Abstract
In the Arctic, areas close to seabird colonies are often characterized by exceptionally rich vegetation communities linked with the high nutrient subsidies transported by seabirds from the marine environment to the land. These areas also support soil invertebrate communities of which springtails (Collembola) often represent the most abundant and diverse group. Our study focused on springtail community composition in the vicinity of seabird (little auk, great skua and glaucous gull) nesting areas in different parts of Svalbard (Magdalenefjorden, Isfjorden and Bjørnøya), and on their comparison with adjacent areas not impacted by seabirds. Out of a total of 35 springtail species recorded, seven were found only within the ornithogenically influenced sites. Although geographical location was the strongest factor differentiating these springtail communities, ornithogenic influence was also significant regardless of the location. When each location was considered separately, seabirds were responsible for a relatively small but strongly significant proportion (8.6, 5.2 and 3.9%, respectively, for each site) of total springtail community variability. Species whose occurrence was positively correlated with seabird presence were Folsomia coeruleogrisea, Friesea quinquespinosa, Lepidocyrtus lignorum and Oligaphorura groenlandica near Magdalenefjorden, Arrhopalites principalis, Folsomia bisetosella and Protaphorura macfadyeni in Isfjorden, and Folsomia quadrioculata on Bjørnøya.
To access the supplementary material for this article, please see supplementary files under Article Tools online.
Arctic terrestrial ecosystems are generally regarded as being relatively simple, species-poor and characterized by short food chains. Very strong seasonality, a short, cold growing season, nutrient deficiency, permafrost, scant liquid water and regular freeze–thaw cycles strongly restrict primary and secondary production (Ims & Ehrich Citation2013). Low energy and limited snow- and ice-free land, the relatively young age of contemporary Arctic terrestrial ecosystems, and spatial isolation contribute to generally low species diversity, especially in the case of higher plants and vertebrate herbivores and predators (Payer et al. Citation2013). However, spatial heterogeneity in, for instance, temperature, precipitation, wind exposure, hydrology, geomorphology (elevation), proximity to coastlines and soil chemistry create environmental gradients and complex mosaics of habitats that may support considerable diversification of communities of smaller organisms such as invertebrates (Hertzberg et al. Citation2000; Sinclair & Sjursen Citation2001; Ims & Ehrich Citation2013). Even very small patches of suitable favourable habitats surrounded by a hostile environment, such as individual Carex tussocks embedded in cyanobacteria-covered ground (Hertzberg et al. Citation1994; Hertzberg et al. Citation2000; Ims et al. Citation2004), Azorella cushion plants on sub-Antarctic Marion Island (Hugo et al. Citation2004), cryoconite holes (De Smet & Van Rompu Citation1994) and glacier mice (Coulson & Midgley Citation2012), may provide viable habitats for these animals.
Most of the Arctic invertebrate fauna inhabit soil and soil surface environments, and these organisms can develop much higher abundance, species diversity and food web complexity in suitable habitats than any other non-microbial eukaryotes on the land (Hodkinson & Coulson Citation2004; Hodkinson Citation2013; Coulson et al. Citation2014). Springtails (Collembola) are often the most abundant and diverse group (Bengston et al. Citation1974; Uvarov & Byzova Citation1995; Birkemoe & Leinaas Citation2000). Although it is widely assumed that springtails play essential roles in many key polar ecosystem processes, such as decomposition, energy flow and nutrient cycling, mainly through grazing on microorganisms and physical alteration of soil and litter (Hopkin Citation1997; Rusek Citation1998; Bardgett & Chan Citation1999; Filser Citation2002), detailed knowledge about the distribution and autecology of most species is lacking (Hogg et al. Citation2006; Hodkinson Citation2013).
