Abstract
Objective: In Zhejiang Province, there are several highly developed cities near the coast and several relatively under-developed mountain areas. Analysis of the composition of bacteria isolated from patients as well as their antibiotic resistance profile from various areas of this province, and tracing of such data year-by-year, will help to delineate the bacterial resistance profile of these areas and to understand how the stage of socio-economical development impacts on the composition of clinical micro-flora and their resistance profile.
Methods: In order to investigate variation in resistance rates and isolation rates of Enterobacteriaceae, from 2000 to 2009 in Zhejiang Province, China, Enterobacteriaceae isolated from 15 hospitals located in different regions of the province were surveyed.
Results: The total numbers of the Enterobacteriaceae isolated increased more than 20-fold from 2000 to 2009. Among the Enterobacteriaceae, Escherichia coli and Klebsiella pneumoniae were the dominant isolates. The percentage of E. coli and K. pneumoniae that produced detectable extended-spectrum ?-lactamases (ESBLs) increased from 2000 to 2007, and then declined slightly in 2008 and 2009. The percentages of K. pneumoniae and E. coli that were resistant to ceftazidime increased sharply from 2000 to 2009. There were remarkable increases in the carbapenem resistant rates during the decade, but they were much higher for the isolates from the developed cities than from the rural areas. In 2002, carbapenem-resistant E. coli was first found in Hangzhou, one of the highly developed cities in Zhejiang Province. By 2009, carbapenem-resistant bacteria were found for all species of Enterobacteriaceae surveyed in almost all areas of the province, although they were more frequently identified in developed areas than in rural areas.
Conclusion: Much restrictive actions have to be taken in terms of rational use of antibiotics and nosocomial control to prevent the further spread of the drug-resistant pathogens.
The reformation and opening up of China in the last two decades of the 20th century was accompanied by socio-economic development and a gradual improvement in the standard of living. The life style of the Chinese people, especially people living in the relatively developed areas of China, has undergone significant changes. Such changes have had a profound impact on micro-flora and resistant bacteria both in the environment as well as in clinical settings. One of the most important factors that contributed to these changes in micro-flora and to the resistance profile of bacteria was the abuse of antibiotics. Thus, antibiotics have been widely used and, in addition, over-doses of various antibiotics have been administered for healthcare and in farming where they are used as animal feed additives. The abuse of antibiotics in medical settings has directly impacted the resistance profile of clinically isolated bacteria. Moreover, antibiotics used for crop cultivation and/or in animal feeds not only alter micro-flora in the environment, which in turn impact on clinical micro-flora, but also increase the chance of exposure of humans to these antibiotics.
Zhejiang Province, which has a population of over 46 million, is located on the east coast of China and is the most economically developed province in China. While it is one of the largest areas in China for farming and aquaculture, this province also has the most highly developed medical system, with the highest medical consumption per capita among all of the provinces in China. However, the level of development among different areas even within this same province is not equal. There are several highly developed cities near the coast such as Hangzhou (the capital city of the province), Ningbo, Wenzhou, and Shaoxing, as well as several relatively under-developed mountain areas such as Lishui and Quzhou. Analysis of the composition of bacteria isolated from patients as well as their antibiotic resistance profile from various areas of this province, and tracing of such data year-by-year, will help to delineate the bacterial resistance profile of these areas and to understand how the stage of socio-economical development impacts on the composition of clinical micro-flora and their resistance profile.
