Abstract
Background
The aim of this study was to assess subgingival microbiological changes in smokers versus non-smokers presenting severe chronic periodontitis after supragingival periodontal therapy (ST).
Methods
Non-smokers (n=10) and smokers (n=10) presenting at least nine teeth with probing pocket depth (PPD) (≥5 mm), bleeding on probing (BoP), and no history of periodontal treatment in the last 6 months were selected. Clinical parameters assessed were plaque index (PI), BoP, PPD, relative gingival margin position (rGMP) and relative clinical attachment level (rCAL). Subgingival biofilm was collected before and 21 days after ST. DNA was extracted and the 16S rRNA gene was amplified with the universal primer pair, 27F and 1492R. Amplified genes were cloned, sequenced, and identified by comparison with known 16S rRNA sequences. Statistical analysis was performed by Student's t and Chi-Square tests (α=5%).
Results
Clinically, ST promoted a significant reduction in PI and PPD, and gain of rCAL for both groups, with no significant intergroup difference. Microbiologically, at baseline, data analysis demonstrated that smokers harbored a higher proportion of Porphyromonas endodontalis, Bacteroidetes sp., Fusobacterium sp. and Tannerella forsythia and a lower number of cultivated phylotypes (p<0.05). Furthermore, non-smokers featured significant reductions in key phylotypes associated with periodontitis, whereas smokers presented more modest changes.
Conclusion
Within the limits of the present study, ST promoted comparable clinical improvements in smokers and non-smokers with severe chronic periodontitis. However, in smokers, ST only slightly affected the subgingival biofilm biodiversity, as compared with non-smokers.
Although smoking is a recognized risk factor for periodontitis Citation1, leading to an increase in periodontal tissue destruction as a consequence of altered production of metalloproteinases (MMP), interleukins and inflammatory markers and host-cell function Citation2 Citation3 Citation4 Citation5 Citation6 , biofilm still remains the primary etiologic factor for the development of destructive periodontal disease Citation1. Thus, the primary goal of periodontal therapy is to target the subgingival biofilm present in periodontally-diseased sites, which is associated with the progressive destruction of the supportive periodontal tissues. It is well documented that conventional therapy, i.e. subgingival scaling and root planing, is effective in the achievement of this goal. Supragingival biofilm control has been shown to play a critical role in the success of periodontal therapy due to its impact on the subgingival biofilm and its inhibition of re-colonization Citation7 Citation8 Citation9 . However, conflicting results regarding the impact of supragingival biofilm control on the composition of established subgingival biofilm in untreated periodontal sites are found in the literature Citation10 Citation11 Citation12 Citation13 Citation14 .
Smoking has been implicated as a factor that reduces the effectiveness of treatment. Smokers show less favorable responses to various kinds of periodontal treatments, such as non-surgical, surgical, regeneration procedures, and mucogingival surgery Citation15 Citation16 Citation17 . The mechanisms by which smoking affects the response to periodontal treatment might be related to the altered inflammatory and immune response that has been observed in smokers and/or to the persistence of pathogenic flora in smokers after treatment Citation2 Citation18 Citation19. Studies have, therefore, aimed to document a possible role of smoking in the oral microbiota and, although no conclusive findings have been reported, some data have demonstrated that there are important differences in the composition of subgingival biofilm between smokers and non-smokers with chronic periodontal disease; which may, in fact, account for the lower response of smokers to therapy Citation20 Citation21 Citation22 .
