Abstract
Obstruction of the thoracic duct may lead to accumulation of a lymphatic fluid rich in triglycerides named chyle. When chyle accumulates in the pleural cavity, it becomes a chylothorax. Malignancy, particularly lymphoma, is the most common cause of chylothorax; however, any pathology leading to obstruction or destruction of the thoracic duct can lead to a chylothorax. This particular case investigates an incidence of chylothorax in sarcoidosis. A 54-year-old African American woman with a medical history of sarcoidosis, congestive heart failure, and smoking presented to the emergency department with complaints of bilateral foot swelling and exertional shortness of breath 3 days in duration. Physical examination was positive for bilateral crepitations with decreased air entry, abdominal ascites, and bilateral 2+ pitting edema. Both chest X-ray and chest CT were positive for stable bilateral pleural effusions (when compared to imaging done 3 years previously), and thoracocentesis and paracentesis were positive for chylous fluid accumulation. Chylothorax was diagnosed, and based on the previous medical history, the lymphadenopathy of sarcoidosis was determined to cause the occlusion of the thoracic duct. Lymphoscintigraphy and surgical intervention were advised; however, the family decided on conservative management and the patient expired intubated in the ICU. Chylothorax is a rare manifestation of sarcoidosis and high index of suspicion should be there to diagnose this, as there is high morbidity and mortality associated with it.
A 54-year-old African American woman with a medical history of sarcoidosis, congestive heart failure, pulmonary hypertension on home oxygen, and smoking (she quit 10 years previously) presented to the emergency department with a chief complaint of bilateral foot swelling and exertional shortness of breath for 3 days in duration.
The patient was on home oxygen 24 h a day and usually able to ambulate 3–4 blocks without having shortness of breath; however, over the past 3 days, she reported a decreased functional capacity limited to one block. Associated with the limited functional capacity was a worsening of her bilateral foot edema. Beyond this, she denied all other systemic complaints and any history of Paroxysmal Nocturnal Dyspnea (PND), chest pain, palpitations, dizziness, or presyncope. She also denied any history of recent travel, sitting for an extended period of time, or leg pain. She reported to be compliant with her home medications, which included steroid therapy for sarcoidosis. She denied family history of respiratory or cardiovascular diseases.
Her vital signs were stable on admission with an oxygen saturation of 93% on room air. Chest examination revealed bilateral decreased air entry over her lung bases associated with crepitations. Her abdominal examination revealed a distended abdomen with ascites. Due to distension, organomegaly could not be assessed. Furthermore, she had bilateral pitting edema of 2+ in both lower extremities. On laboratory examination, her Complete Blood Count (CBC) and Comprehensive Metabolic Panel (CMP) were within the normal range and her initial Arterial Blood Gas (ABG) indicated hypoxia with respiratory acidosis. Investigations indicated an echocardiogram with an ejection fraction of 40–45% and Pulmonary Artery Pressure (PAP) of 50 mmHg (same result from an echocardiogram performed 2 years previously) and her chest X-ray () illustrated moderate bilateral pleural effusions, with underlying atelactasis and infiltrate. Chest CT (Figs. (Citation2–Citation4)) indicated similar moderate-to-large left and small right pleural effusions with bilateral circumferential pleural thickening.
Fig. 1 Chest X-ray during admission showing bi-basilar effusion, atelectasis, infiltrate, and cardiomegaly.
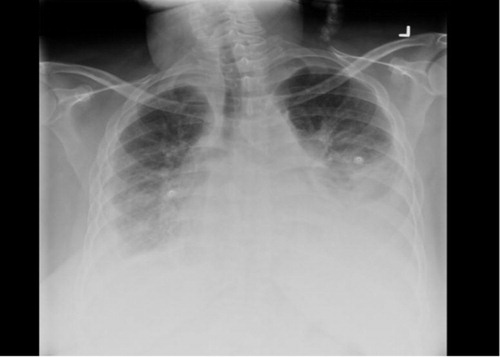
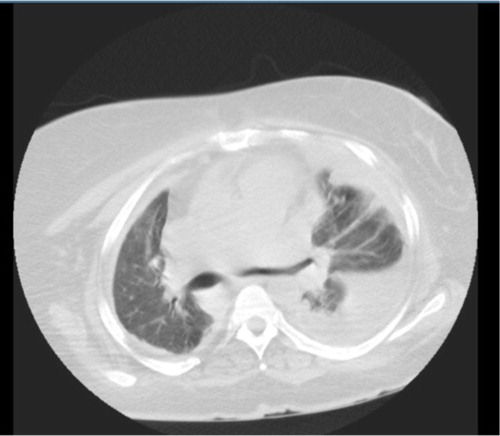
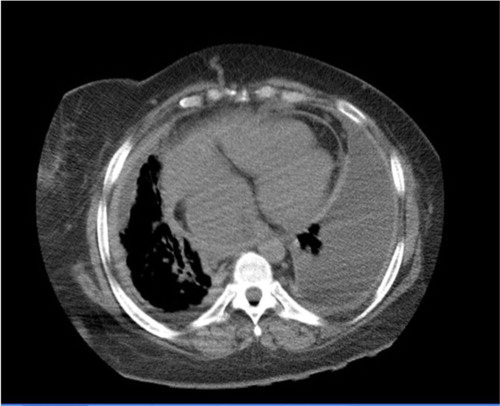
Fig. 3 Chest CT (lung window) at lower lung field showing b/l lower lung pleural effusion, more on the left, and cardiomegaly.
