Abstract
Problem: The nature of the peptides found increased in urine from autism needs verification of their structure, especially those that show opioid activity.
Methods: The peptides were separated on reverse phase C-18 HPLC in Trifluoroacetic acid–acetonitril gradients. Peaks eluting where synthetic opioids appear, and peaks that are common to most autistic children were analyzed by mass spectrometry and fragmentation pattern on a quadropole mass-spectrometer.
Results: We could demonstrate exorphins in the urine from autistic children, and their length varied from one patient to the next.
Conclusion: Exorphins are found in urine of autistic children and may account for their symptoms.
Like most medical and psychiatric diagnoses, autism is a symptomatic description of probable syndromes. Cardinal symptoms are an extreme introversion (aloofness), social indifference, stereotyped repetitive behaviours, language problems, problems with hygiene, temper tantrums and in some cases hyperactivity, irrational fears and decreased but fluctuating insensitivity to pain. Autism was shown to have a considerable genetic disposition Citation1 Citation2 which invariable must entail chemical changes. However, increasing rates point to a toxicological element since genetic drift is a very much slower process. Published data point to mercury (Hg) as a strong candidate Citation3–Citation5.
Table 1. Some known exorphins are listed (one letter code)
An increase in urinary peptides has been found Citation6–Citation9 and also an increase in opioid activity Citation6 Citation8–Citation11. To be relevant to autism the following must apply:
The peptides must be there
Their nature elucidated
Bioactivities must be of relevance
Explain the antibodies found
A food behaviour axis must be possible
Removal of peptide source ought to give improvement
Methods
Diagnosis of patients over many years was based on DSMIII and IV. The children discussed here have all been diagnosed by certified specialists working in several countries (). All were at the time without drugs and had been so for at least 6 months. The degree of handicap was rated by CARS (childhood autism rating scale). Various other tests were used in intervention studies (see later).
Table 2. Area under the 215 nm curve eluting after hippuric acid. Arbitrary units
The prepubertal children were picked out and their age ranged from 2 to 13 years. First morning urine was collected and frozen as soon as possible to −15°C. The urine samples were left to thaw over night in the laboratory cold room (4°C) and creatinin was measured by the Department of Clinical Biochemistry, Rikshospitalet, Norway. Eight-hundred microliters were centrifuged through Spin-X Centrifuge Tube filters from Costar at 3000×g for 20 min at room temperature. The tubes were polypropylene and the filter 0.22 µm Cellulose acetate.
An aliquot equivalent to 250 nanomoles creatinin was applied to reverse phase HPLC columns (Vydac c-18 Peptide/protein columns cat 218TP54 from USA). The columns were eluted with TFA (trifluoroacetic acid) 10 mM and acetonitril gradients as described Citation11. Elution was monitored at 215 nm (peptide bonds), 280 nm (aromatic groups) and 325 nm (indolyl groups). Rate of elution was 1 ml/min and the gradient was started after a 15 min isocratic TFA run.
To determine which peptide peaks should be studied since considerable variation was found both in quantity and properties of eluted peaks, urine from five to ten reasonably similar autistic urines were combined and rerun. Peaks that are common would stay the same or increase, while individual peaks are diluted out. (This technique was learned from P Shattock OBE, Sunderland, UK.) The relevant peaks were lyophilized and stored at −15°C until used further. Peaks that elute co-chromatographically with synthetic standards were also collected off line.
Mass spectroscopy
Lyophilised material from collective runs were re-dissolved in methanol/water (1/1) and 10 mM formic acid and 300 µl of this buffer. After stirring at room temperature this was applied to a P-sciex mass spectrometer by micropump and direct inlet. Prominent ion peaks were fragmented by MS/MS. Each peptide had to have declustering potential and collision energy adjusted for optimal results.
Using Elisa technique anti food protein antibodies were analyzed in serum commercially by Fürsts Laboratory in Oslo, while recently we have been using the research laboratory at the University of Bergen Hospital (Haukeland).
As for ethical considerations, none of our procedures are damaging to the patients.
Late onset and more regressive autism has statistically increased peptide levels compared to early onset autism. Late onset 1520 units±926 units n=25, neonatal onset 570±188 (n=45) and p=0.0001 Mann Whitney U-test, two-tailed.