Of the various bacteria that have been clinically isolated, gram-negative rods, especially the Enterobacteriaceae, are the most prevalent. The CHINET 2008 surveillance of bacterial resistance showed that Enterobacteriaceae account for 52% of clinical gram-negative bacteria, and that Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis, Citrobacter freundii, and Serratia marcescens were the most commonly isolated Enterobacteriaceae Citation1. E. coli accounts for 26.4–27.6% of gram-negative bacteria followed by Klebsiella spp. (13.8–19.6%) and Enterobacter spp. (4.7–5.8%) Citation1 Citation2 Citation3 Citation4 . Penicillin, cephalosporins, aminoglycosides, quinolones, β-lactam/β-lactamase inhibitor combinations, and carbapenems are currently used to treat Enterobacteriaceae infection. The Enterobacteriaceae that predominantly produce extended-spectrum β-lactamases (ESBLs) are E. coli and K. pneumoniae, and ESBL production was detected in close to 56.2 and 43.6%, respectively of these bacteria, consistent with previous studies Citation5 Citation6. ESBL production causes resistance to β-lactams and is usually mediated by plasmids. In addition to carrying β-lactamase genes, these plasmids may encode a number of aminoglycoside- or quinolone-modifying enzymes, which cause resistance to aminoglycosides and quinolones, respectively. Carbapenems that are stable in the presence of bacterially produced β-lactamases (including ESBLs and AmpC) showed strong activity against many gram-positive, gram-negative, and anaerobic bacteria Citation7 Citation8. Therefore, carbapenems are often used as the last choice for treatment of infections that are caused by multidrug-resistant Enterobacteriaceae.
In the present study, we focused on Enterobacteriaceae isolates from 15 hospitals located in widely different areas of Zhejiang Province. We conducted a retrospective investigation of the distribution of the isolates and their drug resistance profile, especially resistance to carbapenems, during the years 2000–2009 to gain an overview of the variation in bacterial drug resistance over this period.
Materials and methods
Hospitals that participated in the survey and bacterial isolates
Fifteen hospitals from 10 cities in Zhejiang Province participated in this survey: the Second Affiliated Hospital of Zhejiang University (Hangzhou), Zhejiang Provincial People's Hospital (Hangzhou), Zhejiang Provincial Hospital of Traditional Chinese Medicine (Hangzhou), Hangzhou First people's Hospital (Hangzhou), Hangzhou Third people's Hospital (Hangzhou), Zhuji People's Hospital of Zhejiang Province (Shaoxing); the First Hospital of Jiaxing (Jiaxing), Huzhou Central Hospital (Huzhou), Ningbo First Hospital (Ningbo), Quzhou Central Hospital (Quzhou), Dongyang People's Hospital (Jinhua), Lishui Central Hospital (Lishui), Taizhou Hospital of Zhejiang Province (Taizhou), Zhoushan Hospital (Zhoushan), the Second Affiliated Hospital of Wenzhou Medical College (Wenzhou). Isolates were collected from aseptically obtained body fluid such as blood, urine, pleural fluid, and ascites from both in-patients and out-patients during January 2000–December 2009.
Antimicrobial susceptibility testing
The identity and susceptibility of isolates were confirmed using the VITEK system (bioMérieux, Hazelwood, MO, USA). E. coli ATCC25922 and K. pneumoniae ATCC700603 were used as reference strains for susceptibility testing. Meropenem and cefoperazone/sulbactam (2:1) were determined by the K-B method as recommended by the Clinical Laboratory Standards Institute (CLSI) of 2009 version Citation9. As no cefoperazone/sulbactam breakpoints are available for Enterobacteriaceae, susceptibility to cefoperazone was referred to in terms of resistance. All of the results were analyzed using WHONET 5.0 software (World Health Organization, Geneva, Switzerland).
Phenotypic test for confirmation of ESBL-producing bacteria
ESBL-producing Enterobacteriaceae were confirmed using an ESBL confirmatory test according to CLSI guidelines Citation9. A 0.5 McFarland standard suspension of each isolate was inoculated on a Mueller-Hinton agar (MHA) plate as for the routine disk diffusion procedure. The plates were incubated for 16–18 h at 35°C. A ≥5 mm increase in a zone diameter for either antimicrobial agent tested in combination with clavulanic acid versus the zone diameter when tested in the absence of these agents was defined as ESBL-positive. E. coli ATCC25922 and K. pneumoniae ATCC700603 were used as reference strains for this ESBL confirmatory test.
Polymerase chain reaction (PCR) and DNA sequencing
A total of 83 non-duplicated clinical carbapenem non-susceptible Enterobacteriaceae were collected from 10 areas of Zhejiang Province. The primers and reaction conditions used to amplify the blaKPC gene were as described previously Citation10. PCR products were sequenced using an ABI3730 Sequencer (Applied Biosystems, Foster City, CA), and the sequences were compared with the sequences reported in GenBank.
