Abstract
Avian intestinal spirochaetosis (AIS) is a common disease occurring in poultry that can be caused by Brachyspira pilosicoli, a Gram-negative bacterium of the order Spirochaetes. During AIS, this opportunistic pathogen colonises the lower gastrointestinal (GI) tract of poultry (principally, the ileum, caeca, and colon), which can cause symptoms such as diarrhoea, reduced growth rate, and reduced egg production and quality. Due to the large increase of bacterial resistance to antibiotic treatment, the European Union banned in 2006 the prophylactic use of antibiotics as growth promoters in livestock. Consequently, the number of outbreaks of AIS has dramatically increased in the UK resulting in significant economic losses. This review summarises the current knowledge about AIS infection caused by B. pilosicoli and discusses various treatments and prevention strategies to control AIS.
Controlled animal husbandry is essential in order to ensure safe and sustainable food production. Animal husbandry is commonly practiced in developed and some developing countries (Citation1) as reported by the USDA and Eurostat (Citation2, Citation3). The constant optimisation of breeding techniques and increased production efficiencies has reduced significantly the price of meat and dairy products over the years, providing wider access to products derived from animals in countries where they were not traditionally consumed (Citation1). Therefore, there is a growing interest in improving breeding methods to improve animal welfare, reduce production costs, and ensure higher safety and better quality for consumers. In this context, it is particularly relevant to reduce diseases of animal production, especially those that have zoonotic potential. Gastrointestinal (GI) diseases are common in production animals, and their incidence has increased in large-scale farming industry due to intensive farming practices, which facilitate rapid spread of infection between animals (Citation4). GI disorders in such facilities often result from the colonisation of the GI tract by pathogenic microorganisms, particularly at certain times in the production cycle such as weaning (Citation5). Brachyspira pilosicoli that induces intestinal spirochaetosis (IS) is an emerging pathogen causing infections in a number of species, including poultry, which is the subject of this review. While Brachyspira spp. are found in intensive husbandry, Brachyspira spp. infection are particularly common in free-range and organic farms (Citation6, Citation7) due to the higher exposure of flocks to wild birds and the environment, which act as infection vectors/reservoirs (Citation7).
IS is a generic name given to largely diarrhoeal disease caused by the colonisation of the lower GI tract by Spirochaetes of the genus Brachyspira, and more specifically for poultry by B. pilosicoli (Citation4, Citation7), B. alvinipulli (Citation8), and B. intermedia that are Gram-negative, spiral organisms with flexible outer-membrane and inter-membrane polar flagella (8–10 depending on species) possessing single circular genome comprising 4–5,000 genes and a guanine–cytosine (GC) ratio of 27%. Pathogenic Brachyspira spp are presented in with their host range and pathogenicity (Citation9). Other Brachyspira spp. (not listed for brevity) are non-pathogenic but may be found in mixed infections. Also B. hampsonii is a newly described type pathogen in several species including poultry, which is yet to be defined and accepted as a new species. B. pilosicoli is an opportunistic pathogen generally associated with swine and poultry but has also been reported to infect other animals including dogs, horses, monkeys, turkeys, geese, and humans (Citation10–Citation13).
Table 1 List of Brachyspira species, their host, and pathogenicity
Avian intestinal spirochaetosis (AIS), caused by the colonisation of the lower GI tract by bacteria of the genus Brachyspira in birds, generally occurs in breeder and egg-laying chickens but also increasingly in broilers. The infection triggers severe diarrhoea accompanied by loss of weight, which has been associated with increased morbidity among flocks with 5–10% mortality if untreated with concurrent loss of egg production in layers (Citation14–Citation17). It often occurs by transmission of the spirochaetes via the faecal–oral route and can be transferred between livestock buildings by farmers (Citation4, Citation18) (Citation19). Increasing number of recent publications have reported the presence of Brachyspira species in farms all over the world (Citation20). This observation could result from several parameters such as the 2006 European Union ban on the prophylactic use of antibiotics (Citation4, Citation21), the modification of animal housing, and finally the development of improved detection methods for this specific genus (Citation22, Citation23). Thus, the impact of this disease on animal welfare and production is of high concern to the poultry industry enhancing needs for novel intervention strategies to reduce the spread of AIS.
Here, we review the current knowledge on AIS caused by B. pilosicoli and discuss the therapeutic and prophylactic strategies currently investigated (including antibiotics and probiotics). Vaccine development to protect from swine dysentery (SD), a disease caused by Brachyspira hyodysenteriae infection in pigs (Citation24), is also in process. Similarly, the development of autogenous vaccines for AIS (Citation25) is just emerging. As progress regarding these interventions is still extremely limited, it will not be discussed further in this review.
Overview of the disease
Signs and symptoms
B. pilosicoli-induced AIS is generally observed in egg-laying chickens over 10 weeks old in large rearing farms (Citation4, Citation26). Numerous cases have been reported worldwide, especially in Europe, the US, and Australia, where intensive farming offers suitable conditions for development and spread of various GI infections including those caused by Brachyspira.
Symptoms of infections by B. pilosicoli can range from asymptomatic to severe, leading to increased mortality rates in chickens (Citation4, Citation27). Nevertheless, the most common mild/moderate infections are generally characterised by diarrhoea, faeces with altered colour and consistency, which are frequently foamy due to increased gas production (Citation28), so-called cappuccino faeces. This may progress to faeces containing mucus and blood (Citation27). Diagnosis is generally confirmed via bacterial culture or PCR (Citation29).
