Abstract
Background : Porphyromonas gulae are black-pigmented anaerobic bacteria isolated from the gingival sulcus of various animal hosts and are distinct from Porphyromonas gingivalis originating in humans. We previously reported the antigenic similarities of 41-kDa fimbriae between P. gulae ATCC 51700 and P. gingivalis ATCC 33277. In this study, to clarify the presence of another type of fimbriae of P. gulae, we have purified and characterized the secondary fimbrial protein from P. gulae ATCC 51700.
Methods : The secondary fimbrial protein was purified from P. gulae ATCC 51700 using an immunoaffinity column coupling with antibodies against the 41-kDa fimbrial protein. The expression of fimbriae on the cell surface of P. gulae ATCC 51700 was investigated by transmission electron microscopy. The N-terminal amino acid sequence was determined by an amino acid sequencer system.
Results : The molecular mass of this protein was approximately 53-kDa, as estimated by SDS-PAGE. The polyclonal antibodies against the 53-kDa protein did not react with the 41-kDa fimbrial protein of P. gulae ATCC 51700. Immunogold electron microscopy revealed that anti-53-kDa fimbrial serum bound to fimbria on the cell surface of P. gulae ATCC 51700. The amino acid sequence of the N-terminal 15 residues of the 53-kDa fimbrial protein showed only 1 of 15 residues identical to the 41-kDa fimbrial protein.
Conclusion : The 53-kDa fimbriae are different in molecular weight and antigenicity from the 41-kDa fimbrial protein of P. gulae ATCC 51700. These results clearly suggest that the 41-kDa and the 53-kDa fimbriae are distinct types of fimbriae expressed simultaneously by this organism.
Periodontal disease is a significant oral problem in dogs, characterized by halitosis, gingival inflammation, increased periodontal pocket depth, and alveolar bone loss, resulting in loosening and eventual loss of teeth. In humans, much progress has been made in understanding the disease etiology and interaction between the host and periodontal pathogens. Human periodontitis has been associated with subgingival plaque containing elevated levels of specific Gram-negative anaerobic bacteria, including Porphyromonas gingivalis. It possesses virulence factors that include collagenase, lipopolysaccharides, a trypsin-like protease, and fimbriae Citation1. Some reports have shown that P. gingivalis can adhere to other bacteria, erythrocytes, and epithelial cells Citation2–Citation4. Fimbriae in particular play an important role in facilitating the initial interaction between the bacteria and the host Citation5–Citation7. Moreover, P. gingivalis strains possessed two types of fimbriae on the cell surface Citation6 Citation8. We have previously reported that fimbrial protein of Porphyromonas gulae ATCC 51700 had the same size and antigenicity as 41-kDa fimbriae of P. gingivalis ATCC 33277 Citation9. There is little information available regarding periodontal disease in companion animals. A black-pigmented anaerobic bacteria (BPAB) have been isolated from the periodontal pockets of dogs, cats, and several wild animals Citation10–Citation15. In several BPAB, Porphyromonas spp. is the predominant species Citation10 Citation14. Distinct differences have been noted between human and canine Porphyromonas spp. Citation11 Citation12 Citation14 Citation15. P. gingivalis isolates from humans are catalase-negative, whereas P. gingivalis-like organism isolates from canine periodontal pockets are catalase-positive. These catalase-positive P. gingivalis-like organisms may well represent isolates of P. gulae Citation15. The most frequently isolated BPAB in dog and cat periodontal pockets are P. gulae, P. salivosa and P. denticanis Citation11. Each of these isolates was demonstrated to be pathogenic in a mouse model of periodontal disease. In humans, P. gingivalis is the BPAB associated with periodontal destruction Citation16–Citation22. P. gingivalis is considered to be one of the most prominent periodontopathogens, possessing several characteristics of an overt pathogenic organism. P. gingivalis adheres to salivary components Citation23, epithelial cells Citation24–Citation26, erythrocytes Citation4 Citation27, fibronectin-collagen complexes Citation28, and other bacteria Citation2. This adherence capacity is thought to be mediated by various surface proteins. The fimbriae in particular have been suggested to play an important role in facilitating the initial interaction between bacteria and host Citation29. Moreover, the fimbriae mediate bacterial cell-to-cell interaction. It has been reported that the fimbriae of P. gingivalis mediate the adherence between P. gingivalis and Streptococcus gordonii Citation30. In this study, to clarify the presence of another type of fimbriae of P. gulae, we have purified and characterized the secondary fimbrial protein from P. gulae ATCC 51700. The secondary fimbrial protein of P. gulae ATCC 51700 and the 53-kDa fimbrial protein of P. gingivalis strain 381 are immunologically cross-reactive. Moreover, the secondary fimbrial protein gene (mfa1) of P. gulae ATCC 51700 and the 53-kDa fimbrial protein gene of P. gingivalis strain 381 are highly homologous. We suggest that the secondary fimbrial protein of P. gulae could become an effective vaccine antigen to prevent the initiation and progression of periodontitis in companion animals.
