Abstract
Objective
The first aim of this study was to compare the microbiota of different locations (pus, tonsillar fossa, blood) in peritonsillar abscess (PTA) patients in order to optimize the sampling scheme. The second aim was to estimate the occurrence of tonsillitis episodes and macroscopic oropharyngeal signs characteristic of recurrent tonsillitis in PTA patients.
Methods
The study group consisted of 22 consecutive patients with PTA undergoing bilateral tonsillectomy. The PTA was punctured; pus and tonsillar fossa biopsy samples and the peripheral blood cultures were collected. The index of tonsillitis was calculated by multiplying the number of tonsillitis episodes per year by the morbidity period in years. Macroscopic oropharyngeal signs were evaluated and they were as follows: tonsillar sclerosis, obstruction of the tonsillar crypts, scar tissue on tonsils, cryptic debris, and lymphatic tissue aggregates.
Results
The cultures of the pus were positive in 16 out of 22 patients and the cultures of the tonsillar fossa samples were positive in all cases. In total, 62 different organisms were found from tonsillar fossa, pus, and blood samples, which belonged to 5 different phyla and 18 different families.
In the tonsillar fossa, the most frequent bacteria found were Streptococcus spp. In pus samples, the most frequently found bacteria were Streptococcus spp. and bacteria from the Streptococcus milleri group.
Conclusion
PTA patients had mixed anaerobic and aerobic microbiota both in the tissue of the tonsillar fossa and the pus of the peritonsillar space. We demonstrated that the tonsillar fossa specimen is a better material for microbiological analyses, because it reveals more bacteria per culture. PTA patients usually have a low number of tonsillitis episodes in their previous history, but a relatively high number of macroscopic oropharyngeal signs, indicating the sclerotic process in palatal tonsils.
A peritonsillar abscess (PTA) is characterized by a purulent secretion accumulated between the fibrous capsule of the palatine tonsil and the pharyngeal superior constrictor muscle (Citation1, Citation2). It is a suppurative complication of tonsillitis. It commonly occurs in adolescents and young adults, although any age can be affected. Annual reported incidence ranges from 9 to 37 cases per 100,000 persons a year (Citation3, Citation4). The condition may be life-threatening, though in the antibiotics era, the mortality rate due to PTA has dropped considerably. The proper treatment for this infection aims to avoid serious complications which include its extension into deep spaces of the neck. Therefore, the management of PTA usually includes both surgical and antimicrobial therapy. Surgical drainage varies from pus aspiration and/or drainage all the way to acute tonsillectomy (Citation5, Citation6).
Antibacterial treatment relies on the microbial aetiology of PTA and should cover essentially all the bacteria that are considered to be significant causative agents. Several studies have shown a mixed aerobic and anaerobic flora in PTA (Citation1–Citation3, Citation7–Citation11). As the cultures are obtained from an area normally heavily colonized, the aetiological relevance of each bacterium is raised. Owing to the method of sample collection, such culture results include both a proportion of contaminants and bacteria of clinical significance. It is not clear whether the location of bacteria and the site of bacteriological sampling would help to determine microbes having an aetiological role in PTA.
The objective of this study was twofold. The first aim was to compare the microbiota of different locations (pus, tonsillar fossa, blood) in PTA patients in order to optimize the sampling scheme. The second aim was to estimate the occurrence of tonsillitis episodes and macroscopic oropharyngeal signs characteristic of recurrent tonsillitis in PTA patients.
Materials and methods
Patients
The prospective study was performed between November 2011 and May 2012 at the Department of Otorhinolaryngology, Tartu University Hospital. The study group consisted of 22 consecutive patients (16 male and 6 female with median age of 32, range 14–74) with PTA, undergoing bilateral tonsillectomy. The study was approved by the Ethical Committee of the University of Tartu. All patients were included after their informed consent was obtained.
Clinical examination
The diagnosis of PTA was based on the clinical evaluation, confirming pus collection in the peritonsillar space (). The following clinical and demographic data were obtained: age, gender, type of antimicrobial therapy before admission, axillary temperature with an accuracy of 0.1°C, history of previous episodes of recurrent tonsillitis, and length of previous history of tonsillitis.