AIS infection results in a slower growth rate (Citation28–Citation30) and can also be associated with a delay of up to 7 weeks in the onset of lay accompanied by a decrease in egg quality (Citation28–Citation31). Eggs produced by infected hens are usually small, lighter in weight (i.e. 2–6 g less per egg) (Citation28), and are less numerous. Poor-quality shells are prone to cracks and often contaminated by faeces (Citation32). Yolks are generally less coloured with a decrease of 1.5–3 points on the Roche yolk colour fan (Citation28, Citation33). Furthermore, it has been suggested that infection may have long-term consequences on the second generation of chickens hatched from eggs laid by infected hens (Citation28). Indeed, it has been shown that chicks hatched from eggs laid from infected female parents presented similar symptoms (i.e. decreased weight gain, delayed lay onset, wetter and paler faeces) despite the absence of contamination (Citation28). These results raise new hypotheses regarding potential epigenetic variations in response to B. pilosicoli infection.
At a microscopic level, intestinal biopsies of infected chickens displaying the symptoms described above usually reveal the presence of B. pilosicoli fixed to the cells of the intestinal wall (Citation27), which is suspected to be correlated with the degree of enterocyte perturbation (Citation27). Tissues look inflamed, often with some signs of bleeding. The intestinal wall shows evidence of a loss of microvilli (Citation21). The loss of microvilli results in perturbation of the epithelial barrier permeability, which may contribute to the decrease in weight gain and the increased amount of water in faeces. The cytoplasm of enterocytes appears damaged as indicated by abnormal vacuolation, condensation, and fragmentation of the chromatin and cell sloughing (Citation21). This is likely to result in lower nutrient absorption as indicated by increased food consumption in infected chickens (Citation31) accompanied by increased faecal lipid content concomitant with decreased lipid levels in the general circulation (Citation34). The same phenomenon has been observed for carotenoid concentration, which has been found in higher quantity in faeces of infected animals, while lower in blood, and is believed to be the cause of weakened colour intensity of the yolk (Citation28, Citation34).
Characteristics and mechanism of infection
Morphology
B. pilosicoli is a bacterium of the order Spirochaetales, morphologically characterised by a corkscrew-like shape (Citation35) (). It was first identified as a cause of IS in Denmark in 1982 (Citation27). B. pilosicoli can be found in the literature under the former name of Serpulina pilosicoli (Citation27, Citation36). It is a Gram-negative, fastidious, aerotolerant anaerobe that can be exposed to oxygen for a few hours (Citation4, Citation37). The optimum growth temperature is 38.5°C (Citation27), but it can remain viable for 66 days at 4°C in water and survive up to 210 days in pig faeces mixed with soil at 10°C (Citation37).
Fig. 1 Transmission electron microscopy illustrating the flagella of Spirochaetaceae. (A) [Adapted with permission from Yano et al. (Citation40)]. (B) Graphic representation of picture A enhancing the visualisation of the flagella.
![Fig. 1 Transmission electron microscopy illustrating the flagella of Spirochaetaceae. (A) [Adapted with permission from Yano et al. (Citation40)]. (B) Graphic representation of picture A enhancing the visualisation of the flagella.](/cms/asset/8227d47c-b70c-4444-bb7b-97ac2e8d7a9e/zmeh_a_11816025_f0001_ob.jpg)
B. pilosicoli is constituted of a central protoplasmic cylinder covered by a membrane sheet (Citation27). The membrane sheet, also known as the outer membrane, is an important element for the integrity of the bacterium. Several studies have shown that a perturbation of the membrane generally causes the destruction of the flagella and of the periplasmic membrane (Citation38). The composition of the outer membrane is not entirely known despite its high relevance to host-pathogen interactions. Yet it has been shown to be extremely labile due to its high content in sterols (cholesterol and cholestanol), which are responsible for a low resistance to osmotic stress and to low ionic buffers that trigger its destabilisation (Citation39). Between the outer membrane and the protoplasmic cylinder is the periplasm, where the flagella of the bacteria are located. B. pilosicoli possesses between 8 and 10 flagella disposed equally at the poles at each end of the bacterium following the corkscrew shape of the bacterium and overlapping in the centre (Citation4, Citation27) (). This configuration is specific to the Spirochaetes and confers high motility, which constitutes an important virulence factor. The flagella works by producing helical or flat sinusoidal waves (Citation34), which induce a clockwise or anticlockwise movement of the bacteria and enable a non-transversal swim (Citation41, Citation42). A transversal swim is also possible by the simultaneous combination of the two movements (Citation41). Both modes of movement provide B. pilosicoli with the ability to swim through viscous media (Citation43).
Infection process
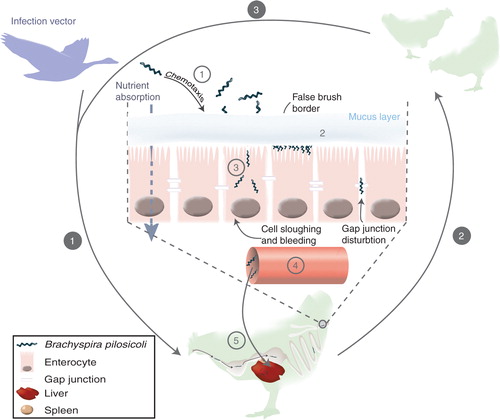
B. pilosicoli infects the lower GI tract of chickens, swine, horses, dogs, humans, and other animals (Citation37). Upon entry via the oral cavity, the bacterium that survives passage through the stomach acid reaches the intestinal lumen. Using chemotaxis, the organism migrates towards the mucus and the intestinal wall (Citation44, Citation45). Indeed, B. pilosicoli has a high number of genes coding for chemotaxis towards the mucus in comparison to other known bacterial species, providing a significant advantage to colonise the host (Citation46). The mucus is a viscous matrix composed of two stratums, the inner and outer layers, which form a physical barrier and protect the intestinal cells from bacterial infections by limiting their motility (Citation44). The unique shape of B. pilosicoli, combined with the production of specific enzymes that hydrolyse the mucus inner layer (sialidase family-like proteins), confers them the ability to swim through this medium and allow them to reach the cell wall (Citation42, Citation44). These are high virulence factors associated with tissue damage (Citation46). Another virulence factor may be the noted sensitivity of B. pilosicoli to the chemo-attractant serine, which is found in high concentration in the mucus secreted by goblet cells (Citation37, Citation38) (Citation47).