Studies of polar (both Arctic and Antarctic) collembolan and other invertebrate assemblages over multiple spatial scales have revealed strong heterogeneity in their distribution and abundance. Between different geographical regions this may be driven by environmental conditions such as temperature and moisture associated with climate (Babenko Citation2000; Bokhorst et al. Citation2008), as well as historical dispersal and colonization processes (Ávila-Jiménez & Coulson Citation2011). In the typical mosaic of High Arctic terrestrial habitats, microtopography and habitat moisture, temperature and habitat quality parameters such as physico-chemical properties of substrate, decomposition rate and food availability affect the invertebrate communities at the scale of meters (Usher & Booth Citation1986; Dollery et al. Citation2006; Caruso & Bargagli Citation2007; Zmudczyńska et al. Citation2012; Hodkinson Citation2013). The size and isolation of habitat patches and especially their plant species composition may further contribute to the variability of springtail assemblages (Hertzberg et al. Citation1994; Hertzberg et al. Citation2000; Ims et al. Citation2004). At the scale of centimetres, invertebrates may be associated with particular plant species and/or local environmental conditions formed beneath them (Coulson et al. Citation1993; Block & Convey Citation1995; Coulson et al. Citation2003; Gwiazdowicz & Coulson Citation2011). Finally, even within relatively homogeneous habitat, clustering of invertebrates may be also explained by inter-species relationships (Usher & Booth Citation1986; Caruso et al. Citation2007; Caruso et al. 2013), pheromone-induced aggregation (Leinaas Citation1983; Usher & Booth Citation1984, Citation1986; Benoit et al. Citation2009), and past stochastic events causing uneven mortality or other demographic phenomena (Coulson et al. Citation2000; Chown & Convey Citation2007).
Within the patchy High Arctic terrestrial ecosystem, particularly favourable habitats for many organisms are found in the vicinity of seabird nesting sites, especially the larger bird colonies which may consist of several hundred thousand individuals (Lindeboom Citation1984; Odasz Citation1994; Stempniewicz et al. Citation2007). These areas are fertilized by nutrients transported by the birds from the marine environment and deposited on land in the form of guano, feathers, egg shells and carcasses (Bokhorst et al. Citation2007; Zwolicki et al. Citation2013). The ornithogenically subsidized areas support exceptionally lush vegetation (Zmudczyńska et al. Citation2008; Zmudczyńska-Skarbek et al. Citation2013) including specific plant communities (Eurola & Hakala Citation1977; Elvebakk Citation1994; Zmudczyńska et al. Citation2009), and populations of herbivores, predators and scavengers (Croll et al. Citation2005; Jakubas et al. Citation2008; Kolb et al. Citation2011).
Collembola feeding on fresh and dead organic matter may specifically graze on or accidentally ingest fungi, algae, bacteria and other microbiota (Hopkin Citation1997; Rusek Citation1998; Worland & Lukešová 2000) and may also attain very high population densities in ornithogenic substrates around seabird colonies (Coulson et al. Citation2014). While the contribution of collembolans to decomposition in the Arctic terrestrial ecosystem is thought to be vital, relatively little attention has been given to their role in ornithogenically influenced habitats (see Mulder et al. Citation2011). Byzova et al. (Citation1995) reported extremely high density and biomass of springtails in the vicinity of a little auk (Alle alle) colony in Hornsund (south-west Spitsbergen), reaching more than 105 ind. and 5 g m−2, these values being higher than reported either in most non-influenced ecosystems or in nutrient-enriched manure heaps. We have also reported similar values both associated with little auks and below a nearby cliff-nesting colony of Brunnich's guillemots (Uria lomvia) and kittiwakes (Rissa tridactyla) (Zmudczyńska et al. Citation2012). High springtail density has also been noted from seabird-influenced sites in another west Spitsbergen fjord, Kongsfjorden (Bengston et al. Citation1974; Sømme & Birkemoe Citation1999), and on Nordaustlandet, the northern-most island of the Svalbard Archipelago (Fjellberg Citation1997). Sømme & Birkemoe (Citation1999) and CitationZmudczyńska et al. (2012) described gradual changes in collembolan community composition with distance from seabird colonies. Specific communities have also been described in areas experiencing the most intensive ornithogenic manuring, with few species strongly represented, including Megaphorura (formerly Onychiurus) arctica, Hypogastrura viatica, Folsomia quadrioculata and Xenylla humicola (Hodkinson et al. 1994; Uvarov & Byzova Citation1995; Fjellberg Citation1997; Sømme & Birkemoe Citation1999; Zmudczyńska et al. Citation2012).