The urines were obtained in Italy by Association Planet Autism; from Serbia by Dr Selakovic, Beograd; from Slovenia by D. Patterson, MD, of Runaway Bay, Queensland, Australia. Controls are Norwegian children provided by education specialist M. Nödland, Stavanger. The age range was 3–12 years for controls, for patients 2–13 years. The level for Scandinavian children has been published previously Citation6 Citation11. High functioning autistic children overlap with controls to a degree which makes urine diagnosis difficult Citation13.
Results
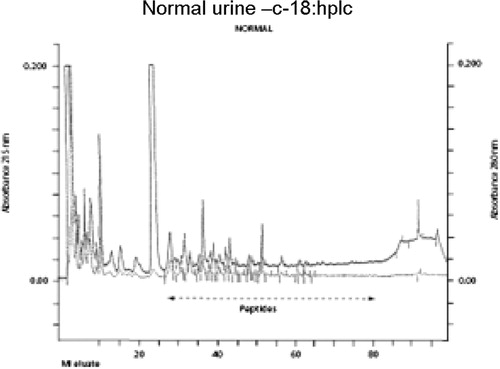
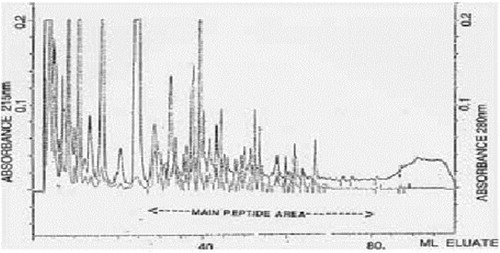
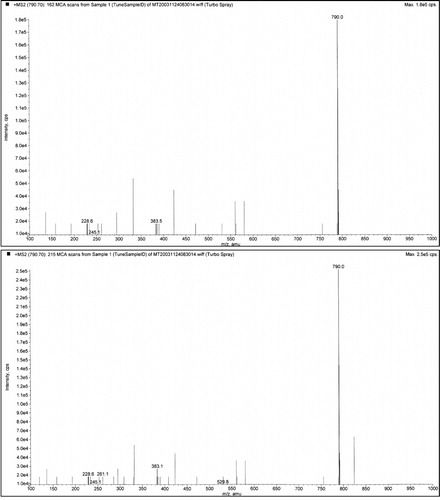
Typical normal and infantile autistic HPLC patterns are seen in and . In we show peaks from the HPLC run where hydrolyzed amino acid analysis is compared with a not hydrolyzed equal aliquot. Clearly a rather massive increase in amino acids demonstrate that most of the peaks eluting after hippuric acid are peptides. In the fragmentation pattern of Glumorphin A5 prove its presence in CPDD children. Likewise in -casomorphin 1–7 is proved present, but is found in only some of the autistic spectrum children. (perhaps 5–10%). Other casomorphins are often found too (see appendix), and more frequently. Finally we can demonstrate that overloading a normal person with gluten and gliadin for three days resulted in finding Glumorphin B5 in urine. The peptide was determined to four decimal places and showed identity through spiking with synthetic glumorphin B5.
Fig. 3. Bottom trace amino acid analysis before hydrolysis, and top trace after hydrolysis in 6 M HCl in closed vials at 1108C for 16 hours. The late eluting (to the right) large peak is ammonia.
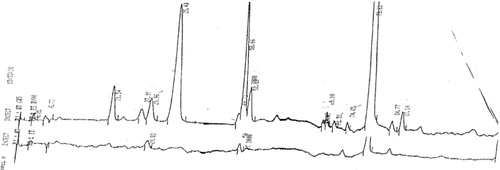
Fig. 4. Demonstration of glumorphin A-5 by MS/MS (fragmentation). Top trace is synthetic standard, and bottom trace Glumorphin A5 isolated from regressive autistic children's urine. Declustering potential was set at 20 and collision energy at 30. The X axis has been extended in bottom trace to secure against hidden ion peaks.
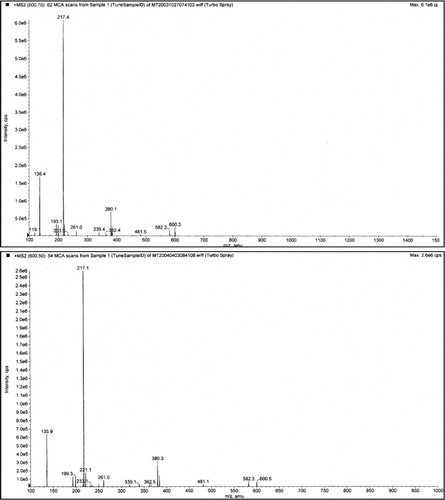
Fig. 5. Casomorphin 1–7. The casomorphin 1–7 standard is the top trace, from CPDD bottom trace. The peak appearing after the intact ion could be removed by increasing the declustering potential. It is shown here to illustrate the strong propensity for peptides to form aggregates and adducts. Declustering here is 20 and collision potential is 70.