Materials and methods
Strains and cultivation conditions
P. gulae ATCC 51700 and P. gingivalis strain 381 and ATCC 33277 were cultivated (5% CO2, 10% H2, and 85% N2) in an anaerobic chamber (ANX-1, HIRASAWA, Japan) at 37°C in pre-reduced brain–heart infusion (BHI) broth (Difco Laboratories, USA) supplemented with yeast extract (0.5%, Difco Laboratories), hemin (5 µg/ml, Wako, Japan), and vitamin K (10 µg/ml, Wako).
Purification of fimbriae from P. gulae ATCC 51700
P. gulae ATCC 51700 was incubated anaerobically for 18 h in BHI broth. The bacterial cell pellet was harvested by centrifugation at 8,000×g for 30 min and washed twice with 20 mM Tris-HCl buffer (pH 8.0) containing 10 mM MgCl2 and 1.5 M NaCl by repeated pipetting. The suspension was subjected to ultrasonication with a 3-mm microtip at 25-W output on the pulse setting with five cycles of 1 min in an icebox, and then the suspension was recentrifuged at 8,000×g for 30 min. After centrifugation, ammonium sulfate was added to the supernatant to 40% saturation and the precipitated proteins were collected by centrifugation and suspended in a small volume of 20 mM Tris-HCl buffer. The suspension was then dialyzed against 20 mM Tris-HCl for a day. The crude fimbrial preparation was applied to a DEAE Sepharose CL-6B anion exchange column equilibrated with 20 mM Tris-HCl (pH 8.0). The column was washed with 20 mM Tris-HCl buffer and then eluted with a linear gradient of 0 to 0.3 M NaCl at room temperature. The 41-kDa and the 53-kDa fimbrial proteins were eluted at 0.15 M NaCl. The fraction containing fimbrial protein was dialyzed against 2 mM Tris-HCl for 1 day and then applied to an immunoaffinity column chromatography (Affi-Gel Hz Immunoaffinity Kit; Bio-Rad, USA) binding the polyclonal antibodies (PAbs) against the 41-kDa fimbriae of P. gingivalis ATCC 33277. The unbound proteins were eluted at phosphate-buffered saline (PBS) containing 0.5 M NaCl and then 41-kDa fimbrial protein bound with the column was eluted at 0.2 M glycine-HCl (pH 2.5).
SDS-PAGE
Protein extracts were heated at 100°C for 5 min in loading buffer (62.5 mM Tris-HCl buffer (pH 6.8) containing 2% SDS, 10% glycerol, 2.5% 2-mercaptoethanol, and 0.1% bromophenol blue). Samples were applied to 12.5% polyacrylamide slab gels with a 4% stacking gel and electrophoresed at 30 mA constant current for 1 h. The proteins were stained with Coomassie brilliant blue R-250. For molecular weight calibration, Precision Plus Protein Unstained Standards (Bio-Rad) were used.
Polyclonal antibodies
PAbs to the 53-kDa fimbrial protein were prepared using purified protein described above as immunogen. BALB/c mice (Nihon SLC, Japan) were injected at multiple sites subcutaneously with 50 µg of the appropriately conjugated protein in Freund's incomplete adjuvant (Difco). After 2 weeks, the mice were injected weekly for 4 weeks with the immunogen. Each mouse was bled after the last booster injection and the antibodies were tested against the corresponding antigen by Western blotting. After an adequate antibody titer was obtained, the mice were bled by cardiac puncture and the sera were prepared and stored at −20°C. PAbs against the purified 41-kDa fimbriae protein were raised in New Zealand White Rabbits (Nihon SLC, Japan). The experimental procedures for this study were reviewed and approved by the Committee of Ethics on Animal Experiments of Kanagawa Dental College.