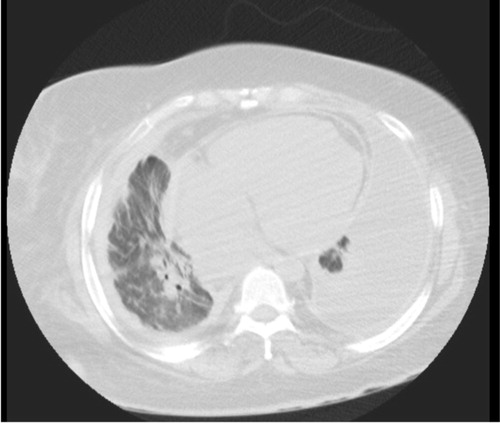
Fig. 4 Chest CT (mediastinal window) at lower lung field showing b/l lower lung pleural effusion, more on the left, bilateral pleural thickening, and cardiomegaly.
The patient was admitted with a diagnosis of Congestive Heart Failure (CHF) exacerbation; however, pneumonia was also considered due to her long-term therapy with steroids for sarcoidosis. After 3 days of medical management for CHF, the patient did not show clinical improvement; therefore, a thoracocentesis was done. A pleural catheter was inserted, and 2,500 mL of chylous fluid was drained. Laboratory analysis of the pleural fluid revealed a WBC count of 110 with lymphocytes 96%, adenosine deaminase <1.6, total cholesterol level, 31 mg/dL; triglycerides, 249 mg/dL; glucose, 106 mg/dL; protein, 3.8 mg/dL; and lactate dehydrogenase, 81 IU/L, without bacterial growth. Based on the pleural fluid, a diagnosis of chylothorax was made. Peritoneal paracentesis was also performed and fluid analysis showed triglycerides of 667 mg/dL.
Due to chylothorax, a fat-free diet with medium chain triglyceride (MCT) oil was initiated; however, the patient started to have worsening shortness of breath with both the chest X-ray and abdominal ultrasound, indicating increasing of both pleural effusion and ascites; therefore, chest tube was inserted and therapeutic thoracocentesis was performed again and the shortness of breath was partially relieved. Because chylothorax leads to critical losses of fluid, lymphocytes, proteins, coagulation factors, and antibodies conservative treatment was started with high-dose octreotide therapy; however, after 10 days of therapy with octreotide, the patient continued to have increasing chylous drainage and ascites.
The patient was maintained on high-dose octreotide and simultaneous arrangement was made for transfer to a center where the patient could have lymphoscintigraphy. The risks and benefits of lymphoscintigraphy and surgical intervention were explained to the patient and her family but they decided to continue with conservative management. The patient continued to deteriorate and subsequently developed fever, hypotension, tachycardia, and hypoxia with leukocytosis. She was intubated and transferred to the ICU but went into cardiac arrest and expired.
Discussion
Chylothorax is a rare manifestation of sarcoidosis and only a few cases have been reported in the literature. Although the exact etiology of chylothorax in sarcoidosis is unknown, it is thought that the adenopathy of sarcoidosis leads to destruction and obstruction of the thoracic duct, which results in an accumulation of chylous fluid in the pleural space (Citation1).
The thoracic duct originates from the cisterna chyli in the abdomen at the level of L1–L2 and enters the thoracic cavity through the aortic hiatus at the level of T12. In the thorax, it ascends in the posterior mediastinum, crossing midline at T5, and draining into the left subclavian vein. Although other anatomic variations may be commonly observed, this course of the thoracic duct is present in 65% of the population (Citation1).
Chyle, which is the fluid that accumulates in chylothorax, is a lymphatic fluid of intestinal origin and has a high content of triglycerides, which in the form of chylomicrons imparts its characteristic white color. It also contains cholesterol, protein, electrolytes, glucose, fat-soluble vitamins, immunoglobulins, and white blood cells, which are predominantly lymphocytes. When functioning appropriately, the daily drainage of the thoracic duct is 1.5–2.4 L; however, this can vary depending on diet, medication, intestinal function, and physical activity (Citation2).