With the concepts discussed above in mind, there is an interest in the possible effect of supragingival biofilm control in the subgingival environment in untreated periodontitis sites in smokers. In smokers, in the only study available, supragingival periodontal therapy has been shown to affect the total bacterial load in the subgingival biofilm with a non-significant tendency towards lower amounts of recognized periodontal pathogens (Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythia) in the treated sites Citation23. However, in a recent and pioneer study in the field, Shchipkova et al. Citation24 demonstrated that not only red complex bacteria, but several other uncultivated phylotypes, including ‘unsuspicious’ species in the subgingival biofilm, may play a role in the disease etiology, highlighting the necessity of expanding the analysis of the periodontal microbiome to open-ended techniques. As such, in the present study, it was hypothesized that supragingival therapy (ST), consisting of supragingival biofilm/calculus removal and dental surface polishing, removal of biofilm retainers, oral hygiene instruction, and reinforcement after 7 days, could affect subgingival biofilm biodiversity at sites with untreated severe chronic periodontitis in smokers. Microbiological analyses were performed using the non-culture-dependent 16S rRNA cloning and molecular sequencing. In addition, smokers and non-smokers were followed clinically with respect to the impact of the ST on the tissue stability.
Materials and methods
Ethics
This study was designed as a parallel, single-arm, and controlled study to evaluate the clinical and microbiological effects of ST on the biodiversity of subgingival biofilm collected in smokers and non-smokers with chronic periodontitis. Subjects included in the present study were examined, treated by ST, and re-evaluated 21 days later. The study was approved by the Institutional Review Board (IRB) and patients received a detailed description of the proposed treatment and gave their informed and written consent.
Patient selection and groups
Potential patients were selected from those referred to the Graduate Clinic of Piracicaba Dental School, University of Campinas – UNICAMP, Brazil. All patients received a complete periodontal examination, including a full-mouth periodontal probing, radiographic examination and complete anamnesis.
The study inclusion criteria were:
diagnosis of chronic periodontitis, according to the criteria of the 1999 International Classification Citation25;
presence of at least 20 teeth;
at least nine teeth presenting probing pocket depth (PPD) ≥ 5 mm with bleeding on probing (BoP; being at least two with PPD ≥ 7 mm);
>35 years of age.
Patients who: (i) were pregnant or lactating; (ii) required antimicrobial pre-medication for the performance of periodontal examination and treatment; (iii) were suffering from any other systemic diseases (cardiovascular, pulmonary, liver, cerebral, or diabetes); (iv) had received antimicrobial treatment in the previous 3 months; (v) were taking long-term anti-inflammatory drugs; and/or (vi) had received a course of periodontal treatment within the last 6 months; were excluded from the study. A parallel, non-blinded and prospective study was designed to enroll the following two groups: non-smoker group (n=10): patients diagnosed with generalized and severe chronic periodontitis and that had never been smokers; and smoker group (n=10): patients diagnosed with generalized and severe chronic periodontitis and that had a smoking habit for at least 10 years and smoked at least 20 cigarettes per day. Sample size was determined by the Bioestat program, using a standard deviation of 1.0 and a power value of 80% to detect a difference between groups of 1.0 mm.
Supragingival therapy (ST): After a full mouth examination and consent in participation, patients of both groups received a full mouth prophylaxis, supragingival calculus, and biofilm removal, using Gracey curettes, an ultrasonic scaler, bicarbonate spray and floss, or interdental brushes, according to the interdental space. Extraction of condemned teeth and biofilm retentive factors removal were also performed. The patients were also individually instructed on how to perform oral self-care, including the Bass technique, inter-dental flossing, and tongue brushing. All the subjects of the study received a standard fluoride dentifrice, toothbrushes, and floss as necessary; and were asked to perform complete oral self-care hygiene at least twice a day. A week after this first instruction session, patients returned to a reinforcement of oral self-care instructions. Twenty-one days after ST, clinical re-evaluation was performed and subgingival samples were collected for microbiological analysis. The same individual (DP) was trained to perform ST in all the patients, whereas another individual was trained to perform baseline and post-therapy clinical assessments (TM).