Once the bacterium is in contact with an intestinal cell, fixation occurs through protein–protein interactions (Citation48), although the exact mechanism has not been fully ascertained. B. pilosicoli attaches vertically to the cell wall by one of its cylinder ends (Citation36, Citation48) and can be found very closely packed on the cell at a density ranging 20–80 bacteria per cell, forming a ‘false brush border’ (Citation37, Citation46). Attachment of the bacterium is not necessarily associated with symptoms of IS (Citation4, Citation37) but an increase in bacterial concentration appears to be directly linked to the intensity of the symptoms (Citation37) as previously mentioned. Adherence of B. pilosicoli to the cell membrane triggers a signal that results in invagination of the apical membrane and internalisation of the bacteria potentially resulting in cell apoptosis. B. pilosicoli can also cross the intestinal barrier by disrupting gap junctions (between cells), which in some cases may allow it to enter the blood stream (Citation4, Citation49). Indeed systemic spread of B. pilosicoli has been reported in one study showing evidence of colonisation of the spleen and liver (Citation50). However, this was not commonly observed, and the mechanism by which the bacterium escapes the immune system is not known yet. The infection process is summarised in .
Fig. 2 Transmission and infection process of Brachyspira pilosicoli. White numbers on grey circles describe the contamination process: 1, transmission of contaminated material in a farm via a vector – wild animals, farmers, water, and other farm animals – to a housed bird via oral route; 2, transmission of the bacterium to the rest of the flock; 3, persistence of infection between birds of a same folk. Grey numbers in white circles describe the infection process once B. pilosicoli has reached the lower digestive tract: 1, chemotaxis attraction of the bacteria towards the mucus and cell wall; 2, attachment of B. pilosicoli on the cells and formation of a ‘false brush border’; 3, invasion of intestinal cells; 4, translocation to the blood stream; 5, systemic infection.
Genetic features
In addition to the aforementioned genetic functions, a recent publication of B. pilosicoli B2904 complete genome by Mappley et al. (Citation46) () identified key genes responsible for some of B. pilosicoli infection- and colonisation-related processes, such as chemotaxis, mobility, adhesion, and host tissue degradation. The B. pilosicoli genome analysis also provided new insights into its metabolism. It revealed numerous genes involved in carbohydrate transport and metabolism, such as phosphoglucomutase that plays a key role in glycolysis. These genetic observations correlated to phenotypic tests using Biolog® technology (which evaluates the cell's ability to respire on a wide range of substrates) demonstrated the ability of B. pilosicoli to use several types of saccharides (e.g. glucose-6-phosphate) and oligosaccharides (e.g. dextrin) as primary carbon sources. Finally, another large section of the genome was allocated to amino acid synthesis and transport. Those results represent a major advancement towards understanding the interrelationship between metabolism and infection.
Fig. 3 Circos circular representation of the complete B. pilosicoli B2904 genome with annotated genes. The genome is orientated from the oriC and also displays the location of dnaA. Circles range from 1 (outer circle) to 7 (inner circle). Circle 1, COG-coded forward strand genes; circle 2, COG-coded reverse strand genes; circle 3, forward strand tRNA; circle 4, reverse strand tRNA; circle 5, forward strand rRNA; circle 6, reverse strand rRNA; circle 7, GC skew ((G-C)/(G + C); red indicates positive values; green indicates negative values). All genes are colour coded according to Cluster of Orthologous Group (COG) functions shown in the key table. [Adapted with permission from Mappley et al. 2012 (Citation46)].
![Fig. 3 Circos circular representation of the complete B. pilosicoli B2904 genome with annotated genes. The genome is orientated from the oriC and also displays the location of dnaA. Circles range from 1 (outer circle) to 7 (inner circle). Circle 1, COG-coded forward strand genes; circle 2, COG-coded reverse strand genes; circle 3, forward strand tRNA; circle 4, reverse strand tRNA; circle 5, forward strand rRNA; circle 6, reverse strand rRNA; circle 7, GC skew ((G-C)/(G + C); red indicates positive values; green indicates negative values). All genes are colour coded according to Cluster of Orthologous Group (COG) functions shown in the key table. [Adapted with permission from Mappley et al. 2012 (Citation46)].](/cms/asset/342db484-9ba4-4a46-9b3e-2f34d5304fd2/zmeh_a_11816025_f0003_ob.jpg)
Impact on the food chain: a zoonotic potential?
Intestinal spirochaetosis is relatively rare in humans as it occurs mostly in immunocompromised patients. In most cases, carriage by the host of the bacteria is often asymptomatic, but following the apparition of any symptoms such as diarrhoea and abdominal pain, IS is confirmed by biopsy (Citation51, Citation52). Only in rare cases did an infection by B. pilosicoli cause death of a patient as a result of septicaemia (Citation53). Such cases have only been observed in elderly and immunocompromised patients or in populations living in dense areas with poor hygiene conditions (Citation54–Citation56).