Western blotting
For immunoblot analysis, the proteins separated by SDS-12.5% PAGE were transferred to a polyvinylidene difluoride membrane (PVDF membrane, Immobilon; Nihon Millipore Kogyo, Japan) at 200 mA for 1 h. The membranes were then treated with TBS (20 mM Tris-HCl, pH 7.4, 0.5 M NaCl) containing 1% bovine serum albumin (BSA) to block unoccupied protein-binding sites. They were then incubated with the PAbs specific for the 53-kDa fimbrial protein of P. gulae ATCC 51700 at 37°C for 1 h, washed in TBS-Tween, incubated for 1 h with goat anti-mouse immunoglobulin G conjugated with horseradish peroxidase, and then immersed in a 4-chloro-1-naphthol (Tokyo Chemical Industry, Japan) solution to develop the color. The reaction was stopped by immersing the membranes in distilled water and the membranes were then dried.
Immunoelectron microscopy
Bacterial cells from 18-h anaerobic culture were harvested by centrifugation at 10,000×g for 1 min and resuspended in PBS (pH 7.4). Copper grids (150 mesh) were covered with a thin film of collodion, which was then coated with carbon. The supported films were made hydrophilic by ion bombardment before use. A drop of cell suspension or purified protein was applied to the specimen grid. For Immunogold labeling, a cell suspension of P. gulae ATCC 51700 was transferred to a collodion-coated film nickel grid. The cells were incubated with 5 µl mouse PAbs against the 53-kDa fimbriae and 5 µl rabbit PAbs to the 41-kDa fimbrial of P. gulae ATCC 51700 (diluted 1:5,000 in PBS containing 1% BSA) at 37°C for 1 h. After five washes with PBS, the cells were incubated with EM Goat anti-mouse IgG: 5-nm gold (BBInternational, UK) and EM Goat anti-rabbit IgG: 10-nm gold (BBInternational, UK) at 37°C for 30 min. Cells were stained with 2% uranyl acetate for 1 min after five washes with PBS. The specimens were examined and photographed with a JEM-200CX electron microscope (Nippon Denshi Co., Japan) operated at 80 kV.
N-terminal amino acid sequences
Purified fimbrial protein was electrophoresed on a 12.5% SDS-polyacrylamide gel and then transferred onto a PVDF membrane operated at 200 mA for 1 h. After the membrane was stained with Coomassie brilliant blue R-250, the purified fimbrial protein band was excised and analyzed using a PPSQ-21 amino acid sequencer system (Shimadzu, Japan).
Nucleotide sequence of a mfa1 gene
P. gulae ATCC 51700 chromosomal DNA was used as the template for amplification of the mfa1 gene. Two pairs of PCR primers, PG1F, PG1R and PG2F, PG2R, were designed based on the mfa1 gene of P. gingivalis strain 381 for amplification of the mfa1 gene with the open reading frame (ORF) and promoter region. PG1F (5'TCCGGATTCTTTTGTTATTTAGTG3’) and PG1R (5'ATAAGGCACAGTGGGGACAT3’) were used to identify the 5’ region of mfa1 gene. PG2F (5'GGTAGCCCAGTACGAAAAGAA3’) and PG2R (5'GAGATACTCCCGAAAAGACAATC3’) were used to identify the 3’ region of mfa1 gene. PCR amplification was performed in a total volume of 50 µl using AccuPrime Pfx DNA polymerase (Invitrogen, USA). The amplification reaction was performed in an iCycler (Bio-Rad) with the following cycling parameters, both PG1 and PG2: initial denaturation at 94°C for 2 min; 35 cycles consisting of 94°C for 1 min, 58°C for 1 min, 72°C for 1 min; and then a final elongation step at 72°C for 7 min. Amplicons were detected by electrophoresis of 10 µl PCR product on a 0.7% agarose gel. Band sizes were confirmed with reference to molecular size markers (Smart Ladder 0.2–10 kbp; NIPPON GENE, Japan). The nucleotide sequence of the gene was determined using a dye-terminator reaction with a model 310 Genetic Analyzer (PE Applied Biosystems, USA).
