral putamen on T1-weighted images. In few case series, patients also had hyperintensities in contralateral caudate (Citation19). Patients can also have bilateral basal ganglia hyperintense signals on T1W images. In the meta-analysis of 53 cases by Oh et al., all patients had hyperintense signals in contralateral putamen on T1W images and six (11%) patients had bilateral basal ganglia lesions (Citation4). Usually, there is hypointensity or no significant alternation in T2-weighted images and on contrast MRI lesions are mostly non-enhancing. Another characteristic finding is the resolution of abnormal signals in follow-up imaging although it may be seen well ahead of clinical improvement (Citation4). We suggest that detailed neuroimaging should be performed including DWI and SWI to exclude infarction, hidden ischemia (perfusion deficit), and hemorrhagic processes, which may affect treatment strategies. CT scan may be normal or may show hyperdensity in contralateral basal ganglia. SPECT studies in patients show hypoperfusion in the corresponding areas (Citation16). PET scan in three patients with chorea and NKH showed markedly reduced rates of cerebral glucose metabolism in the corresponding lesions on T1-weighted images on MRI suggesting evidence of regional metabolic failure (Citation13).
As DM is a widely prevalent disease and hemichorea due to NKH is amenable to rapid sugar control, it is important for physicians to be aware of this condition and consider it in appropriate clinical settings. Why chorea persists in some patients of NKH despite optimal glycemic control is not entirely clear though neuronal loss from ischemia and putaminal microhemorrhage are possible causes. Please see appendix for common questions on this topic.
Conclusion
Involuntary movements, particularly hemichorea, can be a manifestation and rarely be a presenting sign of DM. Usually, hemichorea is associated with NKH. Characteristic MRI feature is hyperintensity at contralateral putamen on T1W images. Hemichorea usually, but not always, responds to glycemic control. Exact pathogenesis is unclear; direct affection of striatum by altered glucose levels is possible.
Conflict of interest and funding
Abhijeet Danve, Supriya Kulkarni and Girija Bhoite have no conflict of interest.
Authors' contributions
All the listed authors have participated actively in the study, have met the requirements for the authorship, and have read and approved the submitted manuscript.
References
- Bedwell SF. Some observations on hemiballismus. Neurology. 1960; 10: 619–22.
- Lin JJ, Chang MK. Hemiballism-hemichorea and NKH. J Neurol Neurosurg Psychiatry. 1994; 57(6): 748–50.
- Verma R, Praharaj HN. Hemichorea-hemiballism as the presenting manifestation of diabetes mellitus. BMJ Case Rep. 2013
- Oh SH, Lee KY, Im JH, Lee MS. Chorea associated with NKH and hyperintensity basal ganglia lesion on T1-weighted brain MRI study; a meta-analysis of 53 cases including 4 present cases. J Neurol Sci. 2002; 200(1–2): 57–62.
- Lin JJ, Lin GY, Shih C, Shen WC. Presentation of Striatal hyperintensity on T1- weighted MRI in patients with HC-HB caused by NKH: report of seven new cases and review of literature. J Neurol. 2001; 248(9): 750–5.
- Ahlskog JE, Nishino H, Evidente VG, Tulloch JW, Forbes GS, Caviness JN, etal. Persistent chorea triggered by hyperglycemic crisis in diabetics. Mov Disord. 2001; 16(5): 890–8.
- Sarah MK, Raymond P, Sashank P, Howard IH. Clinical reasoning: a 52 year old woman with subacute hemichorea. Neurology. 2008; 71(20): 59–62.
- Ohmori H, Hirashima K, Ishihara D, Maeda Y, Hirano T, Uyama E, etal. Two cases of hemiballism-hemichorea with T1-weighted MR image hyperintensities. Intern Med. 2005; 44: 1280–5.
- Wintermark M, Fischbein NJ, Mukherjee P, Yuh EL, Dillon WP. Unilateral putaminal CT, MR, and diffusion abnormalities secondary to nonketotic hyperglycemia in the setting of acute neurologic symptoms mimicking stroke. AJNR Am J Neuroradiol. 2004; 25: 975.
- Branca D, Gervasio O, Le Piane E, Russo C, Aguglia U. Chorea induced by non-ketotic hyperglycaemia: a case report. Neurol Sci. 2005; 26: 275–7.
- Saleh MM, Zacks ES, Katz JS. Delayed recovery of diabetic chorea following correction of hyperglycemia. J Neurol. 2002; 249: 1323–4.
- Driver-Dunckley E, Evidente VG. Hemichorea-hemiballismus may respond to topiramate. Clin Neuropharmacol. 2005; 28: 142–4.
- Hsu JL, Wang HC, Hsu WC. Hyperglycemia-induced unilateral basal ganglia lesions with and without hemichorea: a PET study. J Neurol. 2004; 251: 1486–90.
- Newman RP, Kinkel WR. Paroxysmal choreioathetosis due to hypoglycemia. Arch Neurol. 1984; 41(3): 341–2.
- Altafullah I, Pascual-Leone A, Duvall K, Anderson DC, Taylor S. Putaminal hemorrhage accompanied by hemichorea-hemiballism. Stroke. 1990; 21(7): 1093–4.
- Chang MH, Chiang HT, Lai PH, Sy CG, Lee SS, Lo YY. Putaminal petechial hemorrhage as cause of chorea: a neuroimaging study. J Neurol Neurosurg Psychiatry. 1997; 63(3): 300–3.
- Broderick JP, Hagen T, Brott T, Tomsick T. Hyperglycemia and hemorrhagic transformation of cerebral infarcts. Stroke. 1995; 26: 484–7.
- Nagai C, Kato T, Yamamoto K, Sasaki H. Hyperintense Putamen on T1weighted MR images in case of chorea with hyperglycemia. AJNR. 1994; 16: 124–6.
- Lai PH, Tien RD, Chang MH, Teng MM, Yang CF, Pan HB, etal. Chorea- Ballismus with NKH in primary diabetes mellitus. Am J Neuroradiol. 1996; 17(6): 1057–4.
Appendix
Multiple choice questions
1. Chorea can be a manifestation of which of the following conditions?
A. Non-ketotic hyperglycemia in diabetes
B. Diabetic ketoacidosis
C. Hypoglycemia
D. A and C
Correct answer: D
Both hypoglycemia and non-ketotic hyperglycemia can trigger chorea.
2. Hemichorea due to non-ketotic hyperglycemia is common in which of the following races?
A. Asian
B. White
C. Hispanic
D. Black
Correct answer: A
Majority of reported cases have been Asian women.
3. What is the typical brain MRI finding in patients with diabetic hemichorea?
A. Hypointensity on T1W images in contralateral caudate nucleus
B. Hyperintensity on T1 W images in contralateral putamen
C. Hypointensity on T1W images in ipsilateral putamen
D. Hyperintensity on T2W images in contraletral globus pallidus
Correct answer: B
Majority have hyperintense signals in contralateral putamen on T1-weighted images.
4. Which of the following is the treatment of choice in patients with diabetic hemichorea?
A. Haloperidol
B. Oxcarbazepine
C. Topiramate
D. Adequate glycemic control
Correct answer: D
Majority of patients respond within 24–48 h with adequate glycemic control.
5. Which of the following has been implicated in the pathogenesis of diabetic hemichorea?
A. Ischemia secondary to hyperviscosity or neural injury
B. Putaminal hemorrhage
C. Uninhibited activity of globus pallidus
D. Depletion of GABA
E. All of the above
Correct answer: E
All of the above mechanisms have been reported to contribute to development of diabetic hemichorea.