Table 1 Clinical data
In addition, macroscopic oropharyngeal signs were evaluated and the index of tonsillitis (IT) was calculated as described in our previous studies (Citation12, Citation13). Macroscopic oropharyngeal signs were evaluated on tonsill in the opposite side of PTA and they were as follows: tonsillar sclerosis, obstruction of the tonsillar crypts, scar tissue on tonsils, cryptic debris, and lymphatic tissue aggregates. Tonsillar sclerosis was defined as increased tightness of the tonsillar and peritonsillar tissue together with fixation of the palatine tonsil in the tonsillar fossa. Obstruction of the tonsillar crypts was documented when narrowing of the crypt mouth was observed, resulting in the loss of clear cryptic pattern of the tonsillar surface. Scar tissue on the tonsils was defined as white tissue spots or streaks on the tonsillar surface. Cryptic debris was described as any white or yellow matter in the crypts or in the supratonsillar cleft. Multiple round or elongated yellow-coloured patches on the retropharyngeal mucosa were described as lymphatic tissue aggregates, which are presumably due to enlargement of normal lymphatic structures in the throat. The IT score was calculated by multiplying the number of tonsillitis episodes per year by the morbidity period in years (Citation14). Basically, it represents the total number of tonsillitis episodes the patient has ever had.
Specimen collection
All operations were undertaken under general anaesthesia. The PTA was punctured and pus was aspirated into a sterile syringe. The tonsils were removed by blunt dissection and placed in sterile containers separately. After removal of a tonsil, a tissue biopsy sample was taken from the tonsillar fossa for microbiological analysis. The tissue sample was immediately placed into Stuart transport medium (OXOID, Basingstoke, UK).
Within 5 min of the removal of the first tonsil (the tonsil at the side of the abscess), the peripheral blood cultures were drawn aseptically into BACTEC Plus Aerobic/F and BACTEC Plus Anaerobic/F blood culture bottles (Becton Dickinson, Maryland, Sparks, USA).
Microbiological analyses
All collected specimens were immediately taken to a microbiology laboratory. Pus aspirates and tissue were transported in sterile tubes. All materials were cultured semi-quantitatively in the anaerobic box with loop (10 µl). Blood agar plates (OXOID, Basingstoke, UK) were employed for aerobes, chocolate agar plates for Haemophilus sp., tryptic soy-serum-bacitracin-vancomycin agar (TSBV; Oxoid) for Aggregatibacter sp., and fastidious anaerobe agar (FAA; Lab M, Heywood, UK) for anaerobes. The plates were incubated at 36.6°C either aerobically for 2 days (blood agar), in a CO2-enriched atmosphere for 5 days (chocolate and TSBV agar), or anaerobically for 7 days using the anaerobic glove box (FAA). Bacterial count was evaluated semiquantitatively: the growth in the first sector (+), in the second sector (++), in the third sector (+++), and in the fourth sector (++++). Another amount of pus was inoculated into blood culture bottles (BACTEC Pediatric, Plymouth, UK). Identification of isolated microorganisms was performed using the MALDI Biotyper (Bruker Daltonics, Bremen, Germany).
All blood culture bottles were promptly taken to the laboratory and incubated at 36°C in a fully automated blood culture instrument (Bactec 9050™, Becton Dickinson). The blood culture bottles were cultured for 7 days in total. When evidence of growth was noted, Gram staining and subculture were performed on the relevant plates.
For antibiotic susceptibility testing only the opportunistic pathogens were selected. The selected microorganisms were Streptococcus pneumoniae, S. pyogenes, S. parasanguinis, S. anginosus, S. intermedius, S. constellatus, Rothia mucilaginosa, and Fusobacterium necrophorum. Antimicrobial sensitivity testing was performed for antimicrobials, which included first and second line antibiotics for treating PTA: penicillin, ampicillin/sulbactam, cefuroxime, clindamycin, erythromycin, ciprofloxacin, and metronidazole. Minimum inhibitory concentrations (MICs) were assessed using the E-test method and the guideline ‘Breakpoint tables for interpretation of MICs and zone diameters’ from the European Committee on Antimicrobial Susceptibility Testing (version 5.0, 2015; www.eucast.org) (Citation15). Exact MIC values, which were used to determine sensitivity for each specimen, were as follows: S. parasanguinis – penicillin, ampicillin/sulbactam (MIC susceptible≤0.25; resistant>2); clindamycin (MIC susceptible≤0.5; resistant>0.5); S. milleri group (SMG) – penicillin, ampicillin/sulbactam (MIC susceptible≤0.25; resistant>2); clindamycin (MIC susceptible ≤ 0.5; resistant>0.5); S. pneumoniae – penicillin (MIC susceptible≤0.06; resistant>2); ampicillin/sulbactam (MIC susceptible≤0.5; resistant>2); cefuroxime, erythromycin (MIC susceptible≤0.25; resistant>0.5); clindamycin (MIC susceptible≤0.5; resistant>0.5); ciprofloxacin (MIC susceptible≤0.125; resistant>2); S. pyogenes – penicillin, ampicillin/sulbactam (MIC susceptible≤0.25; resistant>0.25); clindamycin (MIC susceptible≤0.5; resistant>0.5); F. necrophorum – penicillin (MIC susceptible≤0.25; resistant>0.5); ampicillin/sulbactam (MIC susceptible≤4; resistant>8); clindamycin (MIC susceptible≤4; resistant>4); metronidazole (MIC susceptible≤4; resistant>4).