Data analysis of nucleotide sequence and amino acid sequence
Data analyses of nucleotide sequences and deduced amino acid sequences were performed with GENETYX-MAC/DB (Software Development, Japan). Multiple alignment analysis was performed with CLUSTAL W in the DNA Data Bank of Japan (DDBJ; Japan). Sequence data of the mfa1 genes of P. gingivalis strain 381 and ATCC33277 were obtained from DDBJ under accession nos. AB524739 and AB016284, respectively.
Results
Purification of fimbrial protein and immunologic reactions
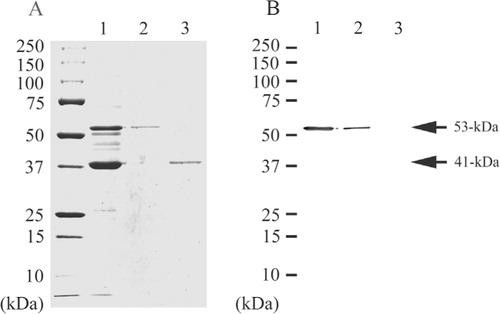
The secondary fimbrial protein of P. gulae ATCC 51700 was purified from the fraction using an immunoaffinity column, from which it was eluted in PBS containing 0.5M NaCl. The protein showed a single band of molecular mass 53-kDa in SDS-PAGE. The 41-kDa fimbrial protein was eluted at 0.2M glycine-HCl (pH 2.5) using the same column (A, lanes 1, 2, and 3). On Western blotting analysis, the PAbs against the secondary fimbrial protein from P. gulae ATCC 51700 reacted with the crude fimbrial preparation and purified the secondary fimbrial protein but did not react with purified 41-kDa fimbrial protein (B, lanes 1, 2, and 3). Thus, these two types of fimbrial proteins did not show cross-reactivity.
Fig. 1. SDS-PAGE and Western blotting of the purified proteins from P. gulae ATCC 51700. Proteins were electrophoresed on a 12.5% SDS-polyacrylamide gel and stained with Coomassie brilliant blue R-250 (A). Western blotting analysis was performed with the PAbs against the 53-kDa fimbrial protein (B). Lane 1, crude fimbrial preparation from P. gulae ATCC 51700; Lane 2, Purified secondary fimbrial protein from P. gulae ATCC 51700; Lane 3, Purified 41-kDa fimbrial protein from P. gulae ATCC 51700.
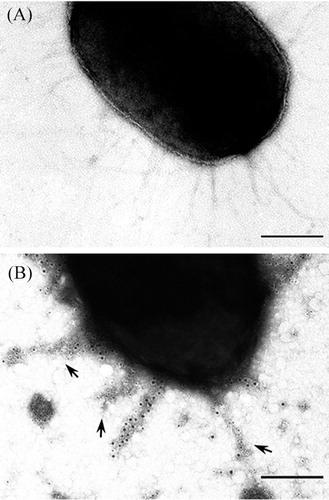
Transmission electron micrograph of immunogold labeling
The expression of fimbriae on the cell surface of P. gulae ATCC 51700 was investigated by transmission electron microscopy (A). In immunogold labeling, the fimbriae of P. gulae ATCC 51700 were labeled with the PAbs against 53-kDa protein (B). An immunogold double-labeling method was used to visualize the binding of two fimbriae-specific PAbs to P. gulae ATCC 51700 cells. The mouse PAbs against the 53-kDa and the rabbit PAbs against the 41-kDa bound to fimbriae on the cell surface, respectively. The 5-nm collodion gold-labeled goat anti-mouse serum bound to the 53-kDa fimbriae. The 10-nm collodion gold bound to the 41-kDa fimbriae. Fimbriae of P. gulae were specifically labeled with two kinds of gold particles. These results suggested that P. gulae ATCC 51700 possesses 53-kDa fimbriae antigenicity distinct from 41-kDa fimbriae. The 53-kDa fimbriae are produced along with 41-kDa fimbriae on the P. gulae ATCC 51700.