Most studies to date have focused on single seabird colonies and locations, and addressed basic parameters of springtail community description, such as overall species richness, density and biomass of Collembola as a whole, or only focusing on the most abundant species. In a previous study of two seabird colonies at Hornsund, we identified significant correlations between springtail density and soil physical and chemical properties, and vegetation biomass (Zmudczyńska et al. Citation2012). Factors significantly influencing the springtail community in that study included the cover of green nitrophilous alga Prasiola crispa (explaining 11% of the total springtail variability), total plant biomass (9%) and soil conductivity (6%). These factors were all clearly associated with distance from the seabird colonies and guano deposition (see also Zwolicki et al. Citation2013).
In the current study, we used multivariate analytical techniques to generate quantitative estimates of, first, the proportion of total variability in the Collembola community explained by seabird influence. To our knowledge, this question has not previously been tested, and multivariate (ordination) approaches have rarely been applied in the study of ornithogenic impact on invertebrate assemblages worldwide (but see Orgeas et al. Citation2003; Towns et al. Citation2009; Kolb et al. Citation2012; Zmudczyńska et al. Citation2012). Second, this approach will permit the identification of springtail species that are specifically linked with seabird-mediated changes in the environment. We analysed collembolan communities collected over a much wider geographical scale than has been achieved previously, ranging from the relatively small island of Bjørnøya (74°N), which is the southernmost island of Svalbard, to the second largest Spitsbergen fjord Isfjorden located in the centre and mildest part of the island (78°N), to the northern-most west Spitsbergen coast close to Magdalenefjorden (79°N). We obtained samples from six seabird nesting sites and respective control areas. This coverage also enabled us to assess the role of geographical location within the archipelago in explaining variability between the springtail communities found. Finally, as other studies have related the occurrence of Collembola and vegetation communities/species (Hertzberg et al. Citation1994; Coulson et al. Citation2003; Ims et al. Citation2004), we evaluated the strength of this relationship in the vicinity of seabird colonies.
Materials and methods
Study area
The study was conducted at three locations within the Svalbard Archipelago, one on Bjørnøya (Bear Island) and two on Spitsbergen (). Within all sampling plots we identified vascular plant species and visually (using a plot-sized quadrat subdivided into 20×20 cm units) estimated the individual species and total moss percentage contributions to vegetation cover.
Bjørnøya (Bjo). This is the southernmost island (176 km2) of the archipelago, midway between the Norwegian mainland and Spitsbergen. Three sites in the vicinity of different seabird species nesting areas, together with respective control sites, were sampled. Site B-A (74°38′N 19°03′E) was close to a relatively large colony of the planktivorous little auk situated on a gentle slope on Alfredfjellet, exposed to the north and descending to Lake Ellasjøen. The upper part of the site consisted of vegetation-covered rock debris, while the lower part approaching the lake shore was flat and waterlogged. Vegetation cover was complete in both parts of the area. Vegetation consisted of vascular plants, mainly Salix sp. and Saxifragaceae (and Equisetum arvense in the waterlogged area), interspersed with compact moss carpets and clumps. Site B-Ac was the control site for B-A, located on Alfredfjellet and parallel to B-A but ca. 500 m from the colony and separated from it by a seasonal stream. Similar plant species to site B-A were present, but the total cover of vegetation was around 20% (see above for method of estimation). Site B-L (74°47′N 18°78′E) was located in the north-west, flat part of the island, close to the cliff edge and to a concentration of nests of the predatory (feeding on fish, large pelagic invertebrates and other seabirds) glaucous gull (Larus hyperboreus), with patches of dense vegetation surrounding each nest. Vascular plants were usually underlain by a dense moss layer (80% moss cover on average), and were dominated by Festuca cf. rubra subsp. arctica (up to 100%), with less than 10% admixture of Oxyria digyna, Saxifraga caespitosa and Draba sp. Site B-S (74°47′N 18°76′E) was located inland from B-L, in close proximity to nests of the predatory (feeding on fish and other seabirds) great skua (Stercorarius skua). Vegetation was similar to that of B-L, but F. cf. rubra subsp. arctica was less abundant (on average 70% cover), with a generally more species rich herb/shrub flora, and more abundant mosses (90%) present. In addition to the species listed above, Salix sp., Cerastium sp. and other Saxifragaceae were present (up to 80, 20, and <1% cover, respectively). Sites B-Lc and B-Sc were the control sites for B-L and B-S, respectively, and were situated beyond the dense vegetation patches surrounding individual nests, on average 3 m from the relevant nest sites. Total vegetation cover values (B-Lc: 90%, B-Sc: 10%), and especially those of vascular plant species (B-Lc: 10%, B-Sc: 3%), were lower than those of the respective nest sites.