Results
Clinical data
The 22 patients who were hospitalized due to PTA included 16 males and 6 females with a median age of 32 (range 14–74). The average duration of symptoms was 3–7 days preoperatively ().
Fifteen patients out of 22 (68.2%) had a fever, but only nine patients (40.9%) had a fever greater than 38°C at presentation. The median temperature was 38.4°C.
Most patients had received antibiotics prior to presentation. Only 6 patients out of 22 (27.3%) had not been treated with antibiotics. Antibiotics that were prescribed usually by general doctors before hospitalization were penicillin V, amoxicillin, cefadroxil, and clindamycin. Three patients took a second course of antibiotics before hospitalization; the second choice was always clindamycin.
Most of the patients (63.6%) had never had problems with their tonsils. Seven patients (31.8%) had one to three episodes a year and only one patient had tonsillitis episodes more often than three times a year. The highest IT score was 36, but the median value was 0 (range 0–36) and the mean value was 4.04. After excluding the one person with the IT score of 36, the mean value of IT dropped to 2.16 ().
The most common macroscopic oropharyngeal sign was cryptic debris that occurred in 83.3% of patients. Tonsillar sclerosis and scar tissue on tonsils was present in 72.2% of cases, obstruction of the tonsillar crypts in 61.1%, and lymphatic tissue aggregates on the retropharyngeal mucosa in 55.6% of the patients. The examination was performed before tonsillectomy on a scale of 0–5 and the median value was 4 (range 0–5).
Microbiological data
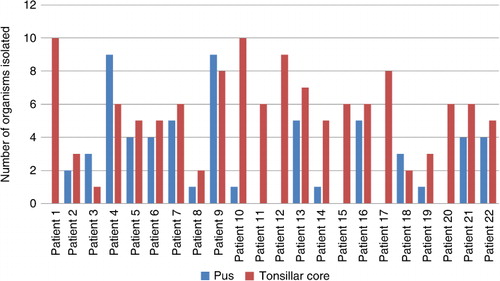
The pus cultures were positive in 16 (72.7%) out of 22 patients and the cultures of the tonsillar fossa samples were positive in all cases. At the same time, only 2 out of 22 blood samples were positive. In total, 62 different organisms were found from tonsillar fossa, pus, and blood samples, which belonged to 5 different phyla and 18 different families (). Among them, 32 different organisms were discovered from the pus specimens, 49 different organisms from the tonsillar fossa, and 3 organisms from the blood samples (). The median number of different organisms found in all tonsillar fossa biopsies was six (range 1–10, mean 5.7 SD ± 2.5) and in all pus samples it was three (range 0–9, mean 2.7 SD ± 2.7) ().
Table 2 Microorganisms isolated from the pus, tonsillar fossa, and blood of peritonsillar abscess patients
In the tonsillar fossa, the most frequent bacteria found were Streptococcus spp., whereas the most frequent specimens were S. parasanguinis (12/22) and S. pneumoniae (12/22). Streptococcus spp. was followed by Neisseria spp. and Actinomyces spp. In the tonsillar fossa biopsy we found six Staphylococcus spp. specimens, but staphylococci were not found in any pus cultures. In addition, R. mucilaginosa was present in seven (31.8%) tonsillar fossa biopsies, but was never found in the pus.
