Abstract
Combined hydroacoustic, video and direct examination by scuba-diving of the underwater meadows of Hornsund, a flagship biodiversity site in Svalbard, revealed 17 species of macroalgae with a biomass, dominated by Laminariales, of as much as 3 kg m−2. The biomass dominants were Laminaria digitata and Saccharina latissima, which were most abundant at depths of between 5 and 10 m. The species data presented are the first records for the fjord and provide a starting point for new research and a baseline for future assessments of climate-induced changes.
The Arctic seas are undergoing rapid change as the climate of the Earth warms and the oceans become more acidic. In addition to fundamental effects on the physiology of individual organisms caused by increased temperature, changes to the physical environment are already underway (Węsławski et al. Citation2011). In fjordic ecosystems increased rates of glacial melt will augment both the supply of fresh water and the volume of suspended particulate matter delivered to these enclosed marine environments. Such changes can significantly change the ecosystem (Zhang et al. Citation2000; Dowdesell & Hagen Citation2004; Gordeev Citation2006). Further modification may result from climate-driven changes in the amount of scour caused by both icebergs and seaice and the length of the period during which fjords are ice-covered (Węsławski et al. Citation1997; Wiencke et al. Citation2006).
A key feature of many high-latitude shallow coastal waters is the extensive macroalgal forests that develop sublittorally. While these meadows are of substantial significance for the functioning of inshore ecosystems, they are highly susceptible to a changing climate. While increased turbidity (Gordeev Citation2006) and ice scour are liable, at least at a decadal scale, to be damaging to macroalgae, the loss of sea ice will substantially increase the area available for the development of new meadows. Long-term increases of suspended matter in the area covered by macroalgae are liable to have a downstream influence on the diversity and productivity of other elements of the biota (Roleda et al. Citation2008; Ronowicz et al. Citation2011).
The importance of the benthic macroalgae to coastal ecosystems cannot be overestimated. Although they cover only about 0.1% of the world's oceanic bottom they contribute about 5% of global productivity (Smith Citation1981), much of which is exported to adjacent habitats (Dunton & Dayton Citation1995). Furthermore, by adding spatial complexity to otherwise monotonic seafloors macroalgae provide additional niches for other animal and plant species and in doing so greatly enhance local biodiversity (Włodarska-Kowalczuk et al. Citation2009).
There are about 9000 species of macroalgae known worldwide, and while Rhodophyta are the richest in taxa (Dawes Citation1998), brown algae (Phaeophyta) are far better known as many species tend to be large. For example Macrocystis pyrifera, found around King George Island (South Shetland Islands) and in South Georgia waters, can reach lengths of up to 45 m (Algaebase Citation2012),while Laminariales, found in the Arctic waters of Svalbard's western fjords, may exceed 4 m (Wiktor et al. Citation1995)
Investigations of Arctic macroalgae were first undertaken in the 19th century by Harvie and Dickie in Canada (Lee Citation1980), by Kjellman (Citation1883) in the Russian Arctic and by Rosenvinge (Wiencke et al. Citation2006) around Greenland. In Svalbard, early investigations were undertaken in 1862 and 1868 by Agardh. More recent work on the area was recorded by Svendsen (Citation1959), who worked in Isfjorden. In Hornsund, limited work on macroalgae inhabiting shallow sublittoral was carried out by Florczyk & Latala (Citation1989), although comprehensive investigations on the ecology and ecophysiology of seaweed have been ongoing in the more northerly Kongsfjorden since 1991 (Wiencke et al. Citation2004).
To date, investigations of Hornsund have concentrated on plankton and zoobenthos, with little attention being given to macroalgae, even though the euphotic zone may extend to 25 m. (This depth varies considerably, depending on the amount of mineral suspension discharged from melting glaciers, streams and rivers.) Recent algal research in the fjord has been restricted to the intertidal and shallow sublittoral (Florczyk & Latala Citation1989; Węsławski et al. Citation1997) largely because of the difficulty of remotely sampling the rocky seabed. The shortage of data on macrophytes constitutes a substantial gap when estimating fjordic productivity and understanding the way in which the Hornsund ecosystem functions.