Isfjorden (Isf). Site I-A (78°24′N 15°34′E) was close to a medium-sized little auk colony in Bjørndalen, on the western slope of Platåberget. Vegetation consisted of a mixed community of mosses (on average 40% cover) and vascular plants (35%), with a considerable proportion of Salix polaris (up to 50%, average 25%) and small admixtures of species such as Poa alpina var. vivipara, Cerastium arcticum, Trisetum arvense, O. digyna and Saxifragacae. Site I-Ac was the control site located parallel to I-A but ca. 1 km from the colony. Vegetation was similar to that of I-A, but mosses were more abundant (on average 55%) with vascular plants including T. arvense, O. digyna and P. alpina var. vivipara correspondingly lower (20%).
Magdalenefjorden (Mag). The sites M-A1 and M-A2 (79°52′N 10°70′E) were situated on the talus slope of Aasefjellet, exposed to the west and descending to the open sea, adjacent to very large little auk colonies. The vascular plant layer was underlain by dense moss, giving up to 95% cover, and mostly consisted of C. arcticum, P. alpina var. vivipara and C. groenlandica. Site M-Ac, the control site for both M-A1 and M-A2, was located on Aasefjellet (ca. 700 m and 500 m from M-A1 and M-A2, respectively), facing north and descending to Hamburgbukta. Boulders that were not overgrown with vegetation composed a significant proportion of this area (up to 60% in some plots), with the remaining area predominantly covered by mosses.
Sampling protocol
The study was conducted in the summer months of July and August, during expeditions to Bjørnøya (2008), Magdalenefjorden (2009) and Isfjorden (2010). Around little auk colonies, samples were collected along line transects down the slopes below the seabird colonies (sites: B-A, I-A, M-A1 and M-A2), and in their respective control sites (B-Ac, I-Ac, and M-Ac). Each transect consisted of 5–9 plots (160×160 cm each) that were located from the transect's starting point (plot 1) as follows: plot 2 (6 m), 3 (15 m), 4 (29 m), 5 (49 m), 6 (79 m), 7 (125 m), 8 (193 m) and 9 (296 m), as described by CitationZmudczynska et al. (2012). Due to practical logistic limitations, on transects B-A and B-Ac every second plot was sampled (plots 1, 3, 5, 7 and 9). We collected three soil cores (together with vegetation cover, see below) from three sites along the same diagonal of each sampling plot (from the centre and the two corners of each square) ().
Table 1 Study areas and sampling pattern.
In the cases of great skua and glaucous gull nesting sites located on flat ground, three plots (100×100 cm each) were sampled in the vicinity of each nest: (plot 1) with a nest situated in the centre, (2) adjoining plot 1, still within the patch of dense vegetation surrounding the nest (both plots included in sites B-L and B-S), and (3) beyond the boundary of the compact vegetation patches, 3 m on average from the nest (sites B-Lc and B-Sc, respectively). We collected one soil core from the centre of each plot, except in plot 1 where the sample was taken adjacent to the nest (). Nests containing eggs or chicks were not sampled. Sampled nests had been recently occupied as evidenced by the presence of down and other nest material, and food scraps, but we cannot be fully certain that they had been occupied in that year's summer season.