In pus samples, the most frequently found bacteria were Streptococcus spp. Bacteria from the SMG, consisting of S. anginosus, S. intermedius, and S. constellatus, were isolated from the pus in 12 cases and from the tonsillar fossa in four cases. Among the 16 positive pus samples, SMG was present in nine (56.2%). A single organism was cultured in four (18.2%) pus cultures, and multiple organisms were present in 12 (54.5%) pus cultures. Out of 22 patients, 6 (27.3%) had no microbiological growth in the pus. S. pyogenes was a monoculture in the pus of two patients; S. constellatus and F. necrophorum were each a single organism in the pus culture of one patient.
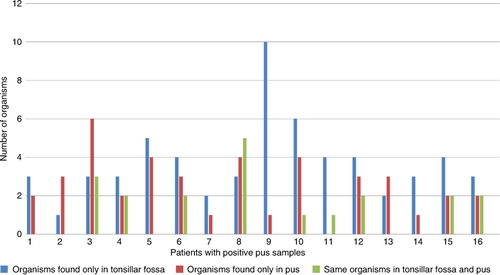
In 9 patients out of the 16 with positive pus samples (56.2%), the same organisms that were found in the pus were also present in the tonsillar fossa biopsy samples (altogether 20 isolates) (). At the same time, all except one pus sample had one or more specimens in the pus that were not found in the tonsillar fossa biopsy.
The blood culture was positive in only two (9.1%) patients. The bacteria found from blood cultures were S. mitis and S. oralis in one patient and Peptostreptococcus anaerobius in another. None of the bacteria were found in the patients’ pus or tonsillar fossa samples.
We also determined the antibiotic susceptibility for opportunistic bacteria. The data are presented in .
Table 3 Antibiotic susceptibility of opportunistic bacteria
Discussion
In the present study, we found mixed aerobic and anaerobic microbiota either in the tonsillar fossa or in the pus evacuated from the peritonsillar space. We demonstrated that the tonsillar fossa specimen is a better material for microbiological analyses, because it reveals more bacteria per culture.
Altogether 49 species were recovered from the tonsillar fossa, which is significantly more than the 32 species recovered from the pus cultures. The most common microorganisms were Streprococcus species, particularly the SMG (S. anginosus, S. intermedius, and S. constellatus). Although the SMG is normally found on the oropharyngeal mucosa, it has been recognized as the aetiological agent of the abscesses in the head and neck region (Citation16–Citation18). We found SMG microorganisms in up to 56.2% of positive cultures. In our previous study, in addition to Streptococcus species, the anaerobic bacteria were the second most common bacteria found in the tonsillar fossa of patients with recurrent tonsillitis (Kasenõmm et al. 2004). In the present study, the number of anaerobes in the tonsillar fossa of PTA patients was significantly lower. This finding is not in accordance with recent studies where the importance of anaerobes in the aetiology of PTA, particularly F. necrophorum, has been questioned (Citation3, Citation6–Citation10). We found F. necrophorum only in one pus culture and in two tonsillar fossa specimens. On the other hand, S. pyogenes, the most important aetiological agent of acute tonsillitis, was recovered from four tonsillar fossa specimens and from six pus cultures out of 22 (27.2%). Interestingly, in our previous study on recurrent tonsillitis we recovered no S. pyogenes from tonsillar core specimens by the culture method, but found it in 29% of cases when using more sensitive PCR. It has been demonstrated that S. pyogenes has a great ability to conceal itself intracellularly, to prevent immune response (Citation19). Our data may suggest that in the case of acute infection, as well as in PTA, S. pyogenes may escape from epithelial cells and play an important role in the pathogenesis of the disease.
We recovered only two positive blood cultures from our PTA patients. The bacteria isolated from blood cultures were viridans streptococci (S. mitis and S. oralis) and P. anaerobius. In other studies, the percentage of positive blood cultures has been higher (Citation20). In our previous study, the rate of positive blood cultures during tonsillectomy was as high as 44% (Citation12). In the present study, we used the same culture media and the same protocol for collecting blood cultures during tonsillectomy. Therefore, we suggest that the significantly lower rate of positive blood cultures is related to preoperative antibacterial therapy. In our previous study, the study group consisted of patients with recurrent tonsillitis in whom tonsillectomy was performed in their relatively healthy state, without preoperative antibiotics. In comparison, among the PTA patients only 6 out of 22 had not taken antibiotics before hospitalization. On the other hand, these data also demonstrate that the use of antibiotics prior to acute tonsillectomy for PTA may be beneficial so as to reduce bacterial translocation and avoid more serious complications. Antibiotics that were used were penicillin V, amoxicillin, cefadroxil, and clindamycin. Because there are no common guidelines for the treatment of PTA in Estonia, the antibiotics spectrum varies.