Despite the rare occurrence of the disease in humans, a major concern is the zoonotic potential of the bacterium (Citation49). Indeed, it has been suggested that B. pilosicoli is able to survive and be transmitted to the consumer via contaminated raw meat from infected chicken (Citation57). Several studies have shown considerable genetic similarities between strains of B. pilosicoli infecting humans, swine, and poultry, suggesting an ability to adapt to various hosts (Citation49). In 2012, Mappley et al. (Citation46) carried out a genetic comparison of three strains of B. pilosicoli isolated from humans, chickens, and pigs, respectively. This study showed that the genotype of these three strains were very similar. However, some differences were noted in the genome size and arrangement and in some putative coding regions for carbohydrate, amino acid, and nucleotide metabolism and transport (Citation46). These data highlighted some fundamental genetic differences that are reflected in their phenotype and may have implications in host specificity and interspecies transmission (Citation46), although this has remained untested till date. More structural rearrangements were observed in the strains isolated from chicken and human in comparison to the strains isolated from pig. Despite these variations, the functional genome comparison showed a high level of similarity in the features of the three strains except for the aforementioned transporters and enzymes (Citation46). Additionally, genes involved in membrane fixation and in β-haemolysis were common to the three strains, which suggests a similar invasion and infection process between the bacteria (Citation46). These genetic and phenotypic data indicate a high degree of similarity in infection processes across species and may support the potential of transmission of bacteria causing IS from farm animals to humans (Citation49) and, therefore, is a realistic issue that requires attention. Prevention of IS spread in animal livestock is currently achieved using antibiotics.
Antibiotics: a controversial solution
Various antibiotics such as the pleuromutilins, macrolides, and lincosamides are currently used to control Brachyspira infections in animals and have been shown to reduce associated symptoms (Citation58, Citation59). The most common antibiotic used in animal husbandry is tiamulin, a member of the pleuromutilin family. By binding with the 50S region of the ribosome, it inhibits amino acid binding during protein synthesis (Citation60). Tiamulin is used widely and has been shown to be efficient at controlling SD, which is a severe GI disease in pigs caused by B. hyodysenteriae, a close relative of B. pilosicoli, at a dose of 7.71 µg/kg of body weight for a 5-day treatment. Nevertheless, the lack of standardised methods and techniques used to calculate the minimum inhibitory concentration (MIC) induces a large disparity in published results. Only two studies describing the impact of tiamulin on B. pilosicoli-induced AIS in chicken have been reported – in 2002, in experimentally infected laying hens (Citation61), and in 2006, in a UK field study (Citation62). Results suggest a positive impact of tiamulin treatment in both studies with a general increase in growth rate, egg production, and decrease of symptoms. Another customer concern is the possible presence of antibiotics and their metabolites in eggs, although this has not been reported in the literature. One report issued by the European Medicine Agency mentioned very low antibiotic residual levels, but these were not sufficient to establish a withdrawal period for eggs [Article 34Citation1 of Directive 2001/82/EC (Citation63)]. Nonetheless, a withdrawal period of 24 h should be applied for meat consumption [Article 34Citation1 of Directive 2001/82/EC (Citation63)].
Furthermore, emerging bacterial resistance to antibiotics is another major concern (Citation58, Citation64). For example, tylosin was a commonly used antibiotic to treat AIS, but resistance has recently emerged, compromising its efficiency and therefore its usage (Citation65). Resistance factors appear as a consequence of an extensive use of antibiotics concomitant with the development of mutations in the bacteria such as on the ribosomal protein (Citation66), which render them less susceptible. This stresses the importance of bacteriological diagnosis that should be used to determine precisely the Brachyspira species responsible of infection followed by antibiotic resistance test on pure culture in order to apply appropriate treatment. In response to the global rise of bacterial resistance and to protect the consumer's safety, the European Commission banned the prophylactic use of antibiotics in livestock in 2006 (Citation67). Indeed, chickens grown in industrial farms used to receive prophylactic antibiotic treatments, which was also associated with increased animal fattening rate (Citation67). Since this interdiction, infection outbreaks by B. pilosicoli have boomed (Citation21, Citation67). Common consequences include reduced egg production, growth delay, higher food consumption, and, in some cases, increased mortality within infected flocks. Since 2006, the economic loss associated with AIS has been estimated to be of approximately £18 million per year in the UK (Burch, D. J. S., 2009 personal communication) pointing to the need for better prevention methods and refined treatments. Prevention of AIS outbreaks can be achieved using appropriate hygiene and biosecurity rules as demonstrated by several studies (Citation68). B. pilosicoli is readily eliminated by standard farm disinfection processes (Citation69), and the potential of vaccination against B. pilosicoli has been explored primarily in pigs and may be applicable in poultry (Citation70). However, treatment is commonly achieved using antibiotics such as linco-spectin and tiamulin at 25 mg/kg of body weight per day, although this dosage regimen is derived from studies in pigs. Recently, we investigated the optimum dose to treat laying hens and demonstrated that 250 ppm given in drinking water over 3 days reduce infection significantly, but the bacterium was still detectable at the end of the study (3 weeks after the end of the treatment) (Citation71). Notwithstanding the use of antibiotic for intervention, it remains crucial to find alternative solutions to prevent AIS to protect animal welfare and consumers.
Probiotics: a potential solution?