Results
Distribution of clinical isolates
Among the all bacteria isolated, Staphylococcus aureus was the most prevalent bacteria in the years 2000 and 2001. However, from 2002 and thereafter, it was replaced by Pseudomonas aeruginosa and followed by Acinetobacter baumannii. The most prominent Enterobacteriaceae bacteria in clinical isolates were K. pneumoniae and E. coli (Supplementary ). Although there was no restrict identification of the origin of the isolates, it was estimated that more than 80% of the isolates were from in-patients, we suspect that majority of the isolates, especially with high resistance, were from the hospital-acquired infections.
Supplementary Table 1. Distribution of clinical isolates in Zhejiang Province from 2000 to 2009
Distribution of clinically isolated Enterobacteriaceae
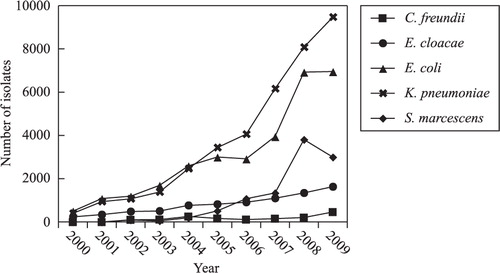
The most commonly isolated Enterobacteriaceae (E. coli, K. pneumoniae, E. cloacae, S. marcescens, and C. freundii) are shown in . The number of isolated Enterobacteriaceae increased dramatically (about 20-fold) from 2000 to 2009. During the first 5 years of the survey (2000–2004), the number of E. coli was higher than that of K. pneumonia. From 2005 onward, K. pneumoniae was the most prevalent species of Enterobacteriaceae, followed by S. marcescens and E. cloacae.
Detection of ESBL-producing bacteria
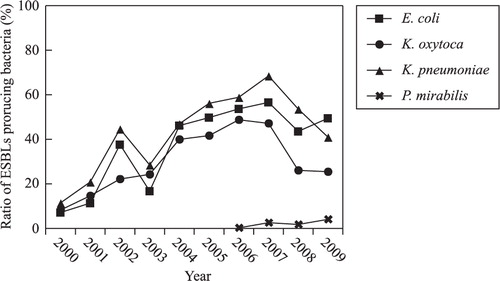
The main bacteria that produced ESBLs were E. coli, K. pneumoniae, Klebsiella oxytoca, and P. mirabilis. The percentage of E. coli and K. pneumoniae that produced ESBLs demonstrated a gradual and remarkable increase from 7.2% in 2000 to 56.6% in 2007, and from 11.5% in 2000 to 68.5% in 2007, respectively, while this percentage decreased in 2008 and 2009. ESBL-producing P. mirabilis was detected from 2006 onward ().
Variation in the antimicrobial susceptibility of Enterobacteriaceae
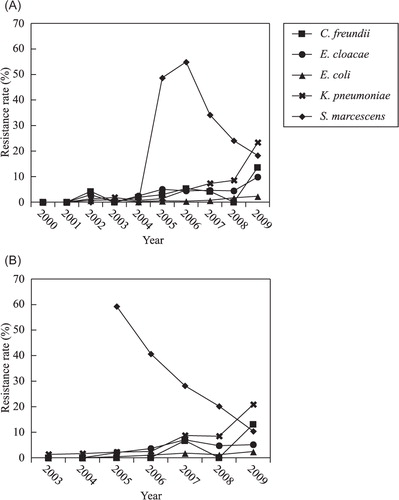
The survey data (Supplementary ) indicated that the percentage of bacteria that were resistant to ceftazidime increased sharply from 5.5% in 2000 to 45.3% in 2009. Some isolates were even resistant to carbapenems (imipenem and meropenem). Compared with E. coli, a higher percentage of K. pneumoniae showed resistance to antimicrobial agents. The percentage of resistant K. pneumoniae increased annually, in particular the percentage of carbapenem-resistant bacteria, which suddenly reached as high as 20.8% in 2009 (). While the percentage of E. cloacae that were resistant to cephalosporins (except for ceftizoxime) remained at about 50%, resistance rate to carbapenems increased yearly, but remained under 10%. Resistance rate of S. marcescens to carbapenem suddenly increased to 40–60% in 2005 and 2006 due to an outbreak of carbapenem-resistant S. marcescens as reported Citation11. Although the number of isolated C. freundii was lower than that of the aforementioned Enterobacteriaceae isolates, their resistance rates also increased annually, and they showed a similar resistance rate to cephalosporins as E. cloacae. The resistance rate of C. freundii to imipenem and meropenem also reached 13.6 and 13.1%, respectively, in 2009.
