Abstract
The human gut microbiota is a complex microbial ecosystem that contributes an important component towards the health of its host. This highly complex ecosystem has been underestimated in its importance until recently, when a realization of the enormous scope of gut microbiota function has been (and continues to be) revealed. One of the more striking of these discoveries is the finding that the gut microbiota and the brain are connected, and thus there is potential for the microbiota in the gut to influence behavior and mental health. In this short review, we outline the link between brain and gut microbiota and urge the reader to consider the gut microbiota as an ecosystem ‘organ’ rather than just as a collection of microbes filling a niche, using the hypothesized role of the gut microbiota in autism spectrum disorder to illustrate the concept.
This paper is part of the Supplement: The Microbiome in Autism Spectrum Disorder. More papers from this supplement can be found at http://www.microbecolhealthdis.net
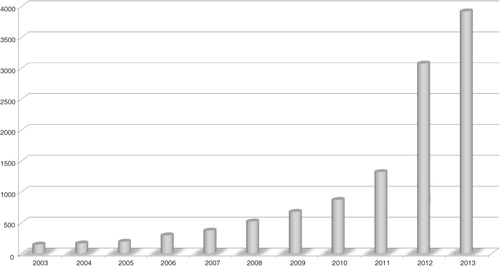
In recent years, research into the human microbiome has captured the imagination of the general public, much in the same way that human genome research permeated public consciousness at the start of the new millennium. As a field of study, human microbiome research has exploded in the last decade (), which has led to a new awareness of the importance of these associated microbes to our overall health. This came as somewhat of a shock to those of us who were raised to think of all microbes as ‘germs’ to be eradicated; instead, we are beginning to see ourselves as microbe managers, tending to the needs of our microbial ‘employees’ for mutual benefit. This short review discusses how human-associated microbes – particularly those in the gut – affect health, and how the widespread phenomenon of gut microbial ‘dysbiosis’ could be driving an epidemic of chronic disease, which may include autism spectrum disorder (ASD).
Origins of the human gut microbiota
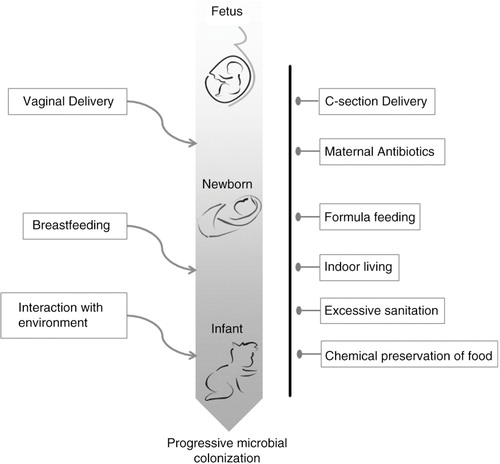
Until recently, babies were believed to be born sterile and only populated by microbes on exposure to their first postdelivery environments (Citation1). However, the process of microbial colonization may begin before birth, with transfer between mother and baby taking place via the placenta (Citation2), and perhaps influenced by changes in the mother's microbiome during pregnancy (Citation3, Citation4). Subsequently, the process of vaginal delivery allows for direct transfer of microbes from the birth canal and the perianal area to the baby Citation5–(Citation7) . Finally, breastfeeding seems to provide and support specific microbes during the early phases of colonization within the infant gut Citation8–(Citation10) . Throughout infancy and early childhood, there are changes in the gut composition that are related to microbial successions, whereby factors such as diet and host immune status appear to confer a ‘permanent resident status’ for some microbes but not others (Citation8, Citation11–Citation13) . This process of building a gut microbiota is still poorly understood, but it is believed to be of critical importance, because there is increasing evidence that a window of time exists for the gut microbiota to develop (Citation13). Beginning at the time of weaning, the microbiota composition stabilizes and matures (Citation12, Citation14); from this point, it can be maintained with only minor changes over many months or years and perhaps even an entire lifetime Citation15–(Citation18) .