The gut microbiota (GM) is estimated to be composed of more than 1,000 species of bacteria (Citation68) which are predominantly Gram-negative (Citation72). They exert an important role for the host as they are involved in its protection from pathogens and in the release of nutrients from the diet, which would otherwise be unavailable to the host (Citation72). Beyond the positive impact of commensal bacteria on the digestive system and associated nutritional benefits, increasing evidence reveals a systemic impact of the GM on the host (Citation73). Probiotics, which are defined as ‘live microorganisms which when administered in adequate amount confer a health benefit on the host’ (Citation74), have been developed to take advantage of this symbiosis. Protection is achieved by increasing the competition between the probiotic and pathogens for cell membrane receptors and nutrients, modulation of the immune system, improvement of the mucosal barrier permeability, secretion of toxins, and lowering the pH of the GI (Citation75, Citation76). Their mechanisms of action vary depending on the probiotic, but most of them remain largely misunderstood.
Only a few studies have investigated the impact of Lactobacillus-based probiotics on B. pilosicoli, and most of them have been carried out in vitro. It has been shown that lactic acid secreted by lactobacilli has similar effects as other acidic compounds and disinfectants on B. pilosicoli, whereby the bactericidal effect is mediated by destabilisation of the cellular wall hence reducing the bacterial viability (Citation77). Another interesting effect of lactic acid is that it induces the formation of ‘spherical bodies’ formed by the retraction and swelling of both ends of the bacterium, which tends to create a sphere shape. At this stage, the bacterium is still viable but in a dormant state (Citation78).
Two promising Lactobacillus species to tackle AIS are L. salivarius and L. reuteri. They are both recognised by the European Food Safety Authority (EFSA), generally regarded as safe (GRAS) and suitable for livestock feeding (Citation79). A recent study has shown that both lactobacilli antagonise motility, growth, and cellular adherence of B. pilosicoli (Citation21). In vitro, it appears that the presence of L. reuteri and L. salivarius reduces markedly the potential of B. pilosicoli to induce apoptosis of intestinal cells (Citation21) by antagonising adhesion to the intestinal epithelium, in a process of competitive exclusion. An in vivo study indicated that Lactobacillus probiotic can prevent potential infection and associated symptoms caused by the pathogen if administered before or during challenge with B. pilosicoli (Citation80), supporting its efficiency as a protective agent against AIS.
Another advantage of using probiotics in farms is their potential as animal growth promoters when used as prophylactic (Citation81–Citation83). In a study by Yoruk et al. (Citation82), it was demonstrated that probiotic consumption by laying hens resulted in decreased mortality and increased egg production without altering quality. Furthermore, the consumption of Lactobacillus-based probiotic during the first 3 weeks of life was shown to increase animal growth, demonstrating their potential as growth promoters in the early stages of life (Citation83). Probiotics may also be useful to prevent infection relapse that is often observed with AIS. Indeed by maintaining a healthy and balanced gut environment, probiotic could potentially be used in order to inhibit B. pilosicoli recurrence post–antibiotic treatment (Citation20).
Conclusions
B. pilosicoli-induced AIS is a growing and underestimated problem in the poultry industry. However, its occurrence and economic burden is not negligible. Antibiotics such as tiamulin are still considered as a gold standard to tackle the infection, although resistance is emerging, which stimulates the need for the development of new interventions. Despite these promising novel therapies, there remains a large gap in the understanding of the pathogen itself, particularly its metabolism, although some new insights were given recently by the genetic mapping of a few strains of B. pilosicoli. Characterising these pathways would provide a major advantage in AIS understanding in order to design more targeted treatments. Finally, combination therapies that use an antibiotic followed by an appropriate probiotic may be worthy of consideration to prevent relapse by strengthening the gut microbial community.
Authors’ contribution
CILR drafted the manuscript with help from LJM, MJW, RML, and SPC. All authors read and approved the final manuscript.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Pretty J , Morison JI , Hine R . Reducing food poverty by increasing agricultural sustainability in developing countries. Agric Ecosyst Environ. 2003; 95: 217–34.
- USDA ERS. USDA ERS history. Glob Supply Chain Stand Poor Global Supply Chains, Standards and the Poor, 8 gr. Available from: http://www.ers.usda.gov/data-products/food-price-outlook.aspx [cited 19 February 2015]..
- Eurostat. Consum. Certain Foodst. per inhabitant Meat – Total (kg/head). Available from: http://epp.eurostat.ec.europa.eu/tgm/table.do?tab=table&plugin=1&language=en&pcode=tsdpc330 [cited 19 February 2015]..
- Hampson DJ , Swayne DE , Glisson JR , McDouglad LR , Nolan LK , Suarez DL , etal. Disease of poultry. 2013; Ames: Blackwell.
- Ghorbani-Dalini S , Kargar M , Doosti A , Sarshar M , Souod N , Golshan M . Quantitation of bacteria in gastric biopsy specimen from patients with gastrointestinal disorders: relationship between counts and clinical features. Int J Infect Dis. 2011; 15: 68.
- Wagenaar J , Bergen MA , Graaf L , Landman WJ . Free-range chickens show a higher incidence of Brachyspira infections in the Netherlands. 2–4 April 2003. Second International Conference on Colonic Spirochaetal Infections in Animals and Humans, Edinburgh, UK.
- Jansson DS , Persson M , Zimmerman U , Johansson KE . Phenotypic and genetic diversity among intestinal spirochaetes (genus Brachyspira) in free-living wild mallards (Anas platyrhynchos) sampled in southern Sweden. Syst Appl Microbiol. 2011; 34: 566–75.
- Stanton TB , Postic D , Jensen NS . Serpulina alvinipulli sp. a new Serpulina species that is enteropathogenic for chickens. Int J Syst Bacteriol. 1998; 48: 669–76.