Clinical parameters
The following clinical parameters were assessed immediately before therapy: full-mouth plaque index (FMPI), according to Ainamo and Bay Citation26, and full-mouth bleeding score (FMBS), according to Mühlemann and Son Citation27; these were calculated after assessing dichotomously the presence of dental biofilm at the site or BoP from the bottom of the pocket when probing with a manual probe.Footnote1 The percentage of total sites that revealed the presence of plaque or bleeding was calculated. All teeth presenting at least one site with PPD ≥ 5 mm were selected for clinical evaluation. These teeth did not present pulpal disease or furcation lesion (in order to avoid bias). From those previously selected teeth, one site (the deepest one) was selected and followed-up during the experimental period, using an individually manufactured acrylic stent in which a groove was made to standardize the measurements. This selection resulted in a mean of 10.0±1.8 sites per patients evaluated during this study. For the sites selected, clinical parameters were:
probing pocket depth (PPD – distance from the bottom of the pocket to the margin);
relative clinical attachment level (rCAL – distance from the bottom of the pocket to the stent margin);
relative gingival margin position (rGMP – distance from the gingival margin to the stent margin).
All parameters were evaluated using a periodontal probe at baseline and 21 days after ST. The examinations were performed by a calibrated examiner (TM). For this calibration, three patients were selected and full mouth rCAL and PPD were measured, twice, within 24 hours, with at least 1 hour between the examinations. The intra-class correlation was calculated for each parameter, resulting in 93.5% reproducibility for rCAL and 94.3% for PPD.
Subgingival biofilm analysis
Subgingival biofilm collection: After a full-mouth examination, all sites previously selected for clinical follow-up were included in the subgingival biofilm analysis, as described. Following the careful removal of supragingival biofilm, the areas were washed with a water spray, isolated with cotton rolls and gently dried. A sterile paper pointFootnote2 was inserted into the bottom of the periodontal pocket for 30 s. Each paper point was placed separately in sterile plastic tubes containing 0.01 M Tris EDTA solution, pH 8.0 (TE). DNA collection and extraction were performed as described by Casarin et al. Citation28. For each patient, samples from the selected sites were pooled together to allow the 16S cloning sequencing.
Cloning and sequencing: First, the 16S rRNA gene was amplified using a universal primer set (27f and 1492r) as described by de Lillo et al. Citation29. Cloning procedures were performed using a TOPO-TA cloning kit,Footnote3 following the manufacturer's instructions. Briefly, the amplicons resulting from universal amplification were cloned into Escherichia coli and then cultured overnight in Luria-Bertani (LB) plates (containing ampicilin and X-gal) for posterior colony selection. After colony selection, each colony was separately cultivated overnight, in LB broth media.Footnote4 The LB broth samples were then centrifuged to form a bacterial pellet containing E. coli that received vectors/bacterial DNA. After vector extraction, the products were purifiedFootnote5 and sequenced.Footnote6 After sequencing, a partial sequence of 600 bp was generated. These sequences were initially aligned and a similarity matrix was constructed from the alignments by the method of Jukes and Cantor Citation30 (Bioedit 7.0 Program Citation31). Phylogenetic trees were constructed by the neighbor joining method (Dotur Program Citation32). Sequences were compared using the HOMD database Citation33 applying a level of 98.5% sequence identity as cut-off. Sequences presenting 98% or greater similarity within a genus were considered as the same species.
Data management and statistical analysis
Clinical parameters were analyzed by Student's t test (for baseline intergroup comparisons) and repeated-measures ANOVA/Tukey (for clinical changes occurring after ST), using the PROC GLM procedure of the SAS program.Footnote7 For microbiological data, a variance-stabilizing transformation, described by Shchipkova et al. Citation24, was used, promoting a normal distribution of the data. After data transformation, Mann–Whitney and Wilcoxon tests (for inter and intragroup analysis, respectively) were used for data analysis. For distribution and frequency analysis, the Chi-Square test was employed. A 5% level of significance was considered for clinical and microbiological data analysis.
Results
Demographic and clinical data
Demographic characteristics and baseline comparisons between groups are displayed in . There were no statistical differences between groups regarding age or gender, as well as in relation to full mouth clinical parameters: plaque, BoP, periodontal probing depth, and clinical attachment level (p>0.05). illustrates the effect of ST on the clinical parameters assessed in the selected sites (PPD ≥ 5 mm). At baseline, intergroup analysis (i.e. non-smokers vs. smokers) showed no significant differences for any of the parameters assessed (p>0.05). The same results were seen after ST, with no differences between the groups (p>0.05). In contrast, intragroup analysis (i.e. baseline vs.after ST, within the same experimental group) demonstrated that ST led to a significant reduction in PI, PPD, and rCAL (p<0.05); whereas no significant changes were found with respect to BoP and GMP, in smokers and non-smokers (p>0.05).