Fig. 2. Transmission electron micrographs of double immunogold labeling. P. gulae ATCC 51700 possessed fimbriae on its cell surface (A). The 5-nm collodion gold-labeled goat anti-mouse serum bound to the 53-kDa fimbriae (arrows). The 10-nm collodion gold bound to the 41-kDa fimbriae. Fimbriae of P. gulae were specifically labeled with gold particles (B). Bars, 0.2 µm.
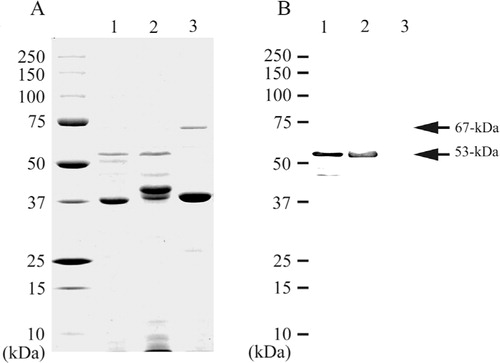
Immunological reactivities between P. gulae and P. gingivalis
Crude fimbrial proteins of P. gulae ATCC 51700 and P. gingivalis strains 381 and ATCC 33277 were analyzed by SDS-PAGE (A, lanes 1, 2, and 3). On Western blotting analysis, the PAbs against the 53-kDa fimbrial protein of P. gulae ATCC 51700 reacted with the 53-kDa band of crude fimbrial protein from P. gingivalis strain 381 but did not react with crude fimbrial protein from P. gingivalis ATCC 33277 (B, lanes 1, 2, and 3).
Fig. 3. SDS-PAGE analysis and Western blotting of crude proteins. Proteins were electrophoresed on a 12.5% SDS-polyacrylamide gel and stained with Coomassie brilliant blue R-250 (A). Western blotting analysis was performed with the PAbs against the secondary fimbrial protein from P. gulae ATCC 51700 (B). Lane: 1, crude fimbrial preparation from P. gulae ATCC 51700; Lane 2, crude fimbrial preparation from P. gingivalis strain 381; Lane 3, crude fimbrial preparation from P. gingivalis ATCC 33277.
Comparison of mfa1 gene
The mfa1 gene was amplified using two pairs of PCR primers, PG1 and PG2, from P. gulae ATCC 51700. The sequence of P. gulae ATCC 51700 mfa1 gene is available from DDBJ (accession no. AB510743). Multiple alignment analysis showed that this gene fragment shares considerably high homology with the 53-kDa fimbrial protein of P. gingivalis strain 381 (89%). The deduced amino acid sequence encoding the 53-kDa fimbrial protein of P. gulae ATCC 51700 showed 92% similarity with the 53-kDa fimbrial protein of P. gingivalis strain 381. However, the deduced amino acid sequence encoding the 67-kDa fimbrial protein of P. gingivalis ATCC 33277 showed 34% similarity with the 53-kDa fimbrial protein of P. gulae ATCC 51700 (). The amino acid sequence of the N-terminal 15 residues of the 53-kDa fimbrial protein of P. gulae ATCC 51700 (AGDNDYNHVGEYGGI) showed 12 of 15 residues identical to the 53-kDa fimbrial protein of P. gingivalis strain 381 (AGDNDYNPIGEYGGV) and 4 of 15 residues identical to the 67-kDa fimbrial protein of P. gingivalis ATCC 33277 (AGDGQDQANPDYHYV) but only 1 of 15 residues identical to the 41-kDa fimbrial protein of P. gulae ATCC 51700 (AFGVADDEAKVAKLT).
Fig. 4. Comparison of predicted amino acid sequences for Mfa1encoded by the mfa1 genes of P. gulae and P. gingivalis strains. Amino acid identities are shown by asterisks. Hyphens are used to indicate the positions of gaps in the multiple alignment. The alignment of the deduced amino acid sequences was performed with the CLUSTALW program of the DNA Data Bank of Japan. The nucleotide sequences had been deposited with DDBJ/EMBL/GenBank under the accession numbers AB510743 for P. gulae ATCC 51700, AB524739 for P. gingivalis 381 and AB016284 for P. gingivalis ATCC33277. The arrow indicates the cleavage site.