Discussion
Some of the peptides found are bio-active. Thus opioid receptor binding Citation14 and analgesia studied by the tail flick test Citation15 have also been found. Furthermore, IV injection caused hyperactivity Citation15 Citation16 and Fos antigen activation in key nuclei of rat brain Citation17, such as the nucleus accumbens. Both analgesia and Fos antigen increase were blocked by naloxone. The opioid peptide also caused decreased uptake of dopamine in striatal synaptosomes and showed in vivo dopaminergic hyperactivity using the Ungerstedt model Citation15.
Also linking autism to dietary proteins was the discovery of antibodies against gluten, gliadin and casein increased in autistic children Citation8, Citation18, Citation19–Citation21.
A Russian group Citation10 found that casomorphin in the blood was inversely related to psychomotor development in infants. Casomorphins have, furthermore, been shown to abrogate separation distress call and reaction in newly hatched chickens Citation22 and also causing social isolation in kittens and pups.
A gut to brain interaction exists
Peptides are formed in the gut from food proteins Citation23 Citation24 and everyone takes up peptides from the gut Citation24 Citation25. If peptidases are inhibited or lacking, this uptake is increased Citation26 Citation27. Opioid peptides also get across the blood–brain barrier Citation28 Citation29.
Antibodies against food proteins increased in autism points to increased uptake of protein. Humans take up intact protein Citation25 Citation30 and intact enzymes Citation31. These proteins can be recovered from mother's milk Citation32 Citation33. Botulinum toxin is a peptidase that is taken up from the gut, crosses the blood–brain barrier and the pre-synaptic membrane and splits one peptide bond in SNAP-25 with fatal results Citation34. Some antibodies, for instance against gliadin Citation35, have direct effects on Purkinje cells of the cerebellum. Considerable intestinal problems are found in autistic spectrum disorders Citation36–Citation38 which would result in increased uptake. Casein and gluten can also release inflammatory cytokines in autism Citation39 and such cytokines increase permeability of endothelial barriers Citation40. In autism increased gut permeability has been established Citation41 Citation42 and, therefore, the prerequisite conditions for the postulated mechanism are present.
Given the established data relating certain food proteins to autism it would be advisable to remove these from the diet. This has been done and quite a number of such studies have been conducted Citation6 Citation7 Citation43–Citation52. The length of the intervention is critical and short term trials such as 14 days are quite inadequate. Double blind dietary studies are very demanding, but single blind pair wise matched and randomly assigned studies Citation50, as well as testing over 4 years to counter act placebo Citation51, are promising, and indicate that dietary intervention is effective.
In we summarize the data from an open 4-year follow up and in the results are shown of matched randomly assigned children with autism. Note that in the 4-year study children that quit the GF/CF diet, the children regressed (0.58). The numbers are small, but since diagnosis is symptomatic not aetiological, pair wise matched randomly assigned studies are much more secure because this increases the probability of comparing similar patients.
Table 3. Year open study of dietary intervention in 15 patients
Table 4. GF/Cf diet with control group for pair wise matched and randomly assigned autistic children Citation52
Overloading normal person with gluten/and gliadin for 3 days gave a measurable peak for glumorphin B5, which could be spiked with B5 (). The peak is determined to four decimal places by mass spectroscopy. It is therefore possible that even with a normal genome but decreased expression number due to lower operator gene activity, insufficient break down capacity may be present in autistic children.
Conflict of interest and funding
K.L. Reichelt is an unpaid consultant to Biomedical Laboratory in Oslo, otherwise the authors’ have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Folstein H, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1975; 113: 297–321.
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995; 25: 63–77. 10.3402/mehd.v23i0.18958.
- Garrecht M, Austin DW. The plausibility of a role for mercury in the etiology of autism: a cellular perspective. Toxicol Environ Chem. 2011; 93: 1251–73. 10.3402/mehd.v23i0.18958.
- Nataf R, Skorupka C, Amet L, Lam A, Springbett A, Lathe R. Porphyrinuria in childhood autistic disorder: implications for environmental toxicity. Toxicol Appl Pharmacol. 2006; 214: 99–108. 10.3402/mehd.v23i0.18958.