Table 1. Clinical and demographic characteristics of participants at baseline (mean±standard deviation)
Table 2. Clinical parameters in smokers and non-smokers, at baseline and after supragingival therapy (ST), at the selected sites
Microbiological data
In total, 1,800 clones were identified, representing 78 different species in non-smoker subjects at baseline and 73 after ST, whereas in smokers 71 species were identified at baseline and 70 were observed after ST. depicts the distribution of clones in the phylum and cultivation status (cultivated and not-yet-cultivated). There was no difference in phylum distribution between smokers and non-smokers, neither at baseline nor after ST. In regards to cultivation status, non-smoker subjects presented a higher prevalence of cultivated indexes compared with smokers at baseline, and at after ST (p<0.05). In addition, 21 days after ST, an increase in cultivated phylotypes was seen only in non-smokers (p<0.05).
Table 3. Distribution by phylum (total number of clones) and culture condition (% of cultivated and not-yet-cultivated phylotypes) in smokers and non-smokers, at baseline and after supragingival therapy (ST)
illustrates the distribution of 16S clonal analysis by genera in non-smokers and smokers, before and after ST. As noted, at baseline, smokers demonstrated significantly greater levels of the genera Fusobacterium and Bacteroides, whereas non-smokers presented higher levels of Eubacterium, Synergistes, and Streptococcus (Chi-Square test, p<0.05).
Figure 1. Distribution by genus (percent total clones) in smokers and non-smokers, at baseline and after supragingival therapy (ST). *Indicates intergroup differences at baseline; †indicates intergroup differences after ST; #indicates intragroup differences after ST (Chi-Square test, p<0.05).
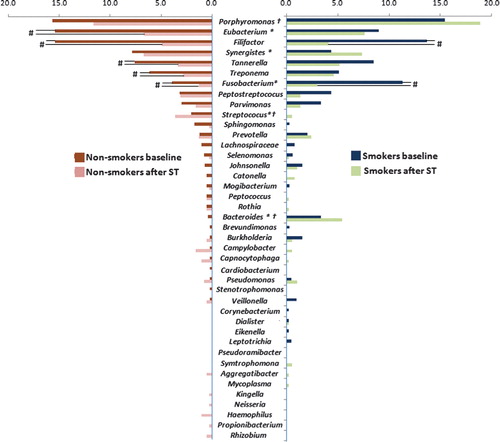
Moreover, ST altered the genera distribution in both groups. In non-smokers, a significant reduction in some genera such as Eubacterium, Filifactor, Tannerella, Treponema, and Fusobacterium was observed, whereas only Filifactor and Fusobacterium were reduced in smokers after ST (p<0.05). The difference in genera distribution was still observed after ST, where Streptococcus was more present in non-smokers (p<0.05) and Bacteroides and Porphyromonas were found more in smokers (p<0.05).
The 20 most detected phylotypes were analyzed separately with regard to their frequency and proportions in the subgingival biofilm (). Significant differences were observed in levels of certain species between current and never-smokers. Smokers presented higher levels of Fusobacterium nucleatum ss. vincentii and Bacteroidetes [G-2] sp. ∣Oral Taxon 274∣ Clone AU126 (p<0.05), whereas non-smokers showed higher levels of Streptococcus constellatus and Eubacterium [11][G-6] nodatum Oral Taxon 694. Moreover, Supragingival Therapy led to a more expressive alteration in the subgingival composition in non-smokers. Twenty-one days after ST, non-smokers presented lower levels of Filifactor alocis, T. forsythia, Eubacterium [XI][G-5] saphenum ∣ Oral Taxon 759 and Eubacterium [XI][G-3] brachy (p<0.05), whereas only T. forsythia presented a significant reduction after ST. Interestingly, a significant increase in Synergistetes [G-3] sp. Oral Clone BH017 and Porphyromonas endodontalis Oral Clone AJ002 was observed in smokers after ST (p<0.05), highlighting the dissimilarities between smokers and non-smokers with regards to their microbial profile.