Climate change is likely to alter the composition and distribution of macroalgal assemblages. During the Arctic summer, meltwater from snow and glaciers discharges substantial quantities of mineral matter into fjord systems (Zajączkowski Citation2002). This sediment reduces light availability and decreases the depth of the euphotic zone; it can also cover macroalgae, thereby decreasing their photosynthetic rate and hence productivity (Roleda et al. Citation2008). Consequently, in the short-term, the overall algal biomass of Hornsund is likely to be reduced.
As the ice melts substantial amounts of floating ice will be mobilized and this will scour existing algae from the intertidal and the shallow subtidal, leaving only taxa, such as Fucus distichus, that colonize small cracks in rocks or live in rock pools (Wiencke et al. Citation2004). At the same time, a shallowing of the euphotic zone will force other subtidal algae to move to the infralittoral, increasing their susceptibility to scour and rendering them more liable to damage by ultraviolet radiation (Aguilera et al. Citation1999). Once the rate of glacial attrition has slowed, the frequency and intensity of scour will decline and shallow waters will once again become suitable for the development of macrophytes. Furthermore, retreating glaciers and ice sheets will leave behind new areas of seafloor suitable for the development of new macroalgal forests. While the short-term future of the macroalgae of the Svalbard coast is liable to be difficult, in the longer term it appears probable that new patterns of macroalgal biodiversity and distribution will arise and the energy that they export will drive further modification of a benthic system which itself will be recovering from substantial environmentally driven changes.
The purpose of the study reported here was to obtain information on the current status and distribution of benthic macrophytes in an Arctic fjord in order to improve our understanding of the likely scale of future changes and to underpin predictions of the form such changes are likely to take.
Material and methods
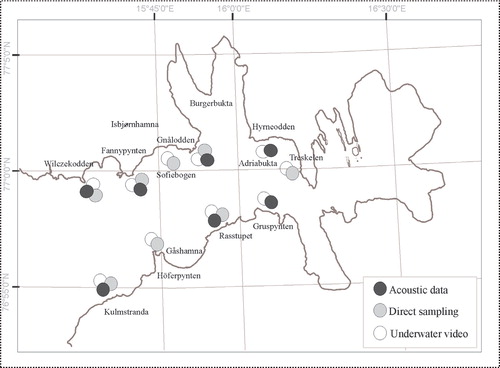
The investigations were conducted in Hornsund fjord (76°N 15°E), on the western coast of southern Spitsbergen, in the archipelago of Svalbard (). Two series of surveys were carried out, the first in late July and early August of 2005 and the second in July 2006. Each survey included three phases: (1) broad-scale mapping of algal distribution using side-scan sonar (Kruss et al. Citation2006); (2) underwater video transects; and (3) quantitative and qualitative sampling by scuba divers at selected sites.
The hydro-acoustic survey was performed along the whole coast of the innermost part of the fjord, with the exception of Brepollen, which could not be sampled along transects between 3 and 40 m. Underwater video was deployed along 10 transects of 100 m in length. The recordings made were saved on compact disc for later review. Subsequently, macroalgal species were identified in individual high-quality images, allowing algal distribution to be mapped. On the basis of the video survey, eight sites were selected for direct sampling () and divers collected triplicate samples from 0.12 m2 from each. Detached from the substratum using knives, algae were placed in polythene bags and transported to the surface where they were fixed in 4% formalin. The preserved material was identified to the species using published identification keys (Zinova Citation1953; Zinova Citation1955; Burrows Citation1991; Wiktor et al. Citation1995). Each specimen was weighed, after drying at 60°C for 24 h. The weight data were used to identify patterns in the distribution and composition of the macroalgal assemblage using the multi-dimensional scaling (MDS) software PRIMER 6.0 (Clarke & Gorley Citation2006), based on Bray Curtis similarity and square root transformed data. This moderate transformation produces an ordination balanced between the abundance of dominant species and the presence of rarer taxa.