- Mappley LJ , La Ragione RM , Woodward MJ . Brachyspira and its role in avian intestinal spirochaetosis. Vet Microbiol. 2014; 168: 245–60.
- Stanton TB , Hampson DJ . Physiology of ruminal and intestinal spirochaets. 1997; Madison, USA: CAB International. 7–45.
- Hovind-Hougen K , Birch-Andersen A , Henrik-Nielsen R , Orholm M , Pedersen JO , Teglbjaerg PS , etal. Intestinal spirochetosis: morphological characterization and cultivation of the spirochete Brachyspira aalborgi . J Clin Microbiol. 1982; 16: 1127–36.
- Stanton TB , Lebo DF . Treponema hyodysenteriae growth under various culture conditions. Vet Microbiol. 2015; 18: 177–90.
- Harris DL , Glock RD , Christensen CR , Kinyon JM . Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction of the disease. Vet Med Small Anim Clin. 1972; 67: 61–4.
- Taylor DJ , Alexander TJ . The production of dysentery in swine by feeding cultures containing a spirochaete. Br Vet J. 1971; 127: 58–61.
- Kinyon JM , Harris DL . Treponema innocens, a new species of intestinal bacteria, and emended description of the type strain of Treponema hyodysenteriae . Int J Syst Bacteriol. 1979; 29: 102–9.
- Stanton TB . Proposal to change the genus designation Serpula to Serpulina containing the species Serpulina hyodysenteriae and Serpulina innocens . Int J Syst Bacteriol. 1992; 42: 189–90.
- Fellström C , Gunnarsson A . Phenotypical characterisation of intestinal spirochaetes isolated from pigs. Res Vet Sci. 1995; 59: 1–4.
- Trott DJ , Stanton TB , Jensen NS , Duhamel GE , Johnson JL , Hampson DJ . Serpulina pilosicoli the agent of porcine intestinal spirochetosis. Int J Syst Bacteriol. 1996; 46: 206–15.
- Oxberry SL , Trott DJ , Hampson DJ . Serpulina pilosicoli, waterbirds and water: potential sources of infection for humans and other animals. Epidemiol Infect. 1998; 121: 219–25.
- Hampson DJ , La T , Phillips ND . Emergence of Brachyspira species and strains: reinforcing the need for surveillance. Porc Heal Manag. 2015; 1: 8.
- Mappley LJ , Tchórzewska MA , Cooley WA , Woodward MJ , La Ragione RM . Lactobacilli antagonize the growth, motility, and adherence of Brachyspira pilosicoli: a potential intervention against avian intestinal spirochetosis. Appl Environ Microbiol. 2011; 77: 5402–11.
- Calderaro A , Piccolo G , Montecchini S , Buttrini M , Gorrini C , Rossi S , etal. MALDI-TOF MS analysis of human and animal Brachyspira species and benefits of database extension. J Proteomics. 2013; 78: 273–80.
- Song Y , La T , Phillips ND , Hampson DJ . Development of a serological ELISA using a recombinant protein to identify pig herds infected with Brachyspira hyodysenteriae . Vet J. 2015; 206: 365–370.
- Hampson DJ , Robertson ID . Experiences with a vaccine being developed for the control of swine dysentery. Aust Vet J. 1993; 70: 8–20.
- Movahedi A , Hampson DJ . Evaluation of recombinant Brachyspira pilosicoli oligopeptide-binding proteins as vaccine candidates in a mouse model of intestinal spirochaetosis. J Med Microbiol. 2010; 59: 353–9.
- Stephens CP , Hampson DJ . Prevalence and disease association of intestinal spirochaetes in chickens in Estern Australia. Avian Pathol. 1999; 28: 447–54.
- Erlamdson K , Klinger E . Intestinal spirochaetosis: epidemiology, micobiology, and clinical significance. Clin Microbiol Newslett. 2005; 27: 91–6.
- Taylor P , Dwars RM , Davelaar FG , Smit HF . Infection of broiler parent hens with avian intestinal spirochaetes: effects on egg production and chick quality. Avian Pathol. 1993; 22: 37–41.
- Jansson DS , Fellström C , Råsbäck T , Vågsholm I , Gunnarsson A , Ingermaa F , etal. Phenotypic and molecular characterization of Brachyspira spp. isolated from laying hens in different housing systems. Vet Microbiol. 2008; 130: 348–62.
- Griffiths IB , Hunt BW , Lister SA , Lamont MH . Retarded growth rate and delayed onset of egg production associated with spirochaete infection in pullets. Vet Rec. 1987; 121: 35–7.
- Taylor P , Stephens CP , Hampson DJ . Experimental infection of broiler breeder hens with the intestinal spirochaete Brachyspira (Serpulina) pilosicoli causes reduced egg production. Avian Pathol. 2002; 31: 37–41.
- Swayne DE , Bermudez AJ , Saqartz KA , Monfort JD , Stoutenburg JW , Hayes JR . Association of cecal spirochetes with pasty vents and dirty eggshells in layers. Avian Dis. 1992; 36: 776–81.
- Beardsworth PM , Hernandez JM . Yolk colour – an important egg quality attribute. Int Poult Prod. 2004; 12: 17–18.
- Dwars RM , Davelaar FG , Smit HF . Spirochaetosis in broilers. Avian Pathol. 1992; 21: 261–73.
- Prapasarakul N , Lugsomya K , Disatian S , Lekdumrongsak T , Banlunara W , Chetanachan P , etal. Faecal excretion of intestinal spirochaetes by urban dogs, and their pathogenicity in a chick model of intestinal spirochaetosis. Res Vet Sci. 2011; 91: 38–43.