Samples were taken with a cylindrical probe (diameter 6 cm) from the soil surface (mainly organic) layer, and included the vegetation covering the area and the underlying soil to a depth of ca. 5 cm. Each sample was sealed in a plastic container and, within a few hours, returned to the laboratory where it was subsequently placed for 48 h in a modified Tullgren apparatus illuminated with 60W bulbs (Barton Citation1995). Extracted springtails were preserved in 96% ethanol and identified to species level following Fjellberg (Citation1998, Citation2007). We calculated frequencies of occurrence (%) and densities of particular species (number of individuals per m2). For cores obtained from the 160×160 cm plots, total springtail species counts for each plot (i.e., the sum of the three samples obtained) were analysed.
Statistical analyses
To test for differences in the springtail species richness between the study areas the non-parametric Mann-Whitney U-test was used on account of the non-normal distributions of data and a relatively low number of sampling plots per group tested. Data were processed using STATISTICA 10.0 (StatSoft, Inc. Citation2011).
Numerical ordination methods were used to describe total (qualitative and quantitative) variability of springtail communities and vegetation: (1) independently of any environmental influence (unimodal indirect gradient analysis: detrended correspondence analysis [DCA]), to describe the general pattern of variability in the studied community; and (2) in relation to environmental variables (unimodal direct gradient analysis: canonical correspondence analysis [CCA]). Two nominal environmental factors were tested: area, determining the geographical location (Bjo, Isf or Mag), and seabird, representing the presence (Seabird) or absence (Control) of a seabird colony in the vicinity of sampling sites. All species data were log-transformed to normalize their distributions. After CCA, a Monte Carlo permutation test was performed (with 499 permutations) to identify which of the factors significantly influenced the model. To calculate the factors’ unique contribution to explaining variability in the springtail species composition we used variation partitioning test (ter Braak & Šmilauer Citation2012). To provide more accurate estimation of variation explained with canonical (CCA) analyses, we adjusted the variation value using the number of degrees of freedom as suggested by Peres-Neto et al. (Citation2006). Each time the results of constrained ordination were compared with those of unconstrained ordination (% variability explained by an environmental factor was divided by % variability explained by one (in the case of seabird) or two (area) axes of the unconstrained analysis). Thus we obtained the efficiency of the environmental factor(s) (%) in explaining the total variability present in the data (ter Braak & Šmilauer Citation2012). In order to relate ordinations based on springtail community composition with those of vegetation composition, we used co-correspondence analysis (CoCA; ter Braak & Šmilauer Citation2012). To explore significant relationships between individual springtail species and the environmental factor seabird we employed t-value biplots (Van Dobben circles) that approximated the t-values of the regression coefficients of a weighted multiple regression (ter Braak & Šmilauer Citation2012). Data were processed using CANOCO 5.0 software (ter Braak & Šmilauer Citation2012).
Results
Within the total of 140 plots (246 samples) studied, we recorded 35 springtail species: 17 on Bjørnøya, 21 in Isfjorden and 30 in Magdalenefjorden (, Supplementary Table S1). The average numbers of species were similar across seabird and control sites in all plots taken together and in each location, except for Magdalenefjorden where it was higher in M-A1 than in M-Ac (Mann-Whitney test, U=3.00, p=0.003). However, in total, more species occurred within seabird sites versus their respective control sites in the case of three little auk colonies: I-A (20) versus I-Ac (18), and M-A1 (18) and M-A2 (23) versus M-Ac (14); and in the case of the great skua nesting area: B-S (12) versus B-Sc (eight). On Bjørnøya, the same number of species was noted close to the little auk colony and within its CONTROL site (seven in both B-A and B-Ac), while the number was lower in B-L (seven) than in B-Lc (nine) adjacent to glaucous gull nests. Seven species occurred only within seabird sites: Arrhopalites principalis (B-A, I-A, M-A1), Ceratophysella longispina (M-A2), C. succinea (B-S, M-A2), Desoria neglecta (M-A1), Mesaphorura macrochaeta (I-A), Pseudanuphorus alticola (M-A2) and Sphaeridia pumilis (M-A1) (Supplementary Table S1). Two species were recorded only on control sites: Agrenia bidenticulata (M-Ac) and Thalassaphorura duplopunctata (B-Ac) (Supplementary Table S1).
Table 2 Number of species recorded in each site and area of the current study, and summed up for the seabird and control sites.