Gut microbial ecosystem diversity
The gut microbiota represents one of the densest ecosystems on Earth, and is composed not only of bacteria (which are the most studied components of this niche) but also of Archaea, yeasts, protists, and viruses. Around 500–1,000 different bacterial species may be present in the gut of a given individual (Citation19, Citation20), although at present the species concept in bacteria is imprecise (because of the propensity for bacteria to share genetic information, for example) (Citation21, Citation22). The colon is the most densely populated compartment, with bacterial numbers reaching 1011–1012 cells per gram of content (Citation23). The viral microbiome (the ‘virome’) load is estimated to be higher than that of the bacterial load, with the majority being viruses that infect bacteria and Archaea (bacteriophage) (Citation24, Citation25). Yeasts and other eukaryotes (e.g. protists) are estimated to make up only a small fraction of the colonic microbiota (Citation26, Citation27).
Diet seems to be an important driver of microbial abundance profiles within an ecosystem (Citation28, Citation29). Given that most humans are omnivores with diverse diets, this is not surprising; availability of a large selection of dietary substrates promotes the need for a large variety of metabolic pathways for processing, and it is the gut microbiota that takes on the lion's share of this work for its host (Citation30). The resultant microbial diversity, and consequent functional redundancy within an ecosystem, supports overall ecosystem resilience and stability (Citation31, Citation32). Since resilience and stability largely define the ability of an ecosystem to resist stress, diversity is key to the overall health of the gut microbiota (Citation33).
The functional redundancy of the gut microbiota can also be seen when looking at the human population as a group. There is much variation in the composition of the gut microbiome between individuals, due in large part to the multitude of environmental factors and host genetic influences that work in combination to build a microbiome (Citation30). However, the species variability that can be seen in the microbiomes of different people belies the fact that functionally these microbiomes can be quite similar (Citation34). Even though the exact species content may differ widely, the composite genes of each microbiota as a whole can encode for a very similar group of proteins, or for proteins of similar functions.
Reduced microbial diversity and disease
Having established the importance of microbial community diversity, it is not surprising that a growing body of literature indicates that many chronic diseases are associated with less diverse gut ecosystems (Citation35, Citation36). At the moment, this phenomenon is mainly associative, as it is difficult to ascertain whether reduced diversity occurs as a result of disease or vice versa. However, in some cases (e.g. Clostridium difficile infection, CDI), disease certainly results from a loss of gut microbiota diversity and robustness (Citation36). It is undoubtedly true (both on the micro and macro scale) that ecosystems which lack functional redundancy are more prone to collapse under perturbational stress. An imbalance within the microbial ecosystem (‘dysbiosis’) of the human gut microbiota could result from many different scenarios (), including: insufficient colonization of an infant (e.g. due to Caesarean section) and/or inadequate nursing with breastmilk; exposure to antibiotics, both as short-term therapy as well as long-term pervasive exposure through the food chain; infection with pathogenic microbes; and consumption of a refined, Western-style diet with little fiber [which is an important food source for colonic bacteria (Citation29, Citation37–Citation40) ]. Recently, proponents of the ‘missing microbiota hypothesis’ have warned that modern lifestyles do not sustain a diverse human microbiome, and that the extinction of important ‘keystone’ microbes could lead to the loss of fundamental functional abilities, which would ultimately contribute to dysbiosis and disease (Citation41).
Fig. 2 Pictorial representation of the routes for, and blockages of, microbial colonization of Westernized humans during early life. On the left of the figure, routes of natural colonization are depicted, while on the right, impediments to natural colonization are shown.
From a microbiological perspective, the loss of keystone species from the microbiome may impact the subsequent behavior of the remaining microbes. For example, when C. difficile is present as part of a diverse gut microbiota, it is unlikely to cause problems to the host because its overall abundance and pathogenic behavior are suppressed by the majority presence of the rest of the microbial species in the ecosystem (Citation36). However, loss of ecosystem diversity, usually brought about by antibiotic use, allows C. difficile to proliferate unchecked, and to upregulate virulence determinants that go on to cause disease (Citation42). In this way, C. difficile behaves somewhat like a hoodlum in a subway station; when the subway station is crowded with people, the hoodlum is likely to behave appropriately, perceiving scrutiny by the crowd on the platform. But if the subway station is deserted, the hoodlum may start to vandalize the area, his/her behavior influenced by the lack of surveillance. The case of C. difficile illustrates the importance of the entire gut microbial ecosystem in disease, rather than individual species. Traditional approaches to clinical microbiology have focused largely on the study and surveillance of specific pathogenic microbial species with well-defined virulence determinants, but our simple ‘one microbe-one disease’ models will have to change to incorporate a more complete understanding of microbiome dysbiosis in infection.