Discussion
We previously reported the existence of 41-kDa fimbriae of P. gulae ATCC 51700 and molecular and antigenic similarities of 41-kDa fimbrial protein between P. gulae ATCC 51700 and P. gingivalis ATCC 33277 Citation9. In this study, we have succeeded in purifying and characterizing secondary fimbrial protein of P. gulae ATCC 51700. The purified secondary fimbrial protein was observed as a single band of 53-kDa by SDS-PAGE analysis, and had antigenicity distinct from that of 41-kDa fimbrial protein (). The N-terminal amino acid sequence of 53-kDa and 41-kDa fimbrial proteins were identical at only 1 of 15 positions. In immunogold labeling, the PAbs against 53-kDa protein bound to fimbrial structures on the surface of P. gulae ATCC 51700 (). The 5-nm collodion gold-labeled goat anti-mouse serum bound to the 53-kDa fimbriae. The 10-nm collodion gold bound to the 41-kDa fimbriae. Fimbriae of P. gulae were specifically labeled with two kinds of gold particles. The immunoelectron-microscopic findings suggested that the PAbs react with unique determinants on major and minor fimbrial proteins. Hamada et al. Citation9 reported that the fimbriae of P. gulae ATCC 51700 was labeled with PAbs against 41-kDa fimbrial protein. Some fimbriae on the cell surface were not labeled with the antibody. Thus, the 53-kDa fimbrial protein was antigenicity distinct from 41-kDa fimbrial protein, demonstrating that two distinctly different fimbriae are expressed by the same P. gulae ATCC 51700. The secondary fimbrial protein purified from P. gingivalis ATCC 33277 markedly induced IL-1α, IL-β, IL-6, and TNF-α cytokine expression in mouse peritoneal macrophages Citation31. It has been reported that the 53-kDa protein of P. gingivalis strain 381 reacted strongly with the serum of patients with periodontal disease Citation32 Citation33. On Western blotting analysis, the 53-kDa fimbrial protein of P. gulae ATCC 51700 and that of P. gingivalis strain 381 showed immunological cross-reactivity (). Moreover, the amino acid sequence of the 15 N-terminal residues of the 53-kDa fimbrial protein of P. gulae ATCC 51700 had 12 of 15 residues identical to those of P. gingivalis strain 381 (). It is possible that P. gulae exists frequently in companion animals and the secondary fimbrial protein of P. gulae plays a role as a strong antigen. These results suggest that the 53-kDa fimbrial protein of P. gulae ATCC 51700 may play an important role in periodontal pathogenicity and the host immune response. It was reported that P. gulae was pathogenic in a mouse model of periodontal disease. Whole-cell bacterin preparation of P. gulae displayed significantly reduced alveolar bone loss Citation34. The 53-kDa protein isolated from P. gingivalis 381 Citation8, which has been demonstrated to be the same molecule as a 53-kDa major outer membrane protein Citation35, has reported as a minor fimbriae and a major immunodominant protein likely to contribute to host–bacteria interaction Citation36. Those two types of minor fimbrial proteins showed no immunological cross-reactivity. The roles of these distinct molecules and the difference in antigenicity in relation to bacterial virulence are yet unclear. The role of minor fimbriae in virulence is less understood. However, the minor fimbriae are necessary for the development of synergistic biofilms between P. gingivalis and S. gordonii via a specific interaction with the streptococcal SspB protein Citation37. Moreover, Lin et al. reported that the minor fimbriae are involved in P. gingivalis autoaggregation and colonization. A mutant with a deficiency in minor fimbriae can bind to a saliva-coated surface but does not form microcolonies as the wild-type strain does. The major fimbriae are required for initial attachment and organization of biofilms. The minor fimbriae promoted bacterial autoaggregation, whereas major fimbriae suppressed it Citation38 Citation39. Our further studies will be directed toward elucidating the biochemical and immunobiological functions of the secondary fimbriae.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
References
- Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998; 62: 1244–63.