- Yong LJ, Pitkow LJ, Ferguson IN. The neuropatho-genesis of mercury toxicity. Mol Psychiatry. 2002; 7: 540–1. 10.3402/mehd.v23i0.18958.
- Reichelt KL, Knivsberg AM, Lind G, Nødland M. Probable etiology and possible treatment of childhood autism. Brain Dysfunct. 1991; 4: 308–19.
- Shattock P, Kennedy A, Rowell F, Berney T. Role of neuropeptides in autism and their relationship with classical neurotransmitters. Brain Dysfunct. 1990; 3: 328–45.
- Cade RJ, Privette RM, Fregly M, Rowland N, Sun Z, Zele V, et al. Autism and schizophrenia: intestinal disorders. Nutr Neurosci. 2000; 3: 57–72.
- Shanahan MR, Venturini AJ, Daiss JL, Friedman AE. Peptide diagnostic markers for human disorders. European Patent Application. 2000; EP 0 969 015 A2: 1–44.
- Kost NV, Sokolov OY, Kurasova OB, Dimitriev AD, Tarakanova JN, Gabaeva MV, et al. Beta-casomorphin-7 in infants on different type of feeding and different levels of psychomotor development. Peptides. 2009; 30: 854–60. 10.3402/mehd.v23i0.18958.
- Reichelt KL, Knivsberg AM. Can the pathophysiology of autism be explained by the nature of the discovered urine peptides?. Nutr Neurosci. 2003; 6: 19–28. 10.3402/mehd.v23i0.18958.
- Sacco R, Curatolo P, Manzi B, Militerni R, Bravaccio C, Frolli A, et al. Principal pathogenetic components and biological endophenotypes in autism spectrum disorders. Autism Res. 2010; 3: 237–52. 10.3402/mehd.v23i0.18958.
- Sponheim E, Myhre AM, Reichelt KL, Aasen OC. Peptidmønstre I urin hos barn med mildere former for autisme. Tidsskr Nor Laegeforen. 2006; 126: 1475–7.
- Reichelt KL, Hole K, Hamberger A, Sælid G, Edminson PD, Braestrup CB, et al. Biologically active peptide-containing fractions in schizophrenia and childhood autism. Adv Biochem Psychopharmacol. 1981; 28: 627–98.
- Hole K, Bergslien AA, Jørgensen H, Berge OG, Reichelt KL, Trygstad OE. A peptide containing fraction from schizophrenia which stimulates opiate receptors and inhibits dopamine uptake. Neuroscience. 1979; 4: 1139–47. 10.3402/mehd.v23i0.18958.
- Sun Z, Cade JR. A peptide found in schizophrenia and autism causes behavioural changes in rats. Autism. 1999; 3: 85–95. 10.3402/mehd.v23i0.18958.
- Sun Z, Cade RJ, Fregly MJ, Privette RM. Beta-casomorphine induces fos-like immunoreactivity in discrete brain regions relevant to schizophrenia and autism. Autism. 1999; 3: 67–83. 10.3402/mehd.v23i0.18958.
- Reichelt KL, Ekrem J, Scott H. Gluten, milk proteins and autism: dietary intervention effects on behavior and peptide secretion. J Appl Nutr. 1990; 42: 1–11.
- Kawashti MI, Amin OH, Rowely NG. Possible immunological disorders in autism. Concomittant autoimmunity and immune tolerance. Egypt J Immunol. 2008; 13: 99–104.
- Trajkovski V, Petlichkovski A, Efinska-Mladenovska O, Trajkov D, Arsov T, Strzova A, et al. Higher plasma concentrations of food specific antibodies in persons with autistic disorder in comparison to their siblings. Focus on Autism and Other Developmental Disabilities. 2008; 23: 176–86. 10.3402/mehd.v23i0.18958.
- Voidani A, O'Bryan T, Green JA, McCandless J, Woeller KN, Vojdani AA, et al. Immune response to dietary proteins, Gliadin and Cerebellar Peptides in Children with Autism. Nutritional Neurosci. 2004; 7: 151–61. 10.3402/mehd.v23i0.18958.
- Panksepp J, Normansell L, Sivily S, Rossi J, Zolovick AJ. Casomorphins reduce separation distress in chickens. Peptides. 1978; 5: 829–31. 10.3402/mehd.v23i0.18958.
- Svedberg J, De Haas J, Leimanstoll G, Paul F, Teschemacher H. Demonstration of beta-casomorphin immunoreactive materials in vitro digests of bovine milk and in small intestinal contents after bovine milk ingestion in adult humans. Peptides. 1985; 6: 825–31. 10.3402/mehd.v23i0.18958.