Almost 20% of variability in springtail species composition was explained by two hypothetical gradients (unconstrained ordination axes), with 12.1% explained by axis 1 and 7.7% by axis 2 (DCA, gradient length=4.00 SD; , a). CCA (constrained ordination) revealed that the area factor (Bjo, Isf, Mag) was responsible for 13.4% of the total collembolan variability, while the seabird factor (Seabird, Control) accounted for 2.1% (). These factors therefore contributed 67.7% (area) and 17.4% (seabird) of the variation explained by the model. The area and seabird factors were independent of each other and did not share the explained variation (variation partitioning test, F=8.8, p=0.002).
Fig. 2 Detrended correspondence analysis (DCA) ordination of plots classified with respect to (a) springtail species composition and (b) vegetation composition of the different study areas.
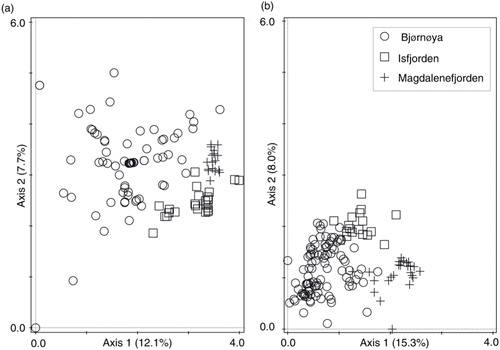
Fig. 3 Canonical correspondence analysis (CCA) ordination of springtail species (triangles) with respect to the influence of different areas—Bjørnøya (Bjo), Isfjorden (Isf) and Magdalenefjorden (Mag)—and the presence (seabird) and absence (control) of a seabird colony in the vicinity of a sampling site (diamonds).
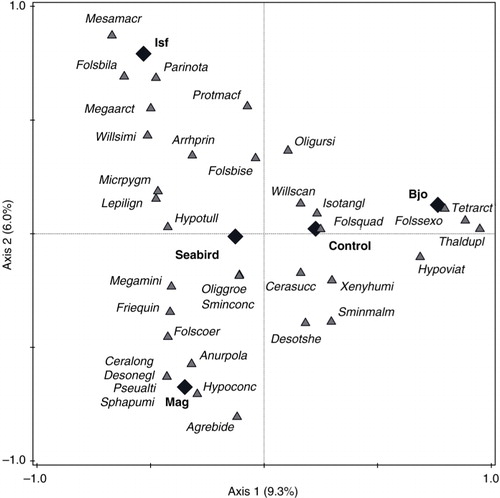
Table 3 Percent of variability in vegetation and collembolan communities explained by hypothetical gradients (axes 1 and 2; detrended correspondence analysis [DCA]) and environmental factors (area and seabird; canonical correspondence analysis [CCA]), with the explaining efficiency of the factors (factors’ contribution to the variation explained by the axes).
The areas studied were distinct with respect to their vegetation composition. The plots from different areas formed distinct groups in the unconstrained ordination (DCA) space based on plant species and moss abundances recorded (b). The area factor explained 19.3% of the total vegetation variability, equating to 83.0% of the variation described with DCA (axis 1: 15.3%, axis 2: 8.0%, gradient length=2.58 SD). The variabilities of springtail and vegetation communities were not significantly correlated (CoCA, p>0.05). Because of the clear differentiation of the three localities (both with respect to collembolan and vegetation diversity, as well as in terms of geographical distance), in subsequent analyses each area was treated separately. Neither springtail nor vegetation variability were significantly co-correlated within any of the individual areas studied (CoCA, p>0.05).