Microbial conversations
One of the more startling revelations in the field of microbiology is that microbes are able to communicate with each other using chemical languages, with ‘words’ largely composed of small molecules and peptides (Citation43). Such communication is now known to govern a wide range of functions, including bacterial movement, gene expression, and community structure (Citation44, Citation45). Furthermore, these same signals may impact the host; small molecules, by their nature, can readily pass through host cells and tissues, and may influence host gene expression and behavior as a result (Citation46, Citation47). This process could, in fact, be to our evolutionary advantage, forging a beneficial connection between host and symbionts. Laboratory mice that are reared under germ-free conditions can exhibit behavioral and gene expression patterns that differ from those that are raised in conventional conditions (Citation48, Citation49). Another striking example of the influence of the gut microbiota on behavior was demonstrated by Bercik et al., who showed that switching the gut microbiota of a timid mouse line (C57Bl/6) with that of a more aggressive mouse line (NIH Swiss) resulted in a concurrent switch in behavioral profiles (Citation50). Bacterial colonization of the gut likely modulates host neural development through signaling pathways that include the use of the vagus nerve, a direct conduit between the gut and the central nervous system (and hence the brain) (Citation51). With the new realization of the influence of the gut microbiota on the brain, is it time that certain diseases traditionally thought to be brain disorders be considered as rooted in the gut microbiota?
The concept of ASD as a consequence of gut microbiota damage
ASD is a pervasive developmental disorder of unknown etiology and widely varying severity. Incidence rates of ASD in North America have risen rapidly in recent decades (Citation52, Citation53), and although it is important to note that these figures have been influenced by changes in diagnostic practices, heightened public awareness, and varying research methodology, there remains a dramatic upward trend that can only be partly explained by these aforementioned factors. Although ASD is traditionally thought to have strong inheritance, single gene disorders and chromosomal abnormalities only account for a minority of ASD cases (Citation54), and Genome Wide Association Studies have found hundreds of genetic variations to be potentially linked with ASD (Citation55); these observations argue against the prevailing view that the disorder is purely genetic in nature. Many patients on the severe end of the autism spectrum present with gastrointestinal comorbidities that can include diarrhea, constipation, bloating, and abdominal pain (Citation56). The propensity for associated GI issues in many ASD children has led some researchers to hypothesize a gut microbial involvement in disease. Molecular profiling methods have been used to look for differences in the compositions of the gut microbiotas of ASD and healthy individuals by examining stool samples, and different studies have yielded different results. Song et al. found significant increases in Clostridium bolteae and Clostridium clusters I and XI (Citation57), Finegold et al. noted an increase of Desulfovibrio spp. (Citation58), and Wang et al. observed higher levels of Sutterella and Ruminococcus spp. (Citation59) in individuals with ASD compared to controls. These may not be conflicting findings; instead, these compositional changes could be indicative of gut dysbiosis in ASD, and certainly there is potential here for the development of disease biomarkers. To date, no causative role has been suggested for these, or any other, individual microbial species, and indeed the functional complexity within the gut microbiota argues against a simple one microbe-one disease model.