The etiology of chylothorax can be grouped as non-traumatic or traumatic. Malignant obstruction of the thoracic duct is the most common non-traumatic cause, of which lymphomas account for 70% of the cases (Citation3). Other non-traumatic causes include sarcoidosis, retrosternal goiter, amyloidosis, obstruction of central veins, diseases affecting lymph vessels such as yellow nail syndrome, lymphangioleiomyomatosis and hemangiomatosis (Gorham's disease), heart failure, and tuberculosis (Citation4, Citation5). Different studies have reported variable rates of pleural involvement in sarcoidosis with approximately 3% of sarcoidosis cases demonstrating pleural involvement, 2.8% demonstrating pleural effusion, and only three cases demonstrating chylothorax (Citation6, Citation7). Chylothorax may be seen in other disease processes as well and unlike the obstructive mechanism seen in sarcoidosis in liver cirrhosis, chylothorax may result from leaks to the pleural space through the diaphragm (Citation8). Chylothorax may also be seen with irradiation of the thorax where it is a late complication (Citation9, Citation10). Traumatic causes of chylothorax usually occur with thoracic surgery involving the posterior mediastinum. Esophageal resection is also commonly associated with chylothorax with the incidence reported as 4% (Citation7), and it may also occur due to complications associated with neck dissection (Citation11) and subclavian vein catheterization (Citation12); however, despite all of the above etiologies, about 10% of chylothoraces are idiopathic (Citation5).
On thoracocentesis, the pleural fluid may be milky, serosanguinous, serous, or bloody depending on the nutritional status of the patient, dietary intake of fat, and the etiology of the chylothorax. Typical pleural fluid in chylothorax is a lymphocytic exudate with low lactate dehydrogenase (Citation13) and pleural fluid triglyceride more than 110 mg/dl. Triglyceride level below 110 mg/dl is non-diagnostic and requires further evaluation for chylomicrons, and the detection of chylomicrons by electrophoresis in pleural fluid confirms the diagnosis (Citation14).
Imaging studies such as CT chest and abdomen may also help in identifying the underlying causes of chylothorax such as lymphadenopathy or mass, and ECHO is indicated if any cardiac involvement is suspected. Lymphoangiogram has a diagnostic value in localizing the site of thoracic duct leak or blockage and has shown therapeutic benefit in some cases (Citation15); however, lymphoscintigraphy is usually a safer and less invasive option (Citation16). The aim of lymphoscintigraphy was to localize the site of thoracic duct obstruction and intervene surgically.
While pleural drainage by intermittent therapeutic thoracentesis or chest tube placement helps in relieving the symptoms associated with chylothorax, the underlying cause should always be addressed and treated. Furthermore, caution should always be exercised with prolonged or high volume drainage of chylous fluid via chest tube since drainage can cause a loss of both electrolytes and immunoglobulins and lead to an increased risk of infections (Citation17).
The conservative approach toward chylothorax aims at reducing lymph flow in the thoracic duct to subsequently facilitate spontaneous closure. This can be achieved by fasting and total parenteral nutrition or dietary restriction of fatty acids since these decrease the production of lymphatic fluid and, therefore, reduce thoracic duct flow. Dietary exclusion of long chain fatty acids and substitution of medium chain fatty acids is the recommended strategy since long chain fatty acids are converted to monoglycerides and triglycerides and transported via lymphatic vessels, but MCTs are directly absorbed in the intestine and transported by the portal vein (Citation1, Citation18). Furthermore, along with changes in diet, pharmacotherapy in the form of a somastatin analog, Octreotide, can be used. Specifically, octreotide inhibits hormonal secretion and absorption in the gastrointestinal tract, which then decreases splanchnic blood flow and the production of lymphatic fluid (Citation19). Chemical pleurodesis may also be considered in cases not responding to conservative therapy (Citation20, Citation21), and in refractory cases, thoracic duct ligation can be done via open thoracotomy or video-assisted thoracoscopic surgery (Citation22). Thoracic duct embolization is also an option, but it is technically challenging (Citation23) and lastly, for those patients who fail conservative treatment pleuroperitoneal shunt can be performed as a palliative procedure (Citation24, Citation25).
Conclusion
Although uncommon, the inflammation associated with sarcoidosis can significantly damage both the thoracic duct and the small lymphatic channels in the lungs, which can then lead to an accumulation of chylous fluid. This case illustrates the fact that chylothorax does occur in sarcoidosis and should be in one of the differentials as there is potential of chylothorax to develop into a significant and unexpected life-threatening complication. Although conservative management is usually preferred, in the case of excessive chylous leaks, pharmacologic therapy may not be the best option and this is especially true if the rate of accumulation of chylous fluid remains unaffected after days of therapy. When conservative measures fail, more aggressive therapeutic options including surgery should always be considered because they can be potentially life-saving.