- Goulbourne PA, Ellen RP. Evidence that Porphyromonas (Bacteroides) gingivalis fimbriae function in adhesion to Actinomyces viscosus. J Bacteriol. 1991; 173: 5266–74.
- Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995; 63: 3878–85.
- Okuda K, Yamamoto A, Naito Y, Takazoe I, Slots J, Genco RJ. Purification and properties of hemagglutinin from culture supernatant of Bacteroides gingivalis. Infect Immun. 1986; 54: 659–65.
- Amano A, Nakagawa I, Okahashi N, Hamada N. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J Periodontal Res. 2004; 39: 136–42. 10.3402/jom.v4i0.19076.
- Hamada N, Sojar HT, Cho MI, Genco RJ. Isolation and characterization of a minor fimbria from Porphyromonas gingivalis. Infect Immun. 1996; 64: 4788–94.
- Yoshimura F, Takahashi K, Nodasaka Y, Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984; 160: 949–57.
- Arai M, Hamada N, Umemoto T. Purification and characterization of a novel secondary fimbrial protein from Porphyromonas gingivalis strain 381. FEMS Microbiol Lett. 2000; 193: 75–81. 10.3402/jom.v4i0.19076.
- Hamada N, Takahashi Y, Watanabe K, Kumada H, Oishi Y, Umemoto T. Molecular and antigenic similarities of the fimbrial major components between Porphyromonas gulae and P. gingivalis. Vet Microbiol. 2008; 128: 108–17. 10.3402/jom.v4i0.19076.
- Allaker RP, de Rosayro R, Young KA, Hardie JM. Prevalence of Porphyromonas and Prevotella species in the dental plaque of dogs. Vet Rec. 1997; 140: 147–8. 10.3402/jom.v4i0.19076.
- Hardham J, Dreier K, Wong J, Sfintescu C, Evans RT. Pigmented-anaerobic bacteria associated with canine periodontitis. Vet Microbiol. 2005; 106: 119–28. 10.3402/jom.v4i0.19076.
- Harvey CE, Thornsberry C, Miller BR. Subgingival bacteria-comparison of culture results in dogs and cats with gingivitis. J Vet Dent. 1995; 12: 147–50.
- Hennet PR, Harvey CE. Anaerobes in periodontal disease in the dog: a review. J Vet Dent. 1991; 8: 18–21.
- Isogai H, Kosako Y, Benno Y, Isogai E. Ecology of genus Porphyromonas in canine periodontal disease. Zentralbl Veterinarmed B. 1999; 46: 467–73.
- Fournier D, Mouton C, Lapierre P, Kato T, Okuda K, Menard C. Porphyromonas gulae sp. nov., an anaerobic, gram-negative coccobacillus from the gingival sulcus of various animal hosts. Int J Syst Evol Microbiol. 2001; 51: 1179–89. 10.3402/jom.v4i0.19076.
- Shah HN, Collins MD. Proposal for reclassification of Bacteroides asaccharolyticus, Bacteroides gingivalis, and Bacteroides endodontalis in a new genus, Porphyromonas. Int J Syst Bacteriol. 1988; 38: 128–31. 10.3402/jom.v4i0.19076.
- Slots J, Genco RJ. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984; 63: 412–21. 10.3402/jom.v4i0.19076.
- Slots J, Listgarten MA. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988; 15: 85–93. 10.3402/jom.v4i0.19076.
- Socransky SS. Relationship of bacteria to the etiology of periodontal disease. J Dent Res. 1970; 49: 203–22. 10.3402/jom.v4i0.19076.
- Tanner ACR, Haffer C, Baratthall GT, Visconti RA, Socransky SS. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979; 6: 278–307. 10.3402/jom.v4i0.19076.
- White D, Mayrand D. Association of oral Bacteroides with gingivitis and adult periodontitis. J Periodont Res. 1981; 16: 259–65. 10.3402/jom.v4i0.19076.
- Zambon JJ, Reynolds HS, Slots J. Black-pigmented Bacteroides spp. in the human oral cavity. Infect Immun. 1981; 32: 198–203.
- Lee JY, Sojar HT, Bedi GS, Genco RJ. Synthetic peptides analogous to the fimbrillin sequence inhibit adherence of Porphyromonas gingivalis. Infect Immun. 1992; 60: 1662–70.