Fig. 3. Annual changes in the resistance rate of various Enterobacteriaceae species against carbapenem from 2000 to 2009. (A) Imipenem, (B) meropenem.
Supplementary Table 2. Variation of antimicrobial susceptibility of Enterobacteriaceae isolated from 2000 to 2009
Distribution of carbapenem-resistant Enterobacteriaceae
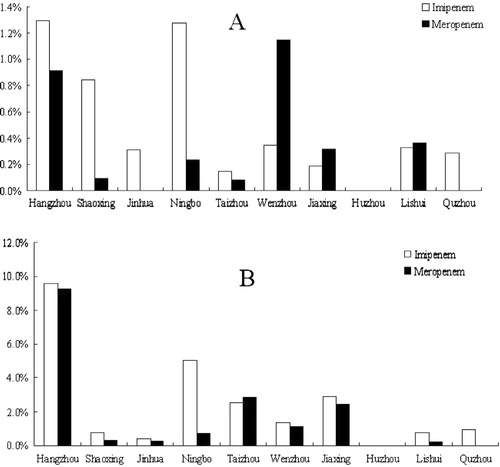
Carbapenem-resistant Enterobacteriaceae were identified from areas throughout the entire province (, ). Since the first identification in 2002 of carbapenem-resistant E. coli in Hangzhou, the capital city of Zhejiang Province, eight species of carbapenem-resistant Enterobacteriaceae have been found in Hangzhou. It is clear from the map () that there was a much higher number of carbapenem-resistant Enterobacteriaceae species in coastal areas than in inland areas. The most prevalent carbapenem-resistant Enterobacteriaceae were E. coli, K. pneumoniae, S. Marcescens, and C. freundii. The resistance rate to imipenem increased annually, especially in 2005–2007, when a high number of carbapenem-resistant S. marcescens was found in Hangzhou, while the resistance rate declined in 2008 and 2009. A previous study reported that all of the carbapenem-resistant S. marcescens isolated from Hangzhou belonged to the same clone Citation11.
Fig. 4. Prevalence of carbapenem-resistant Enterobacteriaceae from 10 areas in Zhejiang Province from 2000 to 2009. The year shown inside the area indicated the year when the first carbapenem-resistant Enterobacteriaceae was identified from this area. The sign of different color shown in the area indicates the different carbapenem-resistant Enterobacteriaceae so far have been identified from this area.
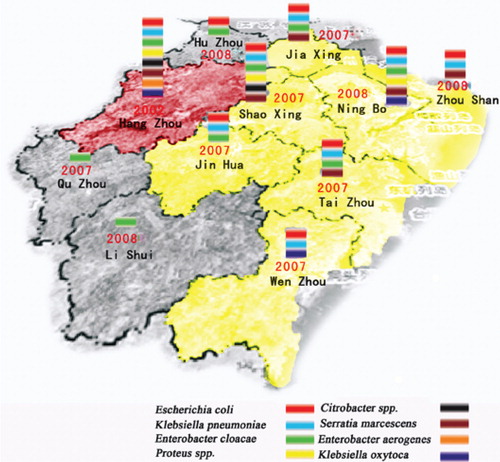
Table 1. Detection of carbapenem-resistant Enterobacteriaceae in different districts of Zhejiang Province
Detection of blaKPC in Zhejiang Province
All of the 82 non-duplicated isolates including isolates of E. coli, K. pneumoniae, E. cloacae, C. freundii, S. marcescens, Enterobacter aerogenes, and K. oxytoca that were collected from different districts of Zhejiang Province harbored the blaKPC-2 gene, which can hydrolyze all β-lactams including carbapenems. This result indicates that blaKPC-2 is prevalent in the Zhejiang Province, which is thus a serious problem in terms of bacterial resistance.