On Bjørnøya, the seabird factor was responsible for 3.9% of the total springtail variability, constituting 30.7% of the variability identified by the theoretical unconstrained analysis (). Of the collembolan species best fitted to the first CCA axis (equivalent to the seabird factor; the species shown in a), Folsomia quadrioculata was significantly positively associated with this explanatory variable while four other species—Desoria tshernovi, Hypogastrura viatica, Tetracanthella arctica and Thalassaphorura duplopunctata—were associated negatively (species selected using Van Dobben circles; ). In Isfjorden, the proportion of variation in collembolan community composition that was influenced by seabird presence was 5.2%, equating to 24.6% of the available variation (). Three species were positively related to seabird impact—Arrhopalites principalis, Folsomia bisetosella and Protaphorura macfadyeni—and two species related to it negatively Lepidocyrtus lignorum and Parisotoma notabilis: (b, ). In Magdalenefjorden, seabird influence accounted for 8.6% of the total springtail variability, or 44.8% of the total available (). Here, four species reacted positively to seabird impact: Folsomia coeruleogrisea, Friesea quinquespinosa, Oligaphorura groenlandica and L. lignorum (which responded negatively in Isfjorden). Among six species that were negatively associated with seabirds in Magdalenefjorden was F. quadrioculata determined as positive in Bjørnøya (c, ). The relationships with seabird influence were still present for nine species (five positive and four negative) when all the areas were analysed together ().
Fig. 4 Canonical correspondence analysis (CCA) ordinations of 10 best-fitted species with respect to the seabird factor (axis 1) in each area. Pie slices based on species percentage occurrence within seabird (black) and control (white) sites. Boldface indicates species that significantly and positively react to the seabird explanatory variable (on the basis of t-value biplot).
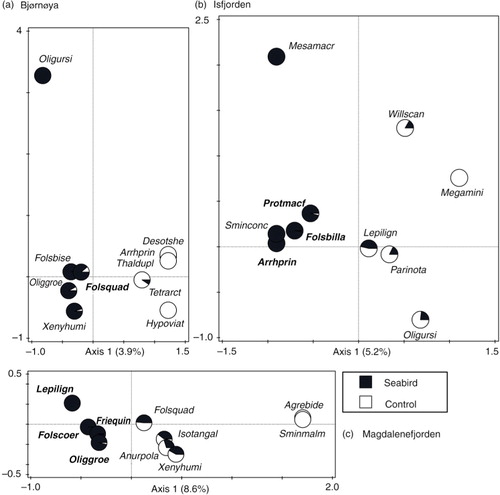
Table 4 Significant positive (+) and negative (−) response of Collembola species for the seabird factor, chosen on the basis of t-value biplots made separately for each area: Bjørnøya (Bjo), Isfjorden (Isf), Magdalenefjorden (Mag) and all the areas together (ALL).
Discussion
Although the key role of colonial seabirds in the enrichment of the otherwise poor Arctic terrestrial ecosystem is relatively well recognized, data on soil invertebrate assemblages inhabiting ornithogenically modified areas are still sporadic. The six previous studies that we are aware of listed species occurring close to rich seabird nesting sites along with, at most, data on overall Collembola density and biomass (Bengston et al. Citation1974; Hodkinson et al. 1994; Byzova et al. Citation1995; Uvarov & Byzova Citation1995; Fjellberg Citation1997; Sømme & Birkemoe Citation1999). Our previous studies at Hornsund have identified some factors significantly correlated with springtail abundance and community composition, including the amount of guano deposited and soil and vegetation parameters that are closely associated with bird colony impact (Zmudczyńska et al. Citation2012; Zwolicki et al. Citation2013). The present study is the first to attempt to estimate quantitatively the proportion of variation in Arctic Collembola communities explained by seabird influence, and one of very few that has addressed this question worldwide (Kolb et al. Citation2011).