Given the importance of the functionality of the microbiota over its precise composition, and also taking into account the importance of the gut microbiota in neural development and function, is it possible that a reduction in the gut microbiota diversity leads to a loss of key signals required for normal brain maturation? This could possibly be triggered by the use of antibiotics in early childhood during the critical window for microbiota development, an event that is commonly (if anecdotally) cited by parents of children with ASD; indeed, several studies report increased use of oral antibiotics in children with ASD compared to neurotypical children Citation60–(Citation63) . If this is the case, the study of individual components of the gut microbiota will likely not be fruitful as the gut microbial ecosystem as a whole entity must be considered. This is currently limited by technical constraints that we and others are trying to address. Advances in the ability to culture whole gut microbial ecosystems in vitro will allow a more holistic view of the structure of the gut microbiota, as well as its potential function in the context of ASD. For example, continuous culture (chemostat) systems allow for the culture of whole, explanted, gut ecosystems under tightly controlled experimental conditions (Citation64), with the added advantage that small molecule metabolites produced by the resident ecosystem can be easily captured and characterized (unpublished observations).
Looking to the future
Is ASD a gut-mediated disease? Clearly there is much work to be done to gain a fuller understanding of what is involved in the etiology of this complex disorder. As with other complex chronic diseases (such as inflammatory bowel disease), ongoing research will likely draw us to the intersection between host genetics and epigenetics, microbiota structure and function, and environmental cues. However, this new and developing view of ASD etiology presents an additional avenue for study.
Conflict of interest and funding
E.A-V is a cofounder of Nubiyota LLC, a company that is engaged in developing and commercializing therapeutic microbial ecosystems for medical use.
Notes
This paper is part of the Supplement: The Microbiome in Autism Spectrum Disorder. More papers from this supplement can be found at http://www.microbecolhealthdis.net
References
- Mackie RI , Sghir A , Gaskins HR . Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999; 69: 1035S–45S. [PubMed Abstract].
- Aagaard K , Ma J , Antony KM , Ganu R , Petrosino J , Versalovic J . The placenta harbors a unique microbiome. Sci Transl Med. 2014; 6: 237ra65.
- Aagaard K , Riehle K , Ma J , Segata N , Mistretta TA , Coarfa C , etal. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012; 7: e36466.
- Koren O , Goodrich JK , Cullender TC , Spor A , Laitinen K , Backhed HK , etal. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012; 150: 470–80.
- Schultz M , Gottl C , Young RJ , Iwen P , Vanderhoof JA . Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr. 2004; 38: 293–7.
- Makino H , Kushiro A , Ishikawa E , Muylaert D , Kubota H , Sakai T , etal. Transmission of intestinal Bifidobacterium longum subsp. longum strains from mother to infant, determined by multilocus sequencing typing and amplified fragment length polymorphism. Appl Environ Microbiol. 2011; 77: 6788–93.
- Dominguez-Bello MG , Costello EK , Contreras M , Magris M , Hidalgo G , Fierer N , etal. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010; 107: 11971–5.
- Penders J , Thijs C , Vink C , Stelma FF , Snijders B , Kummeling I , etal. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006; 118: 511–21.
- Gura T . Nature's first functional food. Science. 2014; 345: 747–9.
- De Leoz ML , Kalanetra KM , Bokulich NA , Strum JS , Underwood MA , German JB , etal. Human milk glycomics and gut microbial genomics in infant feces shows correlation between human milk oligosaccharides and gut microbiota: a proof-of-concept study. J Proteome Res. 2014. [Epub ahead of print]..
- Eggesbo M , Moen B , Peddada S , Baird D , Rugtveit J , Midtvedt T , etal. Development of gut microbiota in infants not exposed to medical interventions. APMIS. 2011; 119: 17–35.
- Schloss PD , Iverson KD , Petrosino JF , Schloss SJ . The dynamics of a family's gut microbiota reveal variations on a theme. Microbiome. 2014; 2: 25.
- Voreades N , Kozil A , Weir TL . Diet and the development of the human intestinal microbiome. Front Microbiol. 2014; 5: 494.
- Koenig JE , Spor A , Scalfone N , Fricker AD , Stombaugh J , Knight R , etal. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011; 108: 4578–85.
- Costello EK , Lauber CL , Hamady M , Fierer N , Gordon JI , Knight R . Bacterial community variation in human body habitats across space and time. Science. 2009; 326: 1694–7.
- David LA , Materna AC , Friedman J , Campos-Baptista MI , Blackburn MC , Perrotta A , etal. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014; 15: R89.