Results and discussion
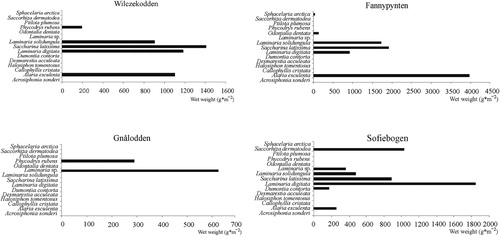
The surveys presented in this paper revealed the occurrence of 17 macroalgae taxa at depths between 3 and 40 m in Hornsund. The algal flora was dominated by Phaeophyta (ca. 53%), followed by Rhodophyta (ca. 41%) and a single taxon of Chlorophyta (ca. 6%). The biodiversity of macroalgae at a particular station ranged from three to 14 species (). The richest macroalgal associations occurred in the southern part of the fjord. Only Laminaria digitata occurred at all of the sites surveyed, although Saccharina latissima and Alaria esculenta (frequency <90%) and Halosiphon tomentosus and the red algae Dumontia contorta (frequency <70%) were also widespread ().
Table 1 Occurrence of macroalgae in Hornsund fjord at the sites surveyed.
Table 2 General occurrence of macroalgae in Honsund fjord by zone and maximum depth sampled. Zones are abbreviated as follows: Arctic (A), high boreal Arctic (HB), boreal Arctic (B) and low boreal Arctic (LB). While the depth of 25 m is not the maximum depth of macroalgae occurrence, it was the limit of the current survey.
At most sites, the brown algae dominated both in terms abundance and biomass (). The overall biomass ranged from 35 g m−2 to 3443 g m−2. Based on macroalgal biomass, MDS plotting (Clarke Citation1993), revealed a well-defined group of five sites (Fannypynten, Höferpynten, Wilczekodden, Sofiebogen and Rasstupet) in the central fjord (north and south sides). Assemblages from the three other sites are substantially different to the “central fjord” association ().
Fig. 3 Mult-dimensional scaling plot depicting the similarity between sites on the basis of qualitative and quantitative macroalgal distribution.
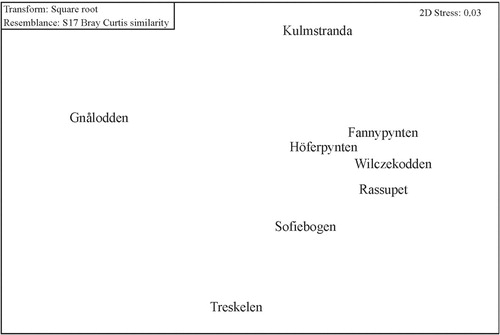
The pattern of distribution of the macroalgal assemblages can be related to the physical environment at each site; at Gnålodden, few algae were observed, which perhaps reflects the intensity of ice-scour. Treskelen, the innermost site, is subject to substantial turbidity which appears to force algae to occupy shallower depths, where they become susceptible the impact of oceanic swell. As a result, biomass and taxonomic richness were reduced. On the other hand, the Kulmstranda's underwater meadows are on the southern bank of the fjord, close to its mouth, where they are sheltered by numerous skerries and underwater rocks; here the assemblage is both rich in taxa and high in biomass. In this area there is little inorganic suspended matter, a pattern typical for fjordic circulation (Svendsen & Thompson Citation1978).
The algal assemblages encountered during the current surveys were far less diverse than those described by Florczyk and Latala (Citation1989), who recorded 48 algal taxa: 13 green, 18 brown and 17 red. This disparity is, at least in part, the result of the different areas sampled. The present survey focused on the zone below 3 m in depth, where underwater meadows occurred and the biomass was highest; the entire intertidal, with its characteristic species-poor assemblage, was omitted. In contrast, Florczyk and Latala studied shallow waters down to 1 m and completed their lists with stranded taxa collected from the lower littoral. Previous researchers collected not only attached algae but also examined stranded drift algae, which might explain the higher concentration of Rhodophyta species in comparison with the material collected by scuba divers or recorded on video.