Table 4. Top 20 most detected phylotype proportions (x value mean) in smokers and non-smokers, at baseline and after supragingival therapy
Discussion
The habit of smoking has been reported to negatively affect periodontal tissues, cell defense, and host response. In addition, microbiological evaluations also indicate a possible influence on subgingival microflora. However, very limited information is available with respect to the effect of ST on the biodiversity of the subgingival biofilm in smokers with chronic periodontitis. With this in mind, it was hypothesized that ST, consisting of standard methods used for supragingival biofilm removal and control, including supragingival biofilm/calculus removal and dental surface polishing, removal of biofilm retainers, oral hygiene instruction and reinforcement after 7 days, would affect subgingival biofilm profile in smokers with severe chronic periodontitis. Subgingival biofilm biodiversity was assessed by the 16S gene cloning technique and clinical parameters were also used to illustrate clinical conditions at baseline and after ST in smokers and non-smokers. Data analyses demonstrated that: (i) there were significant differences in the subgingival biofilm composition, at baseline, in smokers versus non-smokers; (ii) subgingival biodiversity was significantly affected by ST in non-smokers, whereas only a slight effect was observed for smokers; and (iii) clinical response was not affected by dissimilar microbiological outcomes to ST in smokers versus non-smokers.
With non-surgical subgingival therapy as the main treatment modality, most authors report greater reductions in probing depth in non-smokers, compared with smokers Citation19 Citation34 Citation35 Citation36 Citation37 Citation38 . It is, therefore, important to note that there is substantial evidence for the clinical improvement in smokers after treatment, indicating that smoking, as a risk factor, will compromise rather than prevent tissue healing. In non-smokers, ST results in a reduction in gingival bleeding, probing depth, and a gain in attachment level Citation7 Citation9 Citation13 Citation14 Citation39 Citation40. In smokers, however, there was a lack of information on the impact of ST on the periodontal tissues at untreated sites.
Gomes et al., in 2007 Citation40, were the only group to assess the changes in periodontal tissues after a supragingival periodontal therapy. In their study, ST consisted of supragingival calculus removal with hand curettes, extractions, endodontic treatment, placement of temporary restorations, and prostheses. Similarly to the findings of the present study, Gomes et al. Citation40 demonstrated that a comparable clinical outcome was reached for smokers and non-smokers regardless of the severity of the disease. Both groups featured a reduction in plaque and bleeding indices, as well as a reduction in probing depth, and gain in clinical attachment level after 30 days of ST, which was maintained for up to 180 days Citation40. In addition, as previously reported by others, in the present study, data analyses demonstrated that smokers presented a lower reduction in plaque index (PI) than non-smokers. Moreover, as expected, deep pockets presented only a mild change after ST: only a discrete reduction in BoP index was observed in smokers and non-smokers. In summary, available clinical data suggest that smokers and non-smokers with severe chronic periodontitis may similarly benefit from ST.
In the present study, we aimed to determine whether or not ST affected subgingival biofilm biodiversity in both groups. Often, studies have focused on assessing, in smoking and non-smoking conditions, the effect of periodontal therapy on genera associated with periodontal disease, including P. gingivalis, Treponema denticola, and T. forsythia Citation7 Citation11 Citation12 Citation41 Citation42. However, an important study demonstrated that tobacco might also affect the levels of genera not always associated with periodontal disease Citation24. In the present study, using an open-ended approach, a comparison between the subgingival biofilm composition of smokers and non-smokers at baseline (before ST), revealed a higher presence of certain genera, such as Fusobacterium (genus associated with periodontal disease) and Bacteroides; and lower levels of Streptococcus, Synergistes, and Eubacterium. These findings seem to confirm that tobacco exposure may lead to a subgingival biofilm composed not only in a higher proportion by pathogens associated with periodontal disease, but also some species, not commonly included as periodontal pathogens in target-ended or selective techniques.