- Jalanka-Tuovinen J , Salonen A , Nikkila J , Immonen O , Kekkonen R , Lahti L , etal. Intestinal microbiota in healthy adults: temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS One. 2011; 6: e23035.
- Martinez I , Muller CE , Walter J . Long-term temporal analysis of the human fecal microbiota revealed a stable core of dominant bacterial species. PLoS One. 2013; 8: e69621.
- Macfarlane GT , Macfarlane S . Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012; 95: 50–60.
- Zhu B , Wang X , Li L . Human gut microbiome: the second genome of human body. Protein Cell. 2010; 1: 718–25.
- Georgiades K , Raoult D . Defining pathogenic bacterial species in the genomic era. Front Microbiol. 2010; 1: 151. [PubMed Abstract] [PubMed CentralFull Text].
- Hanage WP , Fraser C , Spratt BG . Fuzzy species among recombinogenic bacteria. BMC Biol. 2005; 3: 6.
- Mai V , Morris JG Jr. . Colonic bacterial flora: changing understandings in the molecular age. J Nutr. 2004; 134: 459–64. [PubMed Abstract].
- Mills S , Shanahan F , Stanton C , Hill C , Coffey A , Ross RP . Movers and shakers: influence of bacteriophages in shaping the mammalian gut microbiota. Gut Microbes. 2013; 4: 4–16.
- Reyes A , Haynes M , Hanson N , Angly FE , Heath AC , Rohwer F , etal. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature. 2010; 466: 334–8.
- Parfrey LW , Walters WA , Lauber CL , Clemente JC , Berg-Lyons D , Teiling C , etal. Communities of microbial eukaryotes in the mammalian gut within the context of environmental eukaryotic diversity. Front Microbiol. 2014; 5: 298.
- Schulze J , Sonnenborn U . Yeasts in the gut: from commensals to infectious agents. Dtsch Arztebl Int. 2009; 106: 837–42. [PubMed Abstract] [PubMed CentralFull Text].
- Arumugam M , Raes J , Pelletier E , Le Paslier D , Yamada T , Mende GR , etal. Enterotypes of the human gut microbiome. Nature. 2011; 473: 174–80.
- Sonnenburg ED , Sonnenburg JL . Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014; 20: 778–86.
- Human Microbiome Project C . Structure, function and diversity of the healthy human microbiome. Nature. 2012; 486: 207–14.
- Costello EK , Stagaman K , Dethlefsen L , Bohannan BJ , Relman DA . The application of ecological theory toward an understanding of the human microbiome. Science. 2012; 336: 1255–262.
- Mahowald MA , Rey FE , Seedorf H , Turnbaugh PJ , Fulton RS , Wollam A , etal. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci USA. 2009; 106: 5859–64.
- Lozupone CA , Stombaugh JI , Gordon JI , Jansson JK , Knight R . Diversity, stability and resilience of the human gut microbiota. Nature. 2012; 489: 220–30.
- Ferrer M , Ruiz A , Lanza F , Haange SB , Oberbach A , Till H , etal. Microbiota from the distal guts of lean and obese adolescents exhibit partial functional redundancy besides clear differences in community structure. Environ Microbiol. 2013; 15: 211–26.
- Mondot S , de Wouters T , Dore J , Lepage P . The human gut microbiome and its dysfunctions. Dig Dis. 2013; 31: 278–85.
- Van den Abbeele P , Verstraete W , El Aidy S , Geirnaert A , Van de Wiele T . Prebiotics, faecal transplants and microbial network units to stimulate biodiversity of the human gut microbiome. Microb Biotechnol. 2013; 6: 335–40.
- Antunes LC , Finlay BB . A comparative analysis of the effect of antibiotic treatment and enteric infection on intestinal homeostasis. Gut Microbes. 2011; 2: 105–108.
- Jones ML , Ganopolsky JG , Martoni CJ , Labbe A , Prakash S . Emerging science of the human microbiome. Gut Microbes. 2014; 5: 446–57.
- Neu J , Rushing J . Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol. 2011; 38: 321–31.