Variation in resistant bacteria among different geographic areas
The distribution of this resistance differed according to geographic area. As shown in , resistance rates in economically developed areas (cities of Hangzhou, Ningbo, Shaoxing, Wenzhou, and Jinhua) were much higher than resistance rates in inland areas such as Lishui and Quzhou.
Discussion
There was a remarkable increase in the total numbers of pathogens isolated over the last decade (Supplementary ). There is about 15–20% ‘natural’ increase due to the increase in the number of patients. However, since 2007, there was a jump in the isolate numbers. This was attributed to the changes of the practice of blood-culture. Before that, blood culture was conducted for the blood of a single vial from one side. Since 2007, blood culture was required to be done for four vials (two vials on each side for both anaerobic and aerobic culture), which had remarkably increased the number of isolates. The emergence of ESBL-producing and carbapenem-resistant Enterobacteriaceae has made the treatment of infections caused by such bacteria a serious challenge. Carbapenem-resistant strains belonging to E. coli and K. pneumonia Citation12, the widely reported ‘super bacteria’, are also currently being clinically isolated. A survey of the resistance of Enterobacteriaceae in Zhejiang Province showed that E. coli and K. pneumoniae were the dominant Enterobacteriaceae isolated, and the number of clinical isolates belonging to these species has increased annually. From 2000 to 2004, E. cloacae were in the top 10 isolated species, whereas, after 2005, their numbers declined. In contrast, isolates of S. marcescens reached the top 10 starting in 2007, and these isolates were mainly isolated from five hospitals in Hangzhou. Pulsed-field gel electrophoresis (PFGE) typing showed that most of these S. marcescens isolates belonged to the same clone. The K. pneumoniae species that were isolated from the Second Affiliated Hospital of Zhejiang University were classified into five dominant clones Citation11. Despite the low level of C. freundii that was isolated, its drug resistance in both clinical and environmental settings is worthy of further attention.
The resistance survey also indicated that the most active aminoglycosidic agent against these isolates was amikacin, and the resistance rate to this agent remained below 30%. Resistance rate to amikacin even decreased for some species of Enterobacteriaceae (E. coli, K. pneumoniae, E. Cloacae, and C. freundii) over this decade. Resistance rate to gentamicin also declined over this decade. However, the percent of E. coli resistant to gentamicin remained at over 50%, which was higher than the resistance rate of K. pneumoniae. A high percentage of E. coli (60%) was also resistant to ciprofloxacin and levofloxacin, and this resistance rate was higher than that of other Enterobacteriaceae isolates, consistent with the 2008 CHINET survey of bacterial resistance in China Citation1.
The percentage of E. coli and K. pneumoniae that produced ESBLs gradually increased during this decade. The percentage of E. coli that produced ESBLs increased from 7.2% in 2000 to 56.6% in 2007, and then declined slightly. The percentage of K. pneumoniae that produced ESBLs remained higher than that of E. coli. These results explain why the resistance rate of E. coli to cephalosporin was lower than that of K. pneumoniae. The resistance rate of E. coli to ceftazidime and cefepime clearly increased over the decade, from 5.5 and 10.3% in 2000 to 50 and 52.1% in 2008, respectively. The resistance rate of K. pneumoniae to cefepime increased from 8.3% in 2000 to 65.3% in 2007, and then declined slightly. The resistance rate of E. cloacae, S. marcescens, and C. freundii to cephalosporin increased slightly over the survey period. According to the CLSI guidelines Citation9, new breakpoints against third-generation cephalosporins have been revised, which means that detection of ESBLs is now not required in routine clinical analysis. Further surveys should be carried out to monitor and evaluate the effectiveness of the new breakpoint guidelines.