However, in addition to presenting a reduction in ‘health-associated’ biofilm and an increase in ‘disease-associated’ biofilm, in the present study, smokers showed a lower response to ST, compared with non-smoker subjects as regards the microbiological composition of the subgingival biofilm. Non-smokers had a significant reduction in five species commonly associated with periodontal disease following ST, whereas in smokers only the levels of F. alocis were significantly reduced. In addition, after ST in smokers, disease-associated species, including P. endodontalis, were found to be increased.
P. endodontalis has been listed as one of the potential ‘new species’ associated with periodontal disease Citation43, and the fact that P. endodontalis has additionally been shown to be reduced in subgingival biofilm when the smoking habit is given up Citation44, suggesting this species as an important factor that might contribute to the pathogenesis of periodontal disease in smokers. The apparent reduction in some species in the subgingival biofilm after ST may be explained by the intimate relationship between both supra- and subgingival environments Citation45, and also as a result of the mild, but statistically significant, clinical benefits promoted by ST after 21 days. The probing depth reduction may have led to significant changes in nutrients, oxygen, and microorganisms disposable in periodontal pockets, altering subgingival microflora. Moreover, the reduced microbiological effect of ST on smoker-subgingival microbiota, has been a common goal in previous studies. Furthermore when subgingival scaling and root planing are performed, smokers appear to remain positive for periodontitis-associated species, including P. gingivalis, T. denticola, T. forsythia, P. intermedia, F. nucleatum, and Parvimonas micra Citation41 Citation46 Citation47. Therefore, after ST performance and the assessment of baseline and follow-up parameters, all the patients enrolled in this study were then treated by the conventional subgingival scaling and root planing approach.
In conclusion, in smokers, ST only slightly affected the subgingival biofilm biodiversity, as compared with non-smokers. However, clinically, the response in smokers and non-smokers was similar regardless of the differential impact of ST on the subgingival biofilm composition in these groups of patients.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
Notes
1PCP-15, Hu-Friedy, Chicago, IL, USA.
2#35, Tanari, Manaus, AM, Brazil.
3Invitrogen, San Diego, CA, USA.
4LB-Top Agar, Sigma-Aldrich, Buchs, Switzerland.
5QIAprep miniprep Spin®, Qiagen, Quebec, QC, Canada.
6CHUQ, Centre Hospitalier Universitaire de Québec, Université Laval, Québec, QC, Canada.
7SAS Institute Inc. release 9.02, Cary, NC, USA.
References
- Bergström J. Periodontitis and smoking: an evidence-based appraisal. J Evid Based Dent Pract. 2006; 6: 33–41.
- Boström L, Linder LE, Bergström J. Smoking and crevicular fluid levels of IL-6 and TNF-alpha in periodontal disease. J Clin Periodontol. 1999; 26: 352–7.
- Giannopoulou C, Cappuyns I, Mombelli A. Effect of smoking on gingival crevicular fluid cytokine profile during experimental gingivitis. J Clin Periodontol. 2003; 30: 996–1002.
- Ryder MI. The influence of smoking on host responses in periodontal infections. Periodontol ; 43. 2000; 2007: 267–77.
- César Neto JB, de Souza AP, Barbieri D, Moreno H Jr, Sallum EA, Nociti FH Jr. Matrix metalloproteinase-2 may be involved with increased bone loss associated with experimental periodontitis and smoking: a study in rats. J Periodontol. 2004; 75: 995–1000.
- César-Neto JB, Duarte PM, de Oliveira MC, Tambelli CH, Sallum EA, Nociti FHJr. Smoking modulates interleukin-6: interleukin-10 and RANKL: osteoprotegerin ratios in the periodontal tissues. J Periodontal Res. 2007; 42: 184–91.
- Hellström MK, Ramberg P, Krok L, Lindhe J. The effect of supragingival plaque control on the subgingival microflora in human periodontitis. J Clin Periodontol. 1996; 23: 934–40.