Conflict of interest and funding
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- McGrath EE, Blades Z, Anderson PB. Chylothorax: Aetiology, diagnosis and therapeutic options. Respir Med. 2010; 104(1): 1–8.
- Soto-Martinez M, Massie J. Chylothorax: Diagnosis and management in children. Paediatr Respir Rev. 2009; 10(4): 199–207.
- Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg. 2007; 32(2): 362–9.
- Hillerdal G. Chylothorax and pseudochylothorax. Eur Respir J. 1997; 10(5): 1157–62.
- Hooper C, Lee YC, Maskell N. BTS Pleural Guideline Group. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010; 65(Suppl 2): ii4–17.
- Huggins JT, Doelken P, Sahn SA, King L, Judson MA. Pleural effusions in a series of 181 outpatients with sarcoidosis. Chest. 2006; 129(6): 1599–604.
- Soskel NT, Sharma OP. Pleural involvement in sarcoidosis. Curr Opin Pulm Med. 2000; 6(5): 455–68.
- Romero S, Martín C, Hernandez L, Verdu J, Trigo C, Perez-Mateo M, etal. Chylothorax in cirrhosis of the liver: Analysis of its frequency and clinical characteristics. Chest. 1998; 114(1): 154–9.
- Van Renterghem DM, Pauwels RA. Chylothorax and pleural effusion as late complications of thoracic irradiation. Chest. 1995; 108(3): 886–7.
- McWilliams A, Gabbay E. Chylothorax occurring 23 years post-irradiation: Literature review and management strategies. Respirology. 2000; 5(3): 301–3.
- Runge T, Borbély Y, Candinas D, Seiler C. Bilateral chylothorax following neck dissection: A case report. BMC Res Notes. 2014; 7: 311.
- Ruggiero RP, Caruso G. Chylothorax – a complication of subclavian vein catheterization. J Parenter Enteral Nutr. 1985; 9(6): 750–3.
- Maldonado F, Hawkins FJ, Daniels CE, Doerr CH, Decker PA, Ryu JH. Pleural fluid characteristics of chylothorax. Mayo Clin Proc. 2009; 84(2): 129–33.
- Skouras V, Kalomenidis I. Chylothorax: Diagnostic approach. Curr Opin Pulm Med. 2010; 16(4): 387–93.
- Matsumoto T, Yamagami T, Kato T, Hirota T, Yoshimatsu R, Masunami T, etal. The effectiveness of lymphangiography as a treatment method for various chyle leakages. Br J Radiol. 2009; 82(976): 286–90.
- Ogi S, Fukumitsu N, Uchiyama M, Mori Y. A case of chylothorax diagnosed by lymphoscintigraphy using Tc-99m HSA-DTPA. Clin Nucl Med. 2002; 27(6): 455–6.
- Chalret du Rieu M, Mabrut JY. Management of postoperative chylothorax. J Visc Surg. 2011; 148(5): e346–52.
- Takuwa T, Yoshida J, Ono S, Hishida T, Nishimura M, Aokage K, etal. Low-fat diet management strategy for chylothorax after pulmonary resection and lymph node dissection for primary lung cancer. J Thorac Cardiovasc Surg. 2013; 146(3): 571–4.
- Kalomenidis I. Octreotide and chylothorax. Curr Opin Pulm Med. 2006; 12(4): 264–7.
- Mares DC, Mathur PN. Medical thoracoscopic talc pleurodesis for chylothorax due to lymphoma: A case series. Chest. 1998; 114(3): 731–5.
- Cho HJ, Kim DK, Lee GD, Sim HJ, Choi SH, Kim HR, etal. Chylothorax complicating pulmonary resection for lung cancer: Effective management and pleurodesis. Ann Thorac Surg. 2014; 97(2): 408–13.
- Paul S, Altorki NK, Port JL, Stiles BM, Lee PC. Surgical management of chylothorax. Thorac Cardiovasc Surg. 2009; 57(4): 226–8.
- Nadolski G, Itkin M. Thoracic duct embolization for the management of chylothoraces. Curr Opin Pulm Med. 2013; 19(4): 380–6.
- Epaud R, Dubern B, Larroquet M, Tamalet A, Guillemot N, Maurage C, etal. Therapeutic strategies for idiopathic chylothorax. J Pediatr Surg. 2008; 43(3): 461–5.
- Shimmyo T, Morita K, Mineshita M, Tagaya R, Ando K, Mochizuki A, etal. Pleuroperitoneal shunt for chylothorax and chylopericardium in lung cancer: A case report. Ann Thorac Cardiovasc Surg. 2011; 17(1): 63–6.