- Chabance B, Marteau P, Rambaud JC, Migliore-Samour D, Boynard M, Perrotin P, et al. Casein peptides release and passage to the blood in humans during digestion of milk and yogurt. Biochimie. 1998; 80: 155–65. 10.3402/mehd.v23i0.18958.
- Gardner MLG. Absorption of intact proteins and peptides. Physiology of the Gastrointestinal Tract. Johnson LR. Raven Press: New York, 1994; 1795–820.
- Mahe S, Tome D, Demontier AM, Desjeux JF. Absorption of intact morphiceptin by diisopropylflurophosphate – treated rabbit ileum. Peptides. 1989; 10: 45–52. 10.3402/mehd.v23i0.18958.
- Bouras M, Huneau JF, Tome D. The inhibition of intestinal dipeptidylaminopeptidase-IV promotes the absorption of enterostatin and des-arginine-enterostain across rat jejunum in vitro. Life Sci. 1996; 59: 2147–55. 10.3402/mehd.v23i0.18958.
- Kastin AJ, Nissen C, Schally AV, Coy DH. Additional evidence that small amounts of a peptide can cross the blood–brain barrier. Pharmacol Biochem Behav. 1979; 14: 717–9. 10.3402/mehd.v23i0.18958.
- Ermisch A, Brust P, Kretzchmar R, Buhle HJ. On the blood–brain barrier to peptides (3H) beta-casomorphin-5 uptake by eighteen brain regions in vivo. J Neurochem. 1983; 41: 1229–33. 10.3402/mehd.v23i0.18958.
- Husby S, Jensenius JC, Cant AJ. Passage of un-degraded dietary antigen into the blood of healthy adults. Scand J Immunol. 1984; 22: 83–92. 10.3402/mehd.v23i0.18958.
- Seifert J, Ganser R, Brebdel W. Die resorption eines proteolytisches proteins pflanzlichen ursprunge aus dem magen-darm-trakt in das blut und in die lymphe von erwachsenen ratten. Z Gastroenterol. 1979; 17: 1–8.
- Axelsson I, Jacobsson I, Lindberg T, Benediktsson B. Bovine lactoglobulin in human milk. Acta Paediatr Scand. 1986; 75: 702–7. 10.3402/mehd.v23i0.18958.
- Troncone F, Scarcello A, Donatiello A, Cannataro P, Tabusco A, Auricchio S. Passage of gliadin into human breast milk. Acat Paediatr Scand. 1987; 76: 453–6. 10.3402/mehd.v23i0.18958.
- Langer SZ. 25 years since the discovery of presynaptoic receptors: present knowledge and future perspectives. Trends Pharmacol Sci. 1997; 18: 95–9. 10.3402/mehd.v23i0.18958.
- Hadjivassiliou M, Boscolo S, Davies-Jones GAB, Grünewald RA, Not T, Sanders DS, et al. The humoral response in the pathogenesis of gluten ataxia. Neurology. 2002; 58: 1221–6. 10.3402/mehd.v23i0.18958.
- Horvath K, Papadimitriou JC, Rabaztyn A, Drachenberg C, Tildon IT. Gastrointestinal abnormalities in children with autistic disorder. J Pediatr. 1999; 135: 559–63. 10.3402/mehd.v23i0.18958.
- Torrente F, Ashwood P, Day R, Marchado N, Furlano RI, Anthony A, et al. Small intestinal enteropathy with epithelial IgG and complement deposition in children with regressive autism. Mol Psychiatry. 2002; 7: 375–82. 10.3402/mehd.v23i0.18958.
- Wakefield AJ, Anthony A, Murch SH, Thomson M, Montgomery SM, Davies S, et al. Enerocolitis in children with developmental disorders. Am J Gastroenterol. 2000; 95: 2285–95. 10.3402/mehd.v23i0.18958.
- Jyonouchi H, Sun S, Itokazu N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology. 2002; 46: 76–84. 10.3402/mehd.v23i0.18958.
- Heyman M, Desjeux JF. Cytokine-induced alteration of epithelial barrier to food antigens in disease. Ann NY Acad Sci. 2000; 915: 304–11. 10.3402/mehd.v23i0.18958.
- D'Eufemia P, Celli M, Finnochiaro R, Pacifico L, Viozzi L, Zaccagnini M, et al. Abnormal intestinal permeability in children with autism. Acta Paediatr. 1996; 85: 1076–9. 10.3402/mehd.v23i0.18958.