- Westfelt E, Rylander H, Dahlén G, Lindhe J. The effect of supragingival plaque control on the progression of advanced periodontal disease. J Clin Periodontol. 1998; 25: 536–41.
- Ribeiro Edel P, Bittencourt S, Nociti-Júnior FH, Sallum EA, Sallum AW, Casati MZ. The effect of one session of supragingival plaque control on clinical and biochemical parameters of chronic periodontitis. J Appl Oral Sci. 2005; 13: 275–9.
- Kho P, Smales FC, Hardie JM. The effect of supragingival plaque control on the subgingival microflora. J Clin Periodontol. 1985; 12: 676–86.
- Beltrami M, Bickel M, Baehni PC. The effect of supragingival plaque control on the composition of the subgingival microflora in human periodontitis. J Clin Periodontol. 1987; 14: 161–4.
- Katsanoulas T, Reneè I, Attström R. The effect of supragingival plaque control on the composition of the subgingival flora in periodontal pockets. J Clin Periodontol. 1992; 19: 760–5.
- McNabb H, Mombelli A, Lang NP. Supragingival cleaning 3 times a week. The microbiological effects in moderately deep pockets. J Clin Periodontol. 1992; 19: 348–56.
- al-Yahfoufi ZA, Mombelli A, Wicki A, Lang NP. The effect of plaque control in subjects with shallow pockets and high prevalence of periodontal pathogens. J Clin Periodontol. 1995; 22: 78–84.
- Darby IB, Hodge PJ, Riggio MP, Kinane DF. Clinical and microbiological effect of scaling and root planing in smoker and non-smoker chronic and aggressive periodontitis patients. J Clin Periodontol. 2005; 32: 200–6.
- Stavropoulos A, Mardas N, Herrero F, Karring T. Smoking affects the outcome of guided tissue regeneration with bioresorbable membranes: a retrospective analysis of intrabony defects. J Clin Periodontol. 2004; 31: 945–50.
- Andia DC, Martins AG, Casati MZ, Sallum EA, Nociti-Jr FH. Root coverage outcome may be affected by heavy smoking: a 2-year follow-up study. J Periodontol. 2008; 79: 647–53.
- Boström L, Linder LE, Bergström J. Clinical expression of TNF-alpha in smoking-associated periodontal disease. J Clin Periodontol. 1998; 25: 767–73.
- Renvert S, Dahlén G, Wikström M. The clinical and microbiological effects of non-surgical periodontal therapy in smokers and non-smokers. J Clin Periodontol. 1998; 25: 153–7.
- Salvi GE, Ramseier CA, Kandylaki M, Sigrist L, Awedowa E, Lang NP. Experimental gingivitis in cigarette smokers: a clinical and microbiological study. J Clin Periodontol. 2005; 32: 441–7.
- Natto S, Baljoon M, Dahlén G, Bergström J. Tobacco smoking and periodontal microflora in a Saudi Arabian population. J Clin Periodontol. 2005; 32: 549–55.
- Gomes SC, Piccinin FB, Oppermann RV, Susin C, Nonnenmacher C, Mutters R, et al.. Periodontal status in smokers and never-smokers: clinical findings and real-time polymerase chain reaction quantification of putative periodontal pathogens. J Periodontol. 2006; 77: 1483–90.
- Gomes SC, Nonnenmacher CI, Susin C, Oppermann RV, Mutters R, Marcantonio RA. The effect of a supragingival plaque-control regimen on the subgingival microbiota in smokers and never-smokers: evaluation by real-time polymerase chain reaction. J Periodontol. 2008; 79: 2297–304.
- Shchipkova AY, Nagaraja HN, Kumar PS. Subgingival microbial profiles of smokers with periodontitis. J Dent Res. 2010; 89: 1247–53.
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999; 4: 1–6.
- Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975; 25: 229–35.
- Mühlemann HR, Son S. Gingival sulcus bleeding – a leading symptom in initial gingivitis. Helv Odontol Acta. 1971; 15: 107–13.