The domination of Alaria esculenta and Laminaria spp. that was noted at all the sites between 5 and 15 m () appears to be typical for West Spitsbergen glaciated fiords, having previously described in Kongsfjorden (Dunton & Dayton Citation1995; Hop et al. Citation2002; Wiencke et al. Citation2006).
With a total of 70 species, the algal flora in Svalbard is mainly composed of species also found in other regions on both sides of the North Atlantic, such as Laminaria digitata, Saccharina latissima and Saccorhiza dermatodea, or are typical for the lower sub-littoral, for example, Laminaria solidungula (Florczyk & Latala Citation1989). Assemblages similar to those in Svalbard are recorded from the Canadian High Arctic where they are enriched with species of Pacific origin (Wulff et al. Citation2009). In the Western Canadian Arctic Archipelago (Alaskan Beaufort Sea Shelf) the kelp beds at 5–10 m depth are dominated by Laminaria solidungula, Saccharina latissima and Alaria esculenta (Dunton et al. Citation1982). The same species are found in Hornsund, although intertidal algae do not occur along the Alaskan Beaufort Sea except in small areas protected from ice scour.
On the opposite side of the globe, on the rocky shores of the western Antarctic Peninsula, the total number of recorded species of macroalgae is around 130, although it is expected that intensive taxonomic research in the region is expected to lengthen this list (Wiencke & Clayton Citation2002). Direct comparison of the two habitats is difficult because the biogeographic isolation of the Antarctic results in the presence of a large number of endemic species. In addition, there are different cycles of light Hornsund located at higher latitudes, a subsidiary of another light cycle of longer period of complete darkness. Nevertheless, despite these differences, the vertical distribution of macroalgae shows similar trends. The deeper regions are dominated by large, perennial brown algae of the order Desmarestiales (Wiencke & Clayton Citation2002), which are ecologically similar to Laminariales in the Arctic. In both the Arctic and Antarctic, biomass and species richness decline with increasing latitude (Wulff et al. Citation2009).
The southern and northern shores of Hornsund are biologically separable on the basis of their differing hydrology; while the south shore is influenced by the open sea, the northern shore is physically influenced by meltwater. The results from two contrasting sites indicate that the influence of meltwater, which provides fresh matter, particulate organic matter (POM) and dissolved organic compounds, determines the macroalgae productivity of a particular site and the contribution that the macroalgae make to the carbon budget of the fjord (Duggins & Eckman Citation1997).
The importance of the carbon exported by the macroalgae relates to the periodicity of its supply. Unlike the enormous food resource provided by sea-ice algae and offshore phytoplankton blooms, energy is exported from macroalgal forests throughout the year rather than in short discrete pulses (Eilertsen et al. Citation1989; Wiktor Citation1999). By delivering a persistent and predictable food source, benthic algae are capable of providing the core diet of many benthic animals. On the other hand, the food provided by macroalgae—many of which contain compounds such as phenols—is not necessarily as easily assimilated by benthic animals as are unicellular algae. Nevertheless, Duggins & Eckman (Citation1997) demonstrated that when algae-derived particles are suspended for a long time, especially in turbulent, well-mixed waters, their toxicity declines and palatability increases. For those kelp species, with low polyphenol content, Duggins & Eckman suggested that aging on its own is enough to make debris as valuable a food as is phytoplankton.
The algal inventory from Hornsund fjord provides a good starting point for further research in this area, particularly where this is based on the whole ecosystem approach that is needed to provide valid predictions of climate change impacts. If such predictions are to be made and are to be wide reaching then it is vital to broaden the scope of the collection of the type of biological information examined in the present study. Such an approach is logistically unrealistic over broad areas and therefore the production of intensive inventories in biodiversity flagship sites (selected by European Union) will be essential for modelling and predicting the future.
Acknowledgements
We gratefully thank Alexandra Kruss and Jaroslaw Tęgowski for acoustic images, Sergej Olenin and Darius Daunys for methodology and help in fieldwork and Marta Ronowicz and the team for help in fieldwork and samples collection.