- Riley LW , Raphael E , Faerstein E . Obesity in the United States – dysbiosis from exposure to low-dose antibiotics?. Front Public Health. 2013; 1: 69.
- Blaser MJ , Falkow S . What are the consequences of the disappearing human microbiota?. Nat Rev Microbiol. 2009; 7: 887–94.
- Britton RA , Young VB . Role of the intestinal microbiota in resistance to colonization by Clostridium difficile . Gastroenterol. 2014; 146: 1547–53.
- McNab R , Lamont RJ . Microbial dinner-party conversations: the role of LuxS in interspecies communication. J Med Microbiol. 2003; 52: 541–5.
- Daniels R , Vanderleyden J , Michiels J . Quorum sensing and swarming migration in bacteria. FEMS Microbiol Rev. 2004; 28: 261–89.
- Li Z , Nair SK . Quorum sensing: how bacteria can coordinate activity and synchronize their response to external signals?. Protein Sci. 2012; 21: 1403–17.
- Mayer EA , Savidge T , Shulman RJ . Brain-gut microbiome interactions and functional bowel disorders. Gastroenterol. 2014; 146: 1500–12.
- Russell WR , Hoyles L , Flint HJ , Dumas ME . Colonic bacterial metabolites and human health. Curr Opin Microbiol. 2013; 16: 246–54.
- Diaz Heijtz R , Wang S , Anuar F , Qian Y , Bjorkholm B , Samuelsson A , etal. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011; 108: 3047–52.
- Neufeld KM , Kang N , Bienenstock J , Foster JA . Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011; 23: 255–64. [PubMed Abstract] e119.
- Bercik P , Denou E , Collins J , Jackson W , Lu J , Jury Y , etal. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterol. 2011; 141: 599–609. e591–3.
- Montiel-Castro AJ , Gonzalez-Cervantes RM , Bravo-Ruiseco G , Pacheco-Lopez G . The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Front Integr Neurosci. 2013; 7: 70.
- Developmental Disabilities Monitoring Network Surveillance Year Principal I, Centers for Disease C, & Prevention. Prevalence of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014; 63: 1–21.
- Mitka M . Rising autism rates still pose a mystery. JAMA. 2010; 303: 602.
- Hu WF , Chahrour MH , Walsh CA . The diverse genetic landscape of neurodevelopmental disorders. Annu Rev Genomics Hum Genet. 2014; 15: 195–213.
- Jiang YH , Yuen RK , Jin X , Wang M , Chen N , Wu X , etal. Detection of clinically relevant genetic variants in autism spectrum disorder by whole-genome sequencing. Am J Hum Genet. 2013; 93: 249–63.
- McElhanon BO , McCracken C , Karpen S , Sharp WG . Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014; 133: 872–83.
- Song Y , Liu C , Finegold SM . Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004; 70: 6459–65.
- Finegold SM , Dowd SE , Gontcharova V , Liu C , Henley KE , Wolcott RD , etal. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010; 16: 444–53.
- Wang L , Christophersen CT , Sorich MJ , Gerber JP , Angley MT , Conlon MA , etal. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013; 4: 42.
- Adams JB , Holloway CE , George F , Quig D . Analyses of toxic metals and essential minerals in the hair of Arizona children with autism and associated conditions, and their mothers. Biol Trace Elem Res. 2006; 110: 193–209.
- Adams JB , Romdalvik J , Ramanujam VM , Legator MS . Mercury, lead, and zinc in baby teeth of children with autism versus controls. J Toxicol Environ Health A. 2007; 70: 1046–51.
- Konstantareas MM , Homatidis S . Ear infections in autistic and normal children. J Autism Dev Disord. 1987; 17: 585–94.
- Niehus R , Lord C . Early medical history of children with autism spectrum disorders. J Dev Behav Pediatr. 2006; 27: S120–27.
- McDonald JA , Schroeter K , Fuentes S , Heikamp-Dejong I , Khursigara CM , de Vos WM , etal. Evaluation of microbial community reproducibility, stability and composition in a human distal gut chemostat model. J Microbiol Methods. 2013; 95: 167–74.