- Ochiai K , Adachi Y , Mori K . Unification of the genera Serpulina and Brachyspira, and proposals of Brachyspira hyodysenteriae, Brachyspira innocens and Brachyspira pilosicoli . Microbiol Immunol. 1997; 41: 445–52.
- Falkow S , Rosenberg E , Schleifer KH , Stackebrandt E . The prokaryotes: Vol. 7: Proteobacteria: delta and epsilon subclasses. 2006; New York, NY: Springer. 329–56.
- Gabe JD , Chang RJ , Slomiany R , Andrews WH , McCaman MT . Isolation of extracytoplasmic proteins from Serpulina hyodysenteriae B204 and molecular cloning of the flaB1 gene encoding a 38-kilodalton flagellar protein. Infect Immun. 1995; 63: 142–8.
- Trott D , David P , Zuerner RL , Bularch D , Wannemuehler M , Stasko J . Identification and cloning of the gene encoding BmpC: an outer-membrane lipoprotein associated with Brachyspira pilosicoli membrane vesicles. Microbiology. 2004; 150: 1041–53.
- Yano T , Yamagami R , Misumi K , Kubota C , Moe K , Hayashi T , etal. Misawa, Naoaki. Genetic heterogeneity among strains of Treponema phagedenis-like spirochetes isolated from dairy cattle with papillomatous digital dermatitis in Japan. J Clin Microbiol. 2009; 47: 727–33.
- Charon NW , Goldstein SF . Genetics of motility and chemotaxis of a fascinating group of bacteria: the spirochetes. Annu Rev Genet. 2002; 36: 47–73.
- Li C , Motaleb A , Sal M , Goldstein SF , Charon NW . Spirochete periplasmic flagella and motility. J Mol Microbiol Biotechnol. 2000; 2: 345–54.
- Nakamura S , Adachi Y , Goto T , Magariyama Y . Improvement in motion efficiency of the Spirochete Brachyspira pilosicoli . Biophys J. 2006; 90: 3019–29.
- Naresh R , Hampson DJ . Attraction of Brachyspira pilosicoli to mucin. Microbiology. 2010; 156: 191–7.
- Hopwood D , Pethick D , Hampson D . Increasing the viscosity of the intestinal contents stimulates proliferation of enterotoxigenic Escherichia coli and Brachyspira pilosicoli in weaner pigs. Br J Nutr. 2007; 88: 523–32.
- Mappley LJ , Black ML , Abuoun M , Darby AC , Woodward MJ , Parkhill J , etal. Comparative genomics of Brachyspira pilosicoli strains: genome rearrangements, reductions and correlation of genetic compliment with phenotypic diversity. BMC Genomics. 2012; 13: 454.
- Zuerner RL , Stanton TB , Minion FC , Li C , Charon NW , Trott DJ , etal. Genetic variation in Brachyspira: chromosomal rearrangements and sequence drift distinguish B. pilosicoli from B. hyodysenteriae . Anaerobe. 2004; 10: 229–37.
- Dassanayake RP . Biochemical properties of membrane-associated proteases of Brachyspira pilosicoli isolated from humans with intestinal disorders. J Med Microbiol. 2004; 53: 319–23.
- Hampson DJ , Oxberry SL , La T . Potential for zoonotic transmission of Brachyspira pilosicoli . Emerg Infect Dis. 2006; 12: 869–70.
- Mappley LJ , Tchórzewska MA , Nunez A , Woodward MJ , La Ragione RM . Evidence for systemic spread of the potentially zoonotic intestinal spirochaete Brachyspira pilosicoli in experimentally challenged laying chickens. J Med Microbiol. 2013; 62: 297–302.
- Trott DJ , McLaren AJ , Hampson DJ . Pathogenicity of human and porcine intestinal spirochetes in one-day-old specific-pathogen-free chicks: an animal model of intestinal spirochetosis. Infect Immun. 1995; 63: 3705–10.
- Calderaro A , Gorrini C , Peruzzi S , Piccolo G , Dettori G , Chezzi C . Occurrence of human intestinal spirochetosis in comparison with infections by other enteropathogenic agents in an area of the Northern Italy. Diagn Microbiol Infect Dis. 2007; 59: 157–63.
- Prim N , Pericas R , Español M , Rivera A , Mirelis B , Coll P . Bloodstream infection due to Brachyspira pilosicoli in a patient with multiorgan failure. J Clin Microbiol. 2011; 49: 3697–9.
- Lee JI , Hampson DJ . Intestinal spirochaetes colonizing aborigines from communities in the remote north of Western Australia. Epidemiol Infect. 1992; 109: 133–41.
- Trott DJ , Combs BG , Mikosza AS , Oxberry SL , Robertson ID , Passey M , etal. The prevalence of Serpulina pilosicoli in humans and domestic animals in the Eastern Highlands of Papua New Guinea. Epidemiol Infect. 1997; 119: 369–79.
- Margawani KR . Prevalence, risk factors and molecular epidemiology of Brachyspira pilosicoli in humans on the island of Bali, Indonesia. J Med Microbiol. 2004; 53: 325–32.
- Verlinden M , Pasmans F , Garmyn A , De Zutter L , Haesebrouck F , Martel A . Occurrence of viable Brachyspira spp. on carcasses of spent laying hens from supermarkets. Food Microbiol. 2012; 32: 321–4.