- Childs WC, Gibbons RJ. Selective modulation of bacterial attachment to oral epithelial cells by enzyme activities associated with poor oral hygiene. J Periodontal Res. 1990; 25: 172–8. 10.3402/jom.v4i0.19076.
- Okuda K, Slots J, Genco RJ. Bacteroides gingivalis, Bacteroides asaccharolyticus and Bacteroides melaninogenicus subspecies: cell surface morphology and adherence to erythrocytes and human buccal epithelial cells. Curr Microbiol. 1981; 6: 7–12. 10.3402/jom.v4i0.19076.
- Slots J, Gibbons RJ. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978; 19: 254–64.
- Inoshita E, Amano A, Hanioka T, Tamagawa H, Shizukuishi S, Tsunemitsu A. Isolation and some properties of exohemagglutinin from the culture medium of Bacteroides gingivalis 381. Infect Immun. 1986; 52: 421–7.
- Naito Y, Gibbons RJ. Attachment of Bacteroides gingivalis to collagenous substrata. J Dent Res. 1988; 67: 1075–80. 10.3402/jom.v4i0.19076.
- Isogai H, Isogai E, Yoshimura F, Suzuki T, Kagota W, Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the bacterial fimbriae. Arch Oral Biol. 1988; 33: 479–85. 10.3402/jom.v4i0.19076.
- Lamont RJ, Bevan CA, Gil S, Persson RE, Rosan B. Involvement of Porphyromonas gingivalis fimbriae in adherence to Streptococcus gordonii. Oral Microbiol Immunol. 1993; 8: 272–6. 10.3402/jom.v4i0.19076.
- Hamada N, Watanabe K, Arai M, Hiramine H, Umemoto T. Cytokine production induced by a 67-kDa fimbrial protein from Porphyromonas gingivalis. Oral Microbiol Immunol. 2002; 17: 197–200. 10.3402/jom.v4i0.19076.
- Kokeguchi S, Kato K, Kurihara H, Nishimura F, Murayama Y. Purification and characterization of two major outer membrane proteins from Porphyromonas gingivalis. Dent Jpn (Tokyo). 1990; 27: 29–34.
- Kokeguchi S, Miyamoto M, Ohyama H, Hongyo H, Takigawa M, Kurihara H, et al.. Biochemical properties of major outer membrane proteins of Porphyromonas gingivalis. Microbios. 1994; 77: 247–52.
- Hardham J, Reed M, Wong J, King K, Laurinat B, Sfintescu C, et al.. Evaluation of a monovalent companion animal periodontal disease vaccine in an experimental mouse periodontitis model. Vaccine. 2005; 23: 3148–56. 10.3402/jom.v4i0.19076.
- Hongyo H, Kurihara H, Kokeguchi S, Miyamoto M, Maeda H, Hayakawa M, et al.. Molecular cloning and characterization of the gene encoding a 53 kDa outer membrane protein of Porphyromonas gingivalis. Microbios. 1997; 92: 47–57.
- Kurihara H, Nishimura F, Nakamura T, Nakagawa M, Tanimoto I, Nomura Y, et al.. Humoral immune response to an antigen from Porphyromonas gingivalis 381 in periodontal disease. Infect Immun. 1991; 59: 2758–62.
- Park Y, Simionato MR, Sekiya K, Murakami Y, James D, Chen W, et al.. Short fimbriae of Porphyromonas gingivalis and their role in coadhesion with Streptococcus gordonii. Infect Immun. 2005; 73: 3983–9. 10.3402/jom.v4i0.19076.
- Lin X, Wu J, Xie H. Porphyromonas gingivalis minor fimbriae are required for cell-cell interactions. Infect Immun. 2006; 74: 6011–15. 10.3402/jom.v4i0.19076.
- Kuboniwa M, Amano A, Hashino E, Yamamoto Y, Inaba H, Hamada N, et al.. Distinct roles of long/short fimbriae and gingipains in homotypic biofilm development by Porphyromonas gingivalis. BMC Microbiol. 2009; 9: 105. 10.3402/jom.v4i0.19076.