- Casarin RC, Ribeiro Edel P, Mariano FS, Nociti FH Jr, Casati MZ, Gonçalves RB. Levels of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, inflammatory cytokines and species-specific immunoglobulin G in generalized aggressive and chronic periodontitis. J Periodontal Res. 2010; 45: 635–42.
- de Lillo A, Ashley FP, Palmer RM, Munson MA, Kyriacou L, Weightman AJ, et al.. Novel subgingival bacterial phylotypes detected using multiple universal polymerase chain reaction primer sets. Oral Microbiol Immunol. 2006; 21: 61–8.
- Jukes TH, Cantor CR. Evolution of protein molecules. Mammalian protein metabolism. Munro HNAcademic Press. New York, 1969; 21–132.
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999; 41: 95–8.
- Schloss PD, Handelsman J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Env Micro. 2005; 71: 1501–6.
- Chen T, Yu W-H, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database Article ID baq013. 10.3402/jom.v4i0.8640. Available from: http://database.oxfordjournals.org/cgi/content/full/2010/0/baq013.
- Preber H, Bergström J. Occurrence of gingival bleeding in smoker and non-smoker patients. Acta Odontol Scand. 1985; 43: 315–20.
- Preber H, Linder H, Bergström J. Periodontal healing and periopathogenic microflora in smokers and non-smokers. J Clin Periodontol. 1995; 22: 946–52.
- Grossi SG, Zambon J, Machtei EE, Schifferle R, Andreana S, Genco RJ, et al.. Effects of smoking and smoking cessation on healing after mechanical periodontal therapy. J Am Dent Assoc. 1997; 128: 599–607.
- Preshaw PM, Lauffart B, Zak E, Jeffcoat MK, Barton I, Heasman PA. Progression and treatment of chronic adult periodontitis. J Periodontol. 1999; 70: 1209–20.
- Jin L, Wong KY, Leung WK, Corbet EF. Comparison of treatment response patterns following scaling and root planing in smokers and non-smokers with untreated adult periodontitis. J Clin Dent. 2000; 11: 35–41.
- Nogueira Moreira A, Luna Davila G, Bianchini H, Alonso C, Piovano S. Effect of supragingival plaque control on subgingival microflora and gingivo-periodontal tissues. Acta Odontol Latinoam. 2000; 13: 73–86.
- Gomes SC, Piccinin FB, Susin C, Oppermann RV, Marcantonio RA. Effect of supragingival plaque control in smokers and never-smokers: 6-month evaluation of patients with periodontitis. J Periodontol. 2007; 78: 1515–21.
- Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL Jr, Socransky SS. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol. 1997; 24: 324–34.
- Gomes SC, Nonnenmacher CI, Susin C, Oppermann RV, Mutters R, Marcantonio RA. The effect of a supragingival plaque-control regimen on the subgingival microbiota in smokers and never-smokers: evaluation by real-time polymerase chain reaction. J Periodontol. 2008; 79: 2297–304.
- Kumar PS, Griffen A1, Barton JA, Paster BJ, Moeschberger ML, Leys EJ. New bacterial species associated with chronic periodontitis. J Dent Res. 2003; 82: 338–44.
- Delima SL, McBride BK, Preshaw PM, Heasman PA, Kumar PS. Response of subgingival bacteria to smoking cessation. J Clin Microbiol. 2010; 48: 2344–9.
- Listgarten MA, Mayo HE, Tremblay R. Development of dental plaque on epoxy resin crowns in man. A light and electron microscopic study. J Periodontol. 1975; 46: 10–26.
- Söder B. Neutrophil elastase activity, levels of prostaglandin E2, and matrix metalloproteinase-8 in refractory periodontitis sites in smokers and non-smokers. Acta Odontol Scand. 1999; 57: 77–82.
- Van der Velden U, Varoufaki A, Hutter JW, Xu L, Timmerman MF, Van Winkenholff AJ, et al.. Effect of smoking and periodontal treatment on the subgingival microflora. J Clin Periodontol. 2003; 30: 603–10.