- Pringle M , Landén A , Unnerstad HE , Molander B , Bengtsson B . Antimicrobial susceptibility of porcine Brachyspira hyodysenteriae and Brachyspira pilosicoli isolated in Sweden between 1990 and 2010. Acta Vet Scand. 2012; 54: 54.
- Karlsson M , Fellström C , Gunnarsson A , Landén A , Fellstro C , Lande A . Antimicrobial susceptibility testing of porcine Brachyspira (Serpulina) species isolates. J Clin Microbiol. 2003; 41: 2596–604.
- Schlünzen F , Pyetan E , Fucini P , Yonath A , Harms JM . Inhibition of peptide bond formation by pleuromutilins: the structure of the 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin. Mol Microbiol. 2004; 54: 1287–94.
- Stephens CP , Hampson DJ . Evaluation of tiamulin and lincomycin for the treatment of broiler breeders experimentally infected with the intestinal spirochaete Brachyspira pilosicoli . Avian Pathol. 2002; 31: 299–304.
- Burch DGS , Harding C , Alvarez R , Valks M . Treatment of a field case of avian intestinal spirochaetosis caused by Brachyspira pilosicoli with tiamulin. Avian Pathol. 2006; 35: 211–16.
- European Medicine Agency. Tiamutin – Article 34 Referral – European Medicines Agency. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Tiamutin_34/WC500094801.pdf [cited 18 May 2015]..
- Mortimer-Jones SM , Phillips ND , La T , Naresh R , Hampson DJ . Penicillin resistance in the intestinal spirochaete Brachyspira pilosicoli associated with OXA-136 and OXA-137, two new variants of the class D beta-lactamase OXA-63. J Med Microbiol. 2008; 57: 1122–8.
- Verlinden M , Boyen F , Pasmans F , Garmyn A , Haesebrouck F , Martel A . Antimicrobial susceptibility pattern of Brachyspira intermedia isolates from European layers. Microb Drug Resist. 2001; 17: 485–8.
- Rugna G , Bonilauri P , Carra E , Bergamini F , Luppi A , Gherpelli Y , etal. Sequence types and pleuromutilin susceptibility of Brachyspira hyodysenteriae isolates from Italian pigs with swine dysentery: 2003–2012. Vet J. 2015; 203: 115–19.
- Castanon JIR . History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci. 2007; 86: 2466–71.
- Medhanie GA , Mcewen SA , Slavic D , Guerin MT . Brachyspira spp. and avian intestinal spirochaetosis: an epidemiological review. Worlds Poult Sci J. 2013; 69: 541–52.
- Corona-Barrera E , Smith DGE , Murray B , Thomson JR . Efficacy of seven disinfectant sanitisers on field isolates of Brachyspira pilosicoli . Vet Rec. 2004; 154: 473–4.
- Hampson DJ , Robertson ID , La T , Oxberry SL , Pethick D . Influences of diet and vaccination on colonisation of pigs by the intestinal spirochaete Brachyspira (Serpulina)pilosicoli . Vet Microbiol. 2000; 73: 75–84.
- Woodward MJ , Mappley L , Le Roy C , Claus SP , Davies P , Thompson G , etal. Drinking water application of Denagard® Tiamulin for control of Brachyspira pilosicoli infection of laying poultry. Res Vet Sci. 2015; 103: 87–95.
- Hooper LV . Commensal host-bacterial relationships in the gut. Science. 2001; 292: 1115–18.
- Claus SP , Ellero SL , Berger B , Krause L , Bruttin A , Molina J , etal. Colonization-induced host-gut microbial metabolic Interaction. mBio. 2011; 2: 271–10.
- Food and Agriculture Organization of the United Nation. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. 2001; 1–34. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina, 1–4 October.
- Howarth GS , Butler RN , Salminen S , Gibson GR , Donovan SM . Probiotics for optimal nutrition: from efficacy to guidelines. Adv Nutr. 2012; 3: 720–2.
- Fooks LJ , Gibson GR . Probiotics as modulators of the gut flora. Br J Nutr. 2002; 88: 39–49.
- Bernardeau M , Gueguen M , Smith DGE , Corona-Barrera E , Vernoux JP . In vitro antagonistic activities of Lactobacillus spp. against Brachyspira hyodysenteriae and Brachyspira pilosicoli . Vet Microbiol. 2009; 138: 184–90.
- Wood EJ , Seviour RJ , Siddique ABM , Glaisher RW , Webb RI , Trott DJ . Spherical body formation in the spirochaete Brachyspira hyodysenteriae . FEMS Microbiol Lett. 2006; 259: 9–14.
- European Food Safety Authority. Scientific opinion on the safety and efficacy of Lactobacillus salivarius ( CNCM I-3238) and Lactobacillus casei (ATTC PTA-6135) as silage additives for all species. EFSA J. 2012; 10: 1–14.
- Mappley LJ , Tchórzewska MA , Nunez A , Woodward MJ , Bramley PM , La Ragione RM . Oral treatment of chickens with Lactobacillus reuteri LM1 reduces Brachyspira pilosicoli-induced pathology. J Med Microbiol. 2013; 62: 287–96.
- Awad WA , Ghareeb K , Abdel-Raheem S , Böhm J . Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult Sci. 2009; 1: 49–56.
- Yoruk MA , Gul M , Hayirli A , Macit M . Effects of supplementation of humate and probiotic on egg production and quality parameters during the late laying period in hens. Poult Sci. 2004; 83: 84–8.
- Jinmo Y , Kyu-il K . Effect of feeding diets containing an antibiotic, a probiotic, or yucca extract on growth and intestinal urease activity in broiler chicks animals and diets. Poult Sci. 1992; 76: 381–5.