Almost 20% of total variability of springtail species composition in our data set (comprising 35 species recorded in 140 sampling plots from different habitats and geographical regions of the Svalbard Archipelago) was explained by two hypothetical environmental gradients (unconstrained DCA axes). The most important factor accounting for this variation (68%) appeared to be related to geographical location. We sampled three widely separated parts of the archipelago. At this regionally large spatial scale the sampling areas differed in exposure to the marine environment and, in particular, to the influence of ocean currents and temperatures of the water masses predominating at each (Loeng & Drinkwater Citation2007; Saskaug et al. 2009). For instance, with a mean July temperature of 5.9°C in 1961–1990 (eKlima Citation2014), Isfjorden is the warmest part of Svalbard, mostly due to the large inflow of warm Atlantic water. Bjørnøya, surrounded by cold Arctic water but also in close proximity to Atlantic water masses, experiences large amounts of fog and high winds (Summerhayes & Elton Citation1923; Saskaug et al. 2009), higher precipitation (30 mm in July compared to 18 mm in Isfjorden) and lower summer temperature (4.4°C in July), while being the mildest throughout winter (−8.1°C in January). The key role of geographical factors underlying Collembola distribution has been emphasized by Caruso & Bargagli (Citation2007) and Babenko (Citation2009) in studies sampling different locations across latitudinal gradients in northern Victoria Land (Antarctica) and on the western Taimyr Peninsula (Russian Arctic), respectively. Although these studies did not assess the total species richness in the study areas, both noted that some species did not occur at all localities along each transect. Caruso & Bargagli (Citation2007) demonstrated that variability in springtail species richness between their study sites was the same whether considering distance scales of 10 km or 100 m. DCA ordination of plots in our study revealed a similar pattern, with some samples from Bjørnøya being more similar to those from other Svalbard areas than others within the island itself (a). Furthermore, although the southernmost and also the most extensively sampled location (117 samples, in comparison with 54 from Isfjorden and 75 from Magdalenefjorden), Bjørnøya hosted the lowest number of springtail species (17 as compared with 21 and 30, respectively). We therefore conclude that the inter-area distinctions identified from our data do not represent a latitudinal gradient but, rather, large-scale variability resulting from a range of factors, including local climate, historical dispersal and colonization processes (Babenko Citation2000; Bokhorst et al. Citation2008; Ávila-Jiménez & Coulson Citation2011; Hodkinson Citation2013).
Similarly, geographical factors may underlie the distinctions identified between the study areas in terms of vegetation community composition, accounting for 83% of the variation described by DCA. In this case, the length of the gradient representing beta-diversity (ter Braak & Šmilauer Citation2012) was lower (2.6 SD) than that calculated for Collembola (4 SD) while, as noted above, there was no correlation apparent between the vegetation and collembolan communities. However, the survey scales also differed between these two groups, with springtails being extracted from cores of 6 cm diameter, and vegetation composition documented in complete 160×160 cm or 100×100 cm plots, areas that are likely to host multiple invertebrate microhabitats (Usher & Booth Citation1986; Caruso & Bargagli Citation2007; Hodkinson Citation2013).
Irrespective of the scale considered and the geographical influence, our analyses demonstrate that seabirds exerted significant influence on springtail communities in Svalbard. Seven of the 35 springtail species recorded were present only in the seabird-influenced sites. Collembolan community variability was explained by the seabird factor to a greater extent when each area was considered separately than when data from all the areas were combined (), probably a consequence of the community composition differing in detail between the areas. Hence, the springtail assemblage recorded within any one area contained one to four species that were positively correlated with the seabird influence, without the same relationship being identified in the other study areas. For instance, Folsomia quadrioculata is recognized as a widely distributed species in the Arctic and has been considered to have no clear habitat preferences (Fjellberg Citation1994), although it has also been recorded as notably abundant below bird cliffs (Fjellberg Citation1997; Sømme & Birkemoe Citation1999; Zmudczyńska et al. Citation2012). In the current study, this species’ presence was significantly correlated with seabird influence on Bjørnøya, but negatively correlated in Magdalenefjorden. Other species positively associated with seabird influence in analyses of the remaining areas are known for their occurrence in rich ornithogenic sites, and include both species characteristic of wet (e.g., Oligaphorura groenlandica) and dry (e.g., Lepidocyrtus lignorum) habitats (Fjellberg Citation1994). Those species for which significant responses were not necessarily found in ordinations (likely due to small sample sizes), but were found exclusively within seabird-influenced sites, were also typical of eutrophic and usually wet or moist habitats. Two such species—Sphaeridia pumilis and Pseudanuphorus alticola (both recorded from Magdalenefjorden)—have previously been recorded only sporadically from Svalbard, the former from Kolhamna/Kongsfjorden (Fjellberg Citation1994) and Russebukta/Edgeøya (www.artsobservasjoner.no), and the latter from Jan Mayen (Fjellberg Citation1994), Kinnvika/Nordaustlandet (Coulson