According to our study the commonly used antibiotics penicillin and other beta-lactam antibiotics are suitable as empirical treatment. Antibiotic resistance to penicillin and other beta-lactam is higher only among F. necrophorum. However F. necrophorum was not a very common pathogen in our population.
In a further analysis we studied the IT value in PTA patients. We found that only one patient with PTA out of 22 had an IT score of 36 or higher. The median IT value was 0 (range 0–36) and the average was 4.04. When that single patient with the IT score of 36 was excluded, the average IT value dropped to 2.16. This is a much lower value than in the patients with recurrent tonsillitis in our previous study (Citation13), where the median IT score was 30 (range 6–138). There we found that the IT score of 36 is an optimal prediction for impaired defensive function of palatine tonsils for a patient with recurrent tonsillitis and tonsillectomy was recommended. These data suggest that patients with PTA usually have no, or very few, previous tonsillitis episodes.
We also estimated the presence of five different macroscopic oropharyngeal signs (tonsillar sclerosis, scar tissue on the tonsils, obstruction of tonsillar crypts, cryptic debris, and lymphatic tissue aggregates) in PTA patients on the opposite side. The same macroscopic signs were used in a previous study, where a strong correlation between the occurrence of those signs and the development of tonsillar scarring was demonstrated (Citation12). In the present study, the median rate of macroscopic oropharyngeal signs in PTA patients was 4 out of 5. This finding shows that even when patients had only a few tonsillitis episodes or none at all, most of them had signs characteristic of recurrent inflammation in the tonsillar tissue. Ongoing inflammation in the tonsillar tissue leads, in the long term, to tonsillar scarring and a lower protective function of the tonsils (Citation12). This finding may partially explain the development of PTA despite the absence of recurrent tonsillitis symptoms in these patients.
Conclusions
PTA patients have mixed anaerobic and aerobic microbiota both in the tissue of the tonsillar fossa and the pus of the peritonsillar space, with S. pyogenes and members of the SMG being the most common isolated species. Our results indicate that tonsillar fossa specimens should be preferred over pus sampling during abscess tonsillectomy in order to reveal a possible pathogen.
Second, PTA patients usually have a low number of tonsillitis episodes in their previous history, but a relatively high number of macroscopic oropharyngeal signs, indicating the sclerotic process in palatal tonsils and low immune response that may support the development of mixed oropharyngeal infections.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Acknowledgements
We thank the staff of Tartu University Hospital for technical assistance. This study was supported by the Estonian Ministry of Education and Research (target financing No. SF0180132s08, institutional research funding IUT 34–19, and funding of scientific collections KOGU-HUMB) and the University of Tartu (grant No. SARMBARENG).
References
- Mitchelmore IJ, Prior AJ, Montgomery PQ, Tabaqchali S. Microbiological features and pathogenesis of peritonsillar abscesses. Eur J Clin Microbiol Infect Dis. 1995; 14: 870–7. [PubMed Abstract].
- Prior A, Montgomery P, Mitchelmore I, Tabaqchali S. The microbiology and antibiotic treatment of peritonsillar abscesses. Clin Otolaryngol. 1995; 20: 219–23. [PubMed Abstract].
- Marom T, Cinamon U, Itskoviz D, Roth Y. Changing trends of peritonsillar abscess. Am J Otolaryngol. 2010; 31: 162–7. doi: http://dx.doi.org/10.1016/j.amjoto.2008.12.003 [PubMed Abstract].
- Risberg S, Engfeldt P, Hugosson S. Incidence of peritonsillar abscess and relationship to age and gender: retrospective study. Scand J Infect Dis. 2008; 40: 792–6. doi: http://dx.doi.org/10.1080/00365540802195226 [PubMed Abstract].