References
- Aguilera J. Karsten U. Lippert H. Vögele B. Philipp E. Hanelt D. Wiencke C. Effects of solar radiation on growth, photosynthesis and respiration of marine macroalgae from the Arctic. Marine Ecology Progress Series. 1999; 191: 109–119. 10.3402/polar.v31i0.18900.
- Algaebase. 2012. Macrocystis pyrifera (Linnaeus) C. Agardh. Accessed on the internet at http://www.algaebase.org/search/species/detail/?species_id=4427 on 30 May 2012.
- Burrows E.M. Seaweeds of the British Isles. Chlorophyta. British Museum (Natural History). London, 1991; 2
- Clarke K.R. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology. 1993; 18: 117–143. 10.3402/polar.v31i0.18900.
- Clarke K.R. Gorley R.N. PRIMER v6. User manual/tutorial. PRIMER-E Ltd. Plymouth, 2006
- Dawes C.J. Marine botany. John Wiley & Sons. New York, 1998
- Dowdesell J.A. Hagen J.O. Arctic glaciers and ice caps. Mass balance of the cryosphere. Bamber J.L. Payne A.J. Cambridge University Press. Cambridge, 2004; 527–557.
- Duggins D.O. Eckman J.E. Is kelp detritus a good food for suspension feeders? Effects of kelp species, age and secondary metabolites,. Marine Biology 128. 1997; 489–495.
- Dunton K.H. Dayton P.K. The biology of high latitude kelp. Ecology of fjords and coastal waters. Skjoldal H.R. et al.. Elsevier. Amsterdam, 1995; 499–507.
- Dunton K.H. Reimnitz E.R.K. Schonberg S. An Arctic kelp community in the Alaskan Beaufort Sea. Arctic. 1982; 35: 465–484.
- Eilertsen H.C. Taasen J.P. Weslawski J.M. Phytoplankton studies in the fjords of West Spitsbergen. Physical environment, production in spring and summer. Journal of Plankton Research. 1989; 11: 1245–1260. 10.3402/polar.v31i0.18900.
- Florczyk I. Latała A. The phytobenthos of the Hornsund fiord, SW Spitsbergen. Polar Research. 1989; 7: 29–41. 10.3402/polar.v31i0.18900.
- Gordeev V.V. Fluvial sediment flux to the Arctic Ocean. Geomorphology. 2006; 80: 94–104. 10.3402/polar.v31i0.18900.
- Hop H. Pearson T. Hegseth E.N. Kovacs K.M. Wiencke C. Kwasniewski S. Eiane K. Mehlum F. Culliksen B. Wlodarska-Kowalczuk M. Lydersen C. Weslawski J.M. Cochrane S. Gabrielsen G.W. Leakey R.J.G. Lonne O.J. Zajaczkowski M. Falk-Petersen S. Kendall M. Wangberg S. Bischof K. Voronkov A.Y. Kovaltchouk N.A. Wiktor J. Poltermann M. Prisco G. Papucci C. Gerland S. The marine ecosystem of Kongsfjorden, Svalbard. Polar Research. 2002; 21: 167–208. 10.3402/polar.v31i0.18900.
- Kjellman F.R. The algae of the Arctic Sea. Boktryckeriet. Stockholm, 1883
- Kruss A. Tęgowski J. Wiktor J. Tatarek A. Acoustic estimation of macrophytes in the Hornsund fjord (the Svalbard Archipelago). Hydroacoustics. 2006; 9: 89–96.
- Lee R.K.S. A catalogue of the marine algae of the Canadian Arctic. National Museum of Canada. Ottawa, 1980
- Roleda M.Y. Dethleff D. Wiencke C. Transient sediment load on blades of Arctic Saccharina latissima can mitigate UV radiation effect on photosynthesis. Polar Biology. 2008; 31: 765–769. 10.3402/polar.v31i0.18900.