- de Magistris L, Familian V, Pascotto A, Sapone A, From A, Iardino P, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010; 51: 418–24. 10.3402/mehd.v23i0.18958.
- Knivsberg A-M, Wiig K, Lind G, Nødland M, Reichelt KL. Dietary intervention in Autistic syndromes. Brain Dysfunction. 1990; 3: 315–27.
- Lucarelli S, Frediani T, Zingoni AM, Ferruzzi F, Giardini O, Quintieri F, et al. Food allergy and infantile autism. Panminerva Med. 1995; 37: 137–41.
- Whiteley P, Rodgers J, Savery D, Shattock PA. A gluten-free diet as an intervention for autism and associated spectrum disorders: preliminary findings. Autism. 1999; 3: 45–65. 10.3402/mehd.v23i0.18958.
- Klaveness J, Bigam J. The GFCF kids diet survey. In: The autism Res Unit. Sunderland Univ, ed. Building bridges. 2002, pp. 77–84.
- Rimland B. Parent rating of behavioral effects of biomedical interventions. ARI publ. 2003: 34.
- Kniker WT, Andrews A, Hundley A, Garver C. The possible role of intolerance to milk/dairy and wheat/gluten foods in older children and adults with autism spectrum disorder. The autism Res. Unit, Sunderland Univ, ed. ‘2001: An Autism odyssey’. 2001, pp. 183–91.
- Brudnak MA, Rimland B, Kerry RE, Dailey M, Taylor R, Stayton B, et al. Enzyme-based therapy for autism spectrum disorders-is it worth another look?. Med Hypothesis. 2002; 58: 422–8. 10.3402/mehd.v23i0.18958.
- Whiteley P, Haracopos D, Knivsberg A-M, Reichelt KL, Parlar S, Jacobsen J, et al. The Scan Brit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutrit Neurosci. 2010; 13(2): 87–100. 10.3402/mehd.v23i0.18958.
- Pennesi CM, Klein LC. Effectiveness of the gluten free, caseiun free diet for children diagnosed with autism spectrum disorder.Based on parental report. Nutrit. Neurosci. 2012; 15(2): 85–91.
- Knivsberg A-M. Reichelt KL. Høien T. Nødland M. A randomized, controlled study of dietary intervention in Autistic syndromes. Nutrit. Neurosci. 2002; 5: 251–61. 10.3402/mehd.v23i0.18958.
- Knivsberg A-M, Reichelt KL, Nødland M, Høien T. Austistic sundromes and diet: a follow-up study. Scand J. Educat. Res. 1995; 39: 223–36. 10.3402/mehd.v23i0.18958.
Appendix 1
To ensure safer diagnoses a mass spectrometric fragmentation technique is being developed. In the figure below this is demonstrated for five collected autistic spectrum urines and synthetic standard for casomorphin 1–4. Dr K. Elgøen picked out the two best recognition peaks in the fragmentation pattern. A HPLC peak is even if similar, a like peak, but not necessarily the same as the spiked compound.
As can be seen the urine and the synthetic sample are the same. The autistc children were diagnosed by Prof A. Persico, The Open University, Rome, Italy and is cordially thanked for this help.
Fig. 6. Overloading normal person with gluten and gliadin for three days resulted in a demonstrable glumorphin B 5. Topp trace is spiked with standard peptide, bottom trace urine pattern after three days excessive gluten exposure.

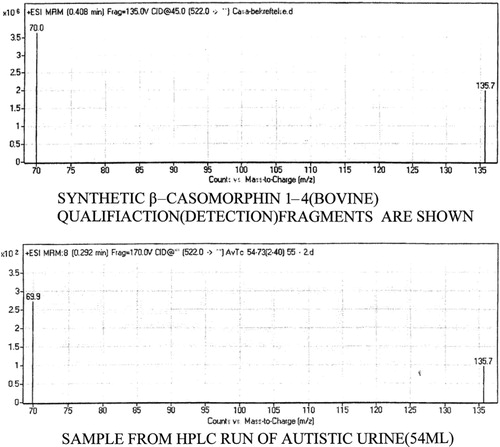
The figure demonstrates qualification ions from standard casomorphin (bovine) 1–4 in the top trace. In the lower trace the same peptide is shown from pooled autistic patients analyzed in a sample equivalent to 250 nanomoles creatinin. They are clearly the same.