- Wikstén J, Blomgren K, Eriksson T, Guldfred L, Bratt M, Pitkäranta A. Variations in treatment of peritonsillar abscess in four Nordic countries. Acta Otolaryngol. 2014; 134: 813–7. doi: http://dx.doi.org/10.3109/00016489.2014.905702.
- Powell EL, Powell J, Samuel JR, Wilson JA. A review of the pathogenesis of adult peritonsillar abscess: time for a re-evaluation. J Antimicrob Chemother. 2013; 68: 1941–50. doi: http://dx.doi.org/10.1093/jac/dkt128 [PubMed Abstract].
- Ehlers Klug T, Rusan M, Fuursted K, Ovesen T. Fusobacterium necrophorum: most prevalent pathogen in peritonsillar abscess in Denmark. Clin Infect Dis. 2009; 49: 1467–72. doi: http://dx.doi.org/10.1086/644616 [PubMed Abstract].
- Klug TE, Henriksen JJ, Fuursted K, Ovesen T. Significant pathogens in peritonsillar abscesses. Eur J Clin Microbiol Infect Dis. 2011; 30: 619–27. doi: http://dx.doi.org/10.1007/s10096-010-1130-9 [PubMed Abstract].
- Gavriel H, Lazarovitch T, Pomortsev A, Eviatar E. Variations in the microbiology of peritonsillar abscess. Eur J Clin Microbiol Infect Dis. 2009; 28: 27–31. doi: http://dx.doi.org/10.1007/s10096-008-0583-6 [PubMed Abstract].
- Hidaka H, Kuriyama S, Yano H, Tsuji I, Kobayashi T. Precipitating factors in the pathogenesis of peritonsillar abscess and bacteriological significance of the Streptococcus milleri group. Eur J Clin Microbiol Infect Dis. 2011; 30: 527–32. doi: http://dx.doi.org/10.1007/s10096-010-1114-9 [PubMed Abstract].
- Jousimies-Somer H, Savolainen S, Mäkitie A, Ylikoski J. Bacteriologic findings in peritonsillar abscesses in young adults. Clin Infect Dis. 1993; 16(Suppl 4): S292–8. [PubMed Abstract].
- Kasenõmm P, Mesila I, Piirsoo A, Kull M, Mikelsaar M, Mikelsaar RH. Macroscopic oropharyngeal signs indicating impaired defensive function of palatine tonsils in adults suffering from recurrent tonsillitis. APMIS. 2004; 112: 248–56.
- Kasenõmm P, Piirsoo A, Kull M, Kull M Jr., Mikelsaar M. Selection of indicators for tonsillectomy in adults with recurrent tonsillitis. BMC Ear Nose Throat Disord. 2005; 5: 7.
- Fujihara K, Goto H, Hiraoka M, Hayashi M, Hotomi M, Tamura S, etal. Tonsillitis index: an objective tool for quantifying the indications for tonsillectomy for recurrent acute tonsillitis. Int J Pediatr Otorhinolaryngol. 2005; 69: 1515–20. [PubMed Abstract].
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 5.0, 2015. Available from: http://www.eucast.org [cited 1 January 2015]..
- Foxton CR, Kapila S, Kong J, Thomson NJ. Streptococcus milleri head and neck abscesses: a case series. Ear Nose Throat J. 2012; 91: 246–54. [PubMed Abstract].
- Han JK, Kerschner JE. Streptococcus milleri: an organism for head and neck infections and abscess. Arch Otolaryngol Head Neck Surg. 2001; 127: 650–4. [PubMed Abstract].
- Hirai T, Kimura S, Mori N. Head and neck infections caused by Streptococcus milleri group: an analysis of 17 cases. Auris Nasus Larynx. 2005; 32: 55–8. [PubMed Abstract].
- Medina E, Rohde M, Chhatwal GS. Intracellular survival of Streptococcus pyogenes in polymorphonuclear cells results in increased bacterial virulence. Infect Immun. 2003; 71: 5376–80. [PubMed Abstract] [PubMed CentralFull Text].
- Klug TE, Henriksen JJ, Rusan M, Fuursted K, Ovesen T. Bacteremia during quinsy and elective tonsillectomy: an evaluation of antibiotic prophylaxis recommendations for patients undergoing tonsillectomy. J Cardiovasc Pharmacol Ther. 2012; 17: 298–302. doi: http://dx.doi.org/10.1177/1074248411423023 [PubMed Abstract].