The Study for Monitoring Antimicrobial Resistance Trends (SMART) in North America, Europe, Latin America, Middle East, Africa, and Asia from 2002 to 2007 showed that imipenem and meropenem showed good activity against Enterobacteriaceae Citation6. Similar results were also reported from other antibiotic resistance surveys. However the resistance rate to carbapenems also increased slightly during the decade Citation5 Citation13 Citation14. Our results were consistent with these previous studies. Carbapenem-resistant K. pneumoniae and E. coli were found in 2002, and carbapenem-non-susceptible P. mirabilis was subsequently found in 2004 Citation15. All β-lactams including carbapenems can be hydrolyzed by blaKPC. KPC was first detected in K. pneumoniae in 2001 Citation10 and was later found in other bacteria, such as other Enterobacteriaceae species, P. aeruginosa, Pseudomonas putida, Acinetobacter spp. and Raoultella spp. Citation16 Citation17 Citation18 . blaKPC-2 was first reported in K. pneumoniae in 2007 in Zhejiang, China Citation19, although carbapenem-resistant E. coli was found in Hangzhou, in the Zhejiang Province, as early as 2002. However, insufficient attention was paid to this phenomenon. Since then, also other carbapenem-resistant Enterobacteriaceae such as K. pneumoniae, E. cloacae, P. mirabilis, C. freundii, S. marcescens, E. Aerogenes, and K. oxytoca have emerged in Zhejiang.
In addition, the percentage of carbapenem-resistant K. pneumoniae that was detected was higher than that of E. coli, especially in 2009. Moreover, PFGE typing showed that the S. marcescens isolates that were isolated in hospitals in Hangzhou during the epidemic of carbapenem-resistant S. marcescens that occurred in 2005–2007 belonged to the same clone, and most of them harbored blaKPC-2 Citation20. Resistance rate to carbapenems decreased remarkably in the following two years. This decrease was attributed to the following two factors. One was enforcement of taking various actions for prevention of bacterial infection such as separating the pathogen carriers and enforcement of hand sanitization of medical professionals by the government through the Nosocomial Infection Control Committee of the province. The second one was the restriction and control of the use of antibiotics by the Chinese Ministry of Hygiene, which has implemented guidelines for the rational use of antibiotics since 2006. In addition to resistance to carbapenems, the resistance rate of Enterobacteriaceae to antibiotics containing enzyme inhibitors (such as sulbactam or tazobactam) also increased over the survey period, and this trend toward increased resistance accorded with the increased resistance to carbapenems. For instance, both K. pneumoniae and S. marcescens showed high resistance to carbapenems, and their resistance rate to antibiotics containing enzyme inhibitors was also very high (2005–2007).
The geographic distribution of carbapenem-resistant Enterobacteriaceae indicated that more resistant isolates were identified from coastal and developed cities than from rural and mountain areas, and that the resistance rate of the isolates from developed cities was much higher than that from rural areas. Hangzhou (the capital city), Wenzhou, Ningbo, and Taizhou are coastal cities, while Shaoxing is an industrially developed area. These areas and cities are economically developed with higher income per capita and more frequent use of antibiotics, especially of broad-spectrum antibiotics, than rural areas such as in Lishui and Quzhou. Economically developed areas have a relatively developed medical system with a higher chance of antibiotic exposure that will increase the possibility of bacterial resistance. Moreover, the higher population density in these areas also increases the chance that resistant pathogens will be transferred among the population. While social-economic development has helped to improve the living standard of the people, it has also changed the micro-flora, especially the pathogens, and increased bacterial resistance, which poses a challenge to our future medical care and infection-control strategies. This finding is in line with our previous report that, during the last several decades, the ratio of Shigella spp. (Shigella flexneri vs. Shigella sonnei) in the province has undergone a shift from a ratio that is typical of developing countries to a ratio that is typical of industrialized countries Citation21.
Currently, the emergence of multi-resistant gram-negative bacteria, especially of Enterobacteriaceae, is a serious problem in Zhejiang, and should be further monitored in the coming years. Much restrictive actions have to be taken in terms of rational use of antibiotics and nosocomial control to prevent the further spread of the multi-resistant pathogens.
Conflict of interest and funding
The authors have no conflict of interest in connection with this paper. This work was partially supported by the JSPS Grant-in-Aid for Scientific Research (A)(21256002).
Acknowledgements
We would like to express our sincere appreciation to the staff members in the 15 hospitals who have participated in this survey program and provided the bacterial isolates and data. Rong Zhang and Tomoaki Ichijo contributed equally to this work.
References
- Wang F, Zhu DM, Hu FP, Ruan FY, Ni YX, Sun JY, et al. CHINET 2008 surveillance of bacterial resistance in China. Chin J Infect Chemother. 2009; 9: 321–9.