- Ronowicz M. Wlodarska-Kowalczuk M. Kukliński P. Patterns of hydroid (Cnidaria, Hydrozoa) species richness and distribution in an Arctic glaciated fjord. Polar Biology. 2011; 34: 1437–1445. 10.3402/polar.v31i0.18900.
- Smith S.V. Marine macrophytes as a global carbon sink. Science. 1981; 211: 838–840. 10.3402/polar.v31i0.18900.
- Svendsen P. The algal vegetation of Spitsbergen. A survey of the marine algal flora of the outer part of Isfjorden. Norsk Polarinst Skrifter 116. Norwegian Polar Institute. Oslo, 1959
- Svendsen H. Thompson R.O.R.Y. Wind-driven circulation in a fjord. Hydrodynamics of Estuaries and Fjords. 1978; 23: 546.
- Węsławski J.M. Kendall M.A. Włodarska-Kowalczuk M. Iken K. Kędra M. Legezynska J. Sejr M.K. Climate change effects on Arctic fjord and coastal macrobenthic diversity. Marine Biodiversity. 2011; 41: 71–85. 10.3402/polar.v31i0.18900.
- Węsławski J.M. ZajĄczkowski M. Wiktor J. Szymelfening M. Intertidal zone of Svalbard. 3. Littoral of a Subarctic, oceanic island: Bjornoya. Polar Biology. 1997; 18: 45–52. 10.3402/polar.v31i0.18900.
- Wiencke C. Clayton M.N. Antarctic seaweeds. Synopses of the Antarctic benthos. A.R.G. Gantner Verlag. Ruggell, 2002; 9
- Wiencke C. Clayton M.N. Gómez I. Iken K. Lüder U.H. Amsler C.D. Karsten U. Hanelt K. Bischof K. Dunton K. Life strategy, ecophysiology and ecology of seaweeds in polar waters. Reviews in Environmental Science and Biotechnology. 2006; 6: 95–126. 10.3402/polar.v31i0.18900.
- Wiencke C. Vögele B. Kovaltchouk N.A. Hop H. Species composition and zonation of marine benthic macroalgae at Hansneset in Kongsfjorden, Svalbard. Reports on Polar and Marine Research. 2004; 492: 55–62.
- Wiktor J. Early spring microplankton development under fast ice covered fjords of Svalbard, Arctic. Oceanologia. 1999; 41: 51–72.
- Wiktor J. Okolodkov J. Vinogradova K. Atlas of the marine flora of southern Spitsbergen. Institute of Oceanology, Polish Academy of Sciences. Gdansk, 1995
- Włodarska-Kowalczuk M. Kuklinski P. Ronowicz M. Legeżyńska J. Gromisz S. Assessing species richness of macrofauna associated with macroalgae in Arctic kelp forests (Hornsund, Svalbard). Polar Biology. 2009; 32: 897–905. 10.3402/polar.v31i0.18900.
- Wulff A. Iken K. Quartino M.L. Al-Handal A. Wiencke C. Clayton M.N. Biodiversity, biogeography and zonation of marine benthic micro- and macroalgae in the Arctic and Antarctic. Botanica Marina. 2009; 52: 491–507. 10.3402/polar.v31i0.18900.
- Zają;czkowski M. On the use of sediment traps in sedimentation measurements in glaciated fjords. Polish Polar Research. 2002; 23: 161–174.
- Zhang T. Hehinbottom J. Barry R.G. Brown J. Further statistics on the distribution of permafrost and ground ice in the Northern Hemisphere. Polar Geography. 2000; 24: 126–131. 10.3402/polar.v31i0.18900.
- Zinova D. Opriedielitiel burych vodorosliej Siviernych moriej SSSR. (Atlas of Phaeophyta from north seas of Russia.). Academy of Sciences of the USSR. Leningrad, 1953
- Zinova D. Opriedielitiel krasnych vodorosliej, Sieviernych moriej SSSR. (Atlas of Rhodophyta from north seas of Russia.). Academy of Sciences of the USSR. Leningrad, 1955