Abstract
The Extracellular RNA (exRNA) Communication Consortium was launched by the National Institutes of Health to focus on the extent to which RNA might function in a non-cell-autonomous manner. With the availability of increasingly sensitive tools, small amounts of RNA can be detected in serum, plasma, and other bodily fluids. The exact mechanism(s) by which RNA can be secreted from cells and the mechanisms for the delivery and uptake by recipient cells remain to be determined. This review will summarize current knowledge about the biogenesis and delivery of exRNA and outline projects seeking to understand the functional impact of exRNA.
This paper is part of the Special Issue: Extracellular RNA Communication Consortium. More papers from this issue can be found at http://www.journalofextracellularvesicles.net
The RNA world and analysis of modern roles for RNA have produced a number of surprises, notably catalytic RNA, the discovery of introns, the prevalence of alternative splicing to generate protein diversity, RNA interference, and riboswitches that link metabolism with gene expression (Citation1). Recent discoveries showing the extent of transcription within eukaryotic genomes have demonstrated that many RNA species exist in cells, but the exact function of most of these transcripts remains unknown (Citation2–Citation4). Many of these RNAs are non-coding. The best-characterized non-coding RNAs are small microRNAs (miRNAs), but many other non-coding RNAs have been identified that appear to play roles in genome defense or chromatin organization (Citation5–Citation9). An underlying assumption for many years has been that RNA function is cell autonomous, especially given the levels of RNAse in extracellular fluids and plasma that function to destroy foreign RNAs, most commonly viral RNA. However, new highly sensitive tools have enabled the discovery of extracellular host RNA in both the bloodstream and multiple body fluids (Citation10). How these RNAs are exported from donor cells, how they are taken up by and/or targeted to specific recipient cells, and how they are released to function inside recipient cells remain mostly unanswered questions. To address these questions, the National Institutes of Health Common Fund launched a new program, the Extracellular RNA (exRNA) Communication Consortium. This review will summarize the projects focusing on biogenesis and delivery mechanisms, and the function of exRNA.
RNA export
To avoid degradation, it is thought that most exRNAs are either encased within membranous vesicles or are tightly associated with proteins and/or lipids. Extracellular vesicles (EV) include exosomes (~40–100 nm) and larger microvesicles but could conceivably also include apoptotic vesicles released upon cell death (Citation11, Citation12) (). Multiple studies have focused on exosomes as carriers of RNA, in part due to RNA sequencing/microarray studies performed from purified exosomes (Citation13–Citation15), as well as localization of miRNA effector complexes associated with multivesicular bodies (MVBs) (Citation16, Citation17). Exosomes are endosomally derived vesicles that form by invagination into MVBs and contain proteins involved in endosomal transport and fusion (Citation18). Normally, MVBs are targeted to lysosomes for degradation, but they can also fuse with the plasma membrane, thereby releasing exosomes into the extracellular space. Deposition of some, but not all, RNAs into exosomes might be explained by import into MVBs during the intraluminal vesiculation process. This explains the presence of miRNAs in exosomes since the RISC machinery that processes miRNAs has been localized to the surface of MVBs (Citation16, Citation17). Exosomes that originate from MVBs and contain miRNAs may not necessarily require ESCRTS for assembly (Citation19, Citation20). Inhibition of ceramide synthesis by blocking neutral sphingomyelinases can cause a decrease in exosome release and secreted miRNAs, although this may be cell specific (Citation21–Citation24). How longer RNAs, particularly mRNAs or long non-coding RNAs are targeted for export into exosomes or microvesicles is not clear (Citation15, Citation25) (Citation26). From microarrays and RNA sequencing experiments, numerous sequences are detected from multiple classes of RNA, including rRNAs and mRNAs, but the abundance and activity of full-length mRNA remain uncertain (Citation13, Citation15) (Citation27, Citation28). Similarly, it is not known if there is a size threshold for long non-coding RNAs. For these reasons, most work has focused on miRNA deposition into exosomes.
Fig. 1. Biogenesis and uptake of exRNA. Endocytosis is commonly mediated via clathrin-coated pits (Citation1) after which endocytic vesicles progress from early (Citation2) to late endosomes, also referred to as multivesicular bodies (MVBs). MVBs often fuse with lysosomes for degradation (Citation4) but can also fuse with the plasma membrane (Citation5) thereby releasing exosomes (40–100 nm) to the extracellular space. Microvesicles are larger fragments of plasma membrane that are shed from almost all cells (Citation6). Extracellular RNA (exRNA) can be detected in exosomes and microvesicles, associated with proteins (Citation7), or as part of lipoprotein particles, particularly HDL. exRNA released from secreting cells can be taken up by recipient cells through receptor-mediated endocytosis (Citation8), by fusion of membranes, or by uptake of RNA–protein complexes or lipoproteins. Although the exact mechanisms remain unclear, exRNA is released inside recipient cells (Citation9) to effect changes in gene expression.
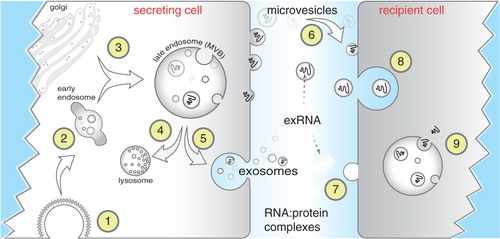
miRNAs can also be found in extracellular fluids in complex with either Argonaute proteins (Citation29) or as part of lipoprotein particles, mostly HDL (Citation30). Quantitative analysis of miRNA content in exosomes has suggested that individual exosomes contain vanishingly small numbers of miRNA molecules, far lower than would be expected if exported miRNAs function stoichiometrically in recipient cells (Citation31). While somewhat controversial, one explanation to account for these results is that different subclasses of exosomes exist, only some of which contain miRNAs or other RNAs. A second possibility is that functional miRNA is transported via Ago2 complexes or as part of HDL particles (). Stoichiometric concerns might be sidestepped in these latter cases but the issue remains as to exactly how miRNAs are packaged into HDL particles or how Ago2 complexes are released from cells. In addition, it is not clear how such miRNAs reassemble into functional RNA Induced Silencing Complexes upon import into recipient cells. A third explanation is that exosomes and exosomal cargo purified from fluids or cell culture medium might not necessarily reflect the extent of continuous transfer that might be occurring between directly adjacent cells.
Experiments comparing cellular miRNA expression profiles to exosomal or HDL profiles are underway. The weight of evidence thus far favours the idea that the 2 profiles are distinct, suggesting that there are likely to be signals that preferentially export specific miRNAs (Citation11, Citation15) (Citation27, Citation30) (Citation32–Citation35).
Delivery of exRNA
After export from donor cells, proposed mechanisms for how RNAs are delivered to recipient cells depend on whether they are part of exosomes (or other vesicles), HDL, or Ago2 complexes (). Receptor-mediated uptake would be expected for HDL-associated miRNAs (scavenger receptor class B type 1) (Citation30) and exosomes by a variety of membrane receptors matched to exosomal ligands (Citation23, Citation27) (Citation36–Citation38). It is not clear how recipient cells would take up Ago2-miRNA complexes. Receptors exist to transfer double-stranded RNA in Caenorhabditis elegans, mammals (Citation39), flies (Citation40), and plants (Citation41). It will be interesting to determine what role such receptors/pathways play in mediating the uptake of exRNA.
Functional transfer of exRNA
The greatest short-term impact of the discovery of exRNA might be the identification of biomarkers of disease because it does not involve functional transfer of RNA to recipient cells (Citation42). However, if RNAs are specifically targeted for export and cells use receptor-mediated mechanisms to uptake specific particles or complexes, a logical extension is that gene expression patterns in recipient cells should be altered. Exosomes can mediate functional effects in recipient cells, but whether RNA is essential and the extent to which gene expression can be altered remain to be precisely determined. In models of colorectal cancer, exosomes released from KRAS mutant cell lines can increase the invasiveness of recipient cells, but this could be due to transfer of mutant KRAS via EGFR ligand signalling or other protein-mediated mechanisms (Citation37, Citation43). Among other examples, exosomal transfer from stromal to breast cancer cells can alter radiation sensitivity (Citation44), exosomes released from cardiosphere-derived cells can promote proliferation of cardiomyocytes (Citation45), glioblastoma microvesicle transfer can promote tumour growth (Citation15), EVs can transfer between endothelial cells and smooth muscle cells for atheroprotective communication (Citation46), exosomal transfer of miR-146a from endothelial cells to cardiomyocytes can alter metabolism (Citation47), and exosomal transfer of miRNAs and miRNA processing machinery can occur between tumorigenic and non-tumorigenic cells (Citation48). In all these cases, exRNA transfer may play a significant role, but the exact extent to which the effects are RNA mediated remains to be determined. For RNA transfer, exosomes can indeed transfer miRNAs but the experiments to demonstrate such transfer often involve culturing recipient cells with largely non-physiological concentrations of exosomes (Citation15, Citation27) (Citation36, Citation49) (Citation50). To attempt to recapitulate more physiological conditions, co-culture experiments, Transwell experiments, systemic injection of exosomes, implantation of Matrigel plugs containing exosomes, and xenograft experiments have all shown that exosomal contents can be transferred to some extent to recipient cells (Citation23, Citation44) (Citation45, Citation49–Citation51).
While many of the above experiments show that exRNA can be delivered, it remains unclear how these RNAs are released for functional activity upon entry into recipient cells. As discussed above, miRNA function is particularly challenging since stoichiometric delivery would seem to be required to effect significant changes in gene expression (Citation31). One possibility is that delivery of exRNAs might induce changes reminiscent of immune activation. miRNAs are ligands of Toll-like receptors and as such could conceivably mediate their effects by activating downstream signalling cascades (Citation52, Citation53). Antiviral immunity is often conferred by recognition of RNAs bearing 5′ disphosphates by Rig-1 (Citation54). Interestingly, transfer of RNA from stromal to breast cancer cells within exosomes stimulates RIG-1 to activate STAT1 signalling (Citation44).
In summary, with the availability of increasingly sensitive tools, there is little doubt that exRNAs exist. However, mechanistic understanding of how RNAs are released from cells and how or whether they can be specifically targeted to recipient cells to alter gene expression patterns remains mostly unknown. To tackle these questions, 5 groups within the exRNA Communication Consortium are studying the biogenesis of exRNA.
exRNA released by glioblastoma alters brain microenvironment (Dr. Xandra Breakefield, Principal Investigator, Harvard Medical School, Massachusetts General Hospital)
The unifying theme of the Breakefield group focuses on biogenesis and release of exRNA by glioblastoma (GBM) and modes of uptake and functional consequences in normal cells in the brain microenvironment. Our overriding hypothesis is that GBM release exRNAs that are taken up by normal cells in their environs and change their gene expression, which, in turn, determines the malignant potential of the tumour and host response. GBMs represent one of the most common and aggressive brain tumours in humans, with time from diagnosis to death being about 1 year and no effective treatments available. Since GBMs are rarely metastatic, they provide a system to study the effects of exRNA in a confined environment with relatively few principal normal cell types in the immediate vicinity. Key questions:
Elucidate basic molecular and cellular mechanisms of exRNA biogenesis in GBM cells, exRNA uptake and function in normal brain cells, using existing and emerging technologies such as RNA interference and gene editing to manipulate these processes.
Characterize the exRNA content and intracellular RNA content of human GBM cells, as well as the intracellular RNA content of normal brain cells, and evaluate functional transfer of exRNAs from GBM cells to brain cells in culture and in GBM mouse brain models.
Evaluate transfer and fate of exRNA in brain cells, including visualizing RNA transfer in EVs, monitoring mRNA translation and miRNA functions, determining possible genomic integration of transposable elements and oncogenes, and evaluating effects of non-coding exRNAs on status of genome methylation.
Study dependence of exRNA cargo composition, formation, and release dynamics as a function of GBM genotype, including activation of EGFR and PDGFRa signalling pathways, which are the 2 most common genetic events in human GBM tumours, as well as changes in GBM exRNA in response to radiation and drug treatment.
Develop and engineer regulators of exRNA release and uptake and tailor fluorescent and other visual labels, vectors, mouse models and reagents for broad applications in monitoring exRNA release, uptake and function in culture and in vivo.
In vivo regulated release and function of extracellular small RNAs (Dr. Robert Blelloch, Principal Investigator, University of California, San Francisco)
The Blelloch group focuses on how different stimuli influence the differential release of exRNAs. They address this question in several different contexts including prostate and liver cancer, immune cell activation, and olfactory sensory input. All these studies use systems that enable fine temporal control of the stimuli, enabling dissection of the sequence of events that occur from the time of the stimulus to the exRNA release. Furthermore, they hypothesize that exRNA release may not only function by altering neighbouring cells, but also by rapidly changing the intracellular content of exRNAs in the source cell. On-going studies are focused on 2 areas:
Strong evidence exists that exRNAs and in particular, extracellular miRNAs, can act as biomarkers of cancer. However, little work has been done to find a direct link between molecular changes, genetic or epigenetic, in cancer cells and the release of miRNAs. Members of our group have done extensive analysis of intracellular changes in the miRNA population following different oncogenic stimuli in the context of liver cells (Citation55). We have also made the surprising discovery that serum exRNAs associated with prostate cancer can change during progression independent of changes in the intracellular pool, suggesting regulative secretion or alternative sources of the miRNAs (Citation56). Our group is using prostate cancer and liver cancer models to build on these findings and to understand how specific signalling pathways are altering both intracellular and exRNA populations.
Signal-induced extracellular miRNA release occurs in healthy cells of the immune system. Many of the signalling networks involved in oncogenesis also mediate immune cell “activation,” inducing growth, proliferation, and differentiation into effectors of immunity. Activated dendritic cells, macrophages, and T cells are all potent sources of exosomes (Citation36, Citation57–Citation59). Indeed, the immunological properties of EVs, particularly exosomes, have long been studied (Citation60). The discovery of their RNA cargo may shed new light on these properties. In addition, T cells and dendritic cells form stable “immune synapses” that direct cell surface molecules, secreted cytokines, and other mediators of intercellular communication towards one another. These close interactions may facilitate sufficient exchange of ex-miRNAs to affect gene expression in recipient cells (Citation23). Ex-miRNAs have also been implicated in communication among different subsets of T cells (Citation61, Citation62), or dendritic cells (Citation36), and between macrophages and cancer cells (Citation57). Even if ex-miRNAs do not meaningfully alter the fate or function of recipient cells in a physiological context, their release very likely affects the source cells that jettison their miRNAs. For example, T-cell activation induces rapid remodelling of the cellular miRNA repertoire through a combination of miRISC degradation and induced ex-miRNA release (Citation58, Citation63). Cellular miRNA “disposal” by extracellular release has also been linked to gain of metastatic behaviour in bladder cancer (Citation64). Thus, common principles and mechanisms may govern ex-miRNA release and function in immune cells and cancer cells.
Neurons, like immune cells, may dynamically redefine their cellular function by jettisoning repressive RNA in response to stimulation. Expelling repressive RNA from locally stimulated synapses – as shown in Goldie et al. (Citation65) – could serve a dual purpose: to both increase local translation and promote the synapse specific strengthening required for long-term memory formation (Citation66) as well as to communicate with neighbouring cells. Evidence that neurons secrete exosome-like vesicles comes from extensive purification and electron microscopic analysis of supernatants from cultured neural stem cells, cultured cortical neurons, and even cultured male C. elegans (Citation56, Citation67) (Citation68). Though the contents of the male specific vesicles promote mating behaviour, what the content of most neuronal exosomes does is still an active area of research. What we do know is that the protein and RNA contents change with neural excitation, differentiation, and the state of the circuit (online resources Exocarta and Vesiclepedia (Citation69)). As cancer cells may utilize miRNAs to create a hospitable tumour environment, neurons may use exRNA to enlist the function of glial support cells to provide an environment that is compatible with neuronal activity. Morel et al. (Citation70) showed that vesicles containing miR-124a and secreted from stimulated neurons are taken up by astrocytes. Within astrocytes, miR-124a indirectly upregulates the glutamate transporter GLT1 thereby limiting glutamate-induced excitotoxicity of neurons by sopping up excess glutamate. The evidence that environmental stimulation increases the expression of regulatory RNAs in C. elegans sensory neurons (Citation71) coupled with the potential for spread of the RNA signal throughout the organism (Citation72) may allow exRNAs to communicate from one cell to the next or even from one animal to the next (Citation73).
Genetic models for exRNA communication (Dr. Michael McManus, Principal Investigator, University of California, San Francisco)
The McManus group seeks to develop a panel of novel genetic and cellular models to clarify and rationalize the mechanisms of exRNA biogenesis, distribution, uptake, and function in vivo.
Development of a highly robust “pOSI-sensor” technology to report on exRNA uptake and activity in cells. In contrast to negative sensors, such sensors allow for the detection of exRNA transport in single cells. This feature is important as it is expected that within tissues, exRNA mode of action will be tightly regulated, notably by cell differentiation, external stimuli, and/or by the expression of specific licensing factors. By combining the use of miRNA pOSI sensors with corresponding miRNA knockout mice, these experiments will be able to exclude the contribution of endogenous miRNA in recipient cells.
Detect local or systemic functional exRNA transfer events in diverse normal or pathological situations such as those occurring in tumour–host communication, secreting epithelia and muscle development, and digestion of breast milk or dietary plant-derived RNAs.
Systematically investigate cell-to-cell miRNA transfer in a wide array of tissues using a mouse chimera approach.
Determine the role of mammalian homologs of C. elegans sid-1 RNA transporter (SidT1 and SidT2). RNA transport defects in SidT1/2 mice will be used to identify exRNAs that require these proteins for their accumulation (or depletion) in cell culture medium and body fluids, and will notably adapt RNA-tagging and pOSI-sensor strategies to efficiently detect their functional transfer.
Investigate the mechanisms of Argonaute release in the extracellular space. Association of Argonaute proteins with the endomembrane system is clearly important for the activity and recycling of small RNAs in cells but its impact on the extracellular release of Argonaute remains unclear. We have developed a unique cellular system allowing us to manipulate the association of Argonautes to a diverse array of endomembranes, which will allow us to identify key cellular compartments supporting extracellular Argonaute accumulation and also identification of cellular co-factors involved in this process. Lastly, we are investigating the behaviour and possible uptake of extracellular Argonautes in vivo using a combination of transgenic and knockout animals.
Definition of serum ribonucleoprotein composition and its regulation and function (Dr. Thomas Tuschl, Principal Investigator, Rockefeller University)
exRNA from pathogens plays a key role as an activator of innate immunity in mammals. However, the presence of host exRNA in serum and other body fluids challenges current models of RNA-based immune recognition. To understand its basis of specificity, it is important to catalogue host exRNAs and their associated proteins in serum, investigate mechanisms leading to circulating ribonucleoprotein (RNP) homeostasis, and identify protein factors contributing to cellular RNA release. Genetic alterations in RNA targets or interacting proteins may contribute to imbalances in normal versus stress-triggered release of RNPs and push adaptive long-term pathogen-directed immunity towards autoimmunity. exRNAs may also play a broader role in extracellular signalling similar to peptide hormones. Specific aims are as follows:
Catalogue and quantify all classes of exRNAs in human serum from normal subjects using RNAseq approaches and examine normal variability of circulating RNA profiles within an individual and between individuals, considering the influence of gender, age, race, and disease.
Determine exRNA composition in patients suffering from systemic lupus erythematosus (SLE), for whom antibodies against different classes of RBPs is a hallmark.
Develop a molecular and mechanistic understanding of stress granule formation and RNA/RNP-mediated innate immune responses. Identify the RNA targets and RNP structures of autoantigens in tRNA stress responses, their turnover, and their immune receptors.
Secreted RNA during colorectal cancer progression: biogenesis, function, and clinical markers (Dr. Robert Coffey, Principal Investigator, Vanderbilt University)
Specific export of exRNAs is likely to be regulated by cellular signalling. However, few studies have addressed the regulatory mechanisms that govern packaging of exRNAs into various carriers and their secretion from cells. Following up on findings that KRAS mutations in colorectal carcinoma affect the RNA and RNA-binding protein content of exosomes, the Coffey group is focusing on regulation of exRNA secretion by oncogenic signalling in colon cancer. The hypothesis is that oncogenic signalling is a key driver of exosomal RNA and protein cargo selection. Based on this hypothesis, the major goals of this project are as follows:
Determine how the profile of exosome-associated circulating RNAs changes as a function of tumour genotype and colorectal cancer progression.
Determine intracellular proteins that mediate RNA trafficking into exosomes in vivo.
Identify modes of exRNA-associated exosome intravasation in vivo.
Determine the export specificity and functional activity of exosomal miRNAs.
Test the hypothesis that late endosomes are scaffolding and export sites for secreted RNAs.
Test the hypothesis that generation of differential subpopulations of exosomes controls RNA export.
Determine the extent to which lipoprotein particles mediates exRNA communication.
Conflict of interest and funding
This work was supported by grants from the National Institutes of Health Common Fund, through the Office of Strategic Coordination and the Office of the NIH Director as part of the Extracellular RNA Communication Consortium 1U19CA179512-01 (Blelloch), 1U19CA179563-01 (Breakefield), 1U19CA179514-01 (Coffey), 1U19CA179513-01 (McManus), and 1U19CA179564-01 (Tuschl).
Notes
This paper is part of the Special Issue: Extracellular RNA Communication Consortium. More papers from this issue can be found at http://www.journalofextracellularvesicles.net
References
- Gesteland RF, Cech TR, Atkins JF. The RNA world. 2006; Huntington, NY: Cold Spring Harbor Laboratory Press.
- Clark MB, Amaral PP, Schlesinger FJ, Dinger ME, Taft RJ, Rinn JL et al. The reality of pervasive transcription. PLoS Biol. 2011; 9: e1000625.
- Jensen TH, Jacquier A, Libri D. Dealing with pervasive transcription. Mol Cell. 2013; 52: 473–84.
- Hangauer MJ, Vaughn IW, McManus MT. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet. 2013; 9: e1003569.
- Siomi MC, Sato K, Pezic D, Aravin AA. PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol. 2011; 12: 246–58.
- Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol. 2013; 14: 475–88.
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004; 5: 522–31.
- Li Y, Lu J, Han Y, Fan X, Ding SW. RNA interference functions as an antiviral immunity mechanism in mammals. Science. 2013; 342: 231–4.
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Ann Rev Biochem. 2012; 81: 145–66.
- Dinger ME, Mercer TR, Mattick JS. RNAs as extracellular signalling molecules. J Mol Endocrinol. 2008; 40: 151–9.
- Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013; 2: 20677.
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014; 30: 255–89.
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007; 9: 654–9.
- Nolte-‘t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH, t Hoen PA. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012; 40: 9272–85.
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008; 10: 1470–6.
- Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009; 11: 1143–9.
- Lee YS, Pressman S, Andress AP, Kim K, White JL, Cassidy JJ et al. Silencing by small RNAs is linked to endosomal trafficking. Nat Cell Biol. 2009; 11: 1150–6.
- Simons M, Raposo G. Exosomes – vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009; 21: 575–81.
- Marsh M, van Meer G. Cell biology. No ESCRTs for exosomes. Science. 2008; 319: 1191–2.
- Schmidt O, Teis D. The ESCRT machinery. Curr Biol. 2012; 22: R116–20.
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008; 319: 1244–7.
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010; 285: 17442–52.
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011; 2: 282.
- Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S. On-going activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013; 4: 2712.
- Batagov AO, Kurochkin IV. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3’-untranslated regions. Biol Direct. 2013; 8: 12.
- Hong BS, Cho JH, Kim H, Choi EJ, Rho S, Kim J et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009; 10: 556.
- Ekstrom K, Valadi H, Sjostrand M, Malmhall C, Bossios A, Eldh M et al. Characterization of mRNA and microRNA in human mast cell-derived exosomes and their transfer to other mast cells and blood CD34 progenitor cells. J Extracell Vesicles. 2012; 1: 18389.
- Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012; 40: 10937–49.
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011; 108: 5003–8.
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011; 13: 423–33.
- Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci USA. 2014; 111: 14888–93.
- Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013; 4: 2980.
- Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Ströbel T, Erkan EP et al. miR-1289 and “zipcode”-like sequence enrich mRNAs in microvesicles. Mol Ther Nucleic Acids. 2012; 1: e10.
- Batagov AO, Kuznetsov VA, Kurochkin IV. Identification of nucleotide patterns enriched in secreted RNAs as putative cis-acting elements targeting them to exosome nano-vesicles. BMC Genomics. 2011; 12(Suppl 3): S18.
- Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MA, Sadek P, Sie D et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014; 8: 1649–58.
- Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012; 119: 756–66.
- Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ et al. Amphiregulin exosomes increase cancer cell invasion. Curr Biol. 2011; 21: 779–86.
- Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Branski P et al. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006; 55: 808–18.
- Shih JD, Hunter CP. SID-1 is a dsRNA-selective dsRNA-gated channel. RNA. 2011; 17: 1057–65.
- Saleh MC, van Rij RP, Hekele A, Gillis A, Foley E, O'Farrell PH et al. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol. 2006; 8: 793–802.
- Yoo BC, Kragler F, Varkonyi-Gasic E, Haywood V, Archer-Evans S, Lee YM et al. A systemic small RNA signalling system in plants. Plant Cell. 2004; 16: 1979–2000.
- Williams Z, Ben-Dov IZ, Elias R, Mihailovic A, Brown M, Rosenwaks Z et al. Comprehensive profiling of circulating microRNA via small RNA sequencing of cDNA libraries reveals biomarker potential and limitations. Proc Natl Acad Sci USA. 2013; 110: 4255–60.
- Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013; 12: 343–55.
- Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014; 159: 499–513.
- Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014; 2: 606–19.
- Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012; 14: 249–56.
- Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013; 123: 2143–54.
- Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014; 26: 707–21.
- Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, Lyle R et al. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep. 2014; 8: 1432–46.
- Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014; 25: 501–15.
- Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013; 21: 185–91.
- Chen X, Liang H, Zhang J, Zen K, Zhang CY. microRNAs are ligands of toll-like receptors. RNA. 2013; 19: 737–9.
- Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R et al. MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012; 109: E2110–6.
- Goubau D, Schlee M, Deddouche S, Pruijssers AJ, Zillinger T, Goldeck M et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5’-diphosphates. Nature. 2014; 514: 372–5.
- Lim L, Balakrishnan A, Huskey N, Jones KD, Jodari M, Ng R et al. MicroRNA-494 within an oncogenic microRNA megacluster regulates G1/S transition in liver tumorigenesis through suppression of mutated in colorectal cancer. Hepatology. 2014; 59: 202–15.
- Wang SY, Shiboski S, Belair CD, Cooperberg MR, Simko JP, Stoppler H et al. miR-19, miR-345, miR-519c-5p serum levels predict adverse pathology in prostate cancer patients eligible for active surveillance. PLoS One. 2014; 9: e98597.
- Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol. 2013; 191: 6250–60.
- de Candia P, Torri A, Gorletta T, Fedeli M, Bulgheroni E, Cheroni C et al. Intracellular modulation, extracellular disposal and serum increase of MiR-150 mark lymphocyte activation. PLoS One. 2013; 8: e75348.
- Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013; 121: 984–95.
- Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014; 14: 195–208.
- Bryniarski K, Ptak W, Jayakumar A, Pullmann K, Caplan MJ, Chairoungdua A et al. Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J Allergy Clin Immunol. 2013; 132: 170–81.
- Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014; 41: 89–103.
- Bronevetsky Y, Villarino AV, Eisley CJ, Barbeau R, Barczak AJ, Heinz GA et al. T cell activation induces proteasomal degradation of argonaute and rapid remodelling of the microRNA repertoire. J Exp Med. 2013; 210: 417–32.
- Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson B et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014; 74: 5758–71.
- Goldie BJ, Dun MD, Lin M, Smith ND, Verrills NM, Dayas CV et al. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014; 42: 9195–208.
- Martin KC, Barad M, Kandel ER. Local protein synthesis and its role in synapse-specific plasticity. Curr Opin Neurobiol. 2000; 10: 587–92.
- Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006; 31: 642–8.
- Sharma P, Schiapparelli L, Cline HT. Exosomes function in cell–cell communication during brain circuit development. Curr Opin Neurobiol. 2013; 23: 997–1004.
- Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012; 10: e1001450.
- Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S et al. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem. 2013; 288: 7105–16.
- Juang BT, Gu C, Starnes L, Palladino F, Goga A, Kennedy S et al. Endogenous nuclear RNAi mediates behavioral adaptation to odor. Cell. 2013; 154: 1010–22.
- Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002; 295: 2456–9.
- Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, Hall DH et al. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol. 2014; 24: 519–25.