- Wang F. CHINET 2005 surveillance of bacterial resistance in China. Chin J Infect Chemother. 2006; 6: 289–95.
- Wang F. CHINET 2006 surveillance of bacterial resistance in China. Chin J Infect Chemother. 2008; 8: 1–9.
- Wang F, Zhu DM, Hu FP, Ruan FY, Ni YX, Sun JY, et al. CHINET 2007 surveillance of bacterial resistance in China. Chin J Infect Chemother. 2008; 8: 325–33.
- Farrell DJ, Turnidge JD, Bell J, Sader HS, Jones RN. The in vitro evaluation of tigecycline tested against pathogens isolated in eight countries in the Asia-Western Pacific region (2008). J Infect. 2010; 60: 440–51.
- Hoban DJ, Bouchillon SK, Hawser SP, Badal RE. Trends in the frequency of multiple drug-resistant Enterobacteriaceae and their susceptibility to ertapenem, imipenem, and other antimicrobial agents: data from the Study for Monitoring Antimicrobial Resistance Trends 2002 to 2007. Diagn Microbiol Infect Dis. 2010; 66: 78–86.
- Nord CE, Lindmark A, Persson I. Susceptibility of anaerobic-bacteria to meropenem. J Antimicrob Chemother. 1989; 24: 113–7.
- Yang YJ, Bhachech N, Bush K. Biochemical-comparison of imipenem, meropenem and biapenem-permeability, binding to penicillin-binding proteins, and stability to hydrolysis by beta-lactamases. J Antimicrob Chemother. 1995; 35: 75–84.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twentieth informational supplement. Available from: http://www.clsi.org/source/orders/free/m100-s20.pdf.
- Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001; 45: 1151–61.
- Cai JC, Zhou HW, Zhang R, Chen GX. Emergence of Serratia marcescens, Klebsiella pneumoniae, and Escherichia coli isolates possessing the plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC-2 in intensive care units of a Chinese hospital. Antimicrob Agents Chemother. 2008; 52: 2014–18.
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010; 10: 597–602.
- Nijssen S, Florijn A, Bonten MJM, Schmitz FJ, Verhoef J, Fluit AC. Beta-lactam susceptibilities and prevalence of ESBL-producing isolates among more than 5000 European Enterobacteriaceae isolates. Int J Antimicrob Agents. 2004; 24: 585–91.
- Rhomberg PR, Jones RN. Summary trends for the Meropenem Yearly Susceptibility Test Information Collection Program: a 10-year experience in the United States (1999–2008). Diagn Microbiol Infect Dis. 2009; 65: 414–26.
- Pfaller MA, Sader HS, Fritsche TR, Jones RN. Antimicrobial activity of cefepime tested against ceftazidime-resistant Gram-negative clinical strains from North American hospitals: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis. 2006; 56: 63–8.
- Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007; 20: 440–58.
- Castanheira M, Deshpande LM, DiPersio JR, Kang J, Weinstein MP, Jones RN. First descriptions of bla(KPC) in Raoultella spp. (R. planticola and R. ornithinolytica): report from the SENTRY Antimicrobial Surveillance Program. J Clin Microbiol. 2009; 47: 4129–30.
- Robledo IE, Aquino EE, Sante MI, Santana JL, Otero DM, Leon CF, et al. Detection of KPC in Acinetobacter spp. in Puerto Rico. Antimicrob Agents Chemother. 2010; 54: 1354–57.
- Wei ZQ, Du XX, Yu YS, Shen P, Chen YG, Li LJ. Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob Agents Chemother. 2007; 51: 763–5.
- Zhang R, Zhou HW, Cai JC, Chen GX. Plasmid-mediated carbapenem-hydrolysing beta-lactamase KPC-2 in carbapenem-resistant Serratia marcescens isolates from Hangzhou, China. J Antimicrob Chemother. 2007; 59: 574–6.
- Zhang R, Zhou HW, Cai JC, Zhang J, Chen GX, Nasu M, et al. Serotypes and extended-spectrum beta-lactamase types of clinical isolates of Shigella spp. from the Zhejiang province of China. Diagn Microbiol Infect Dis. 2011; 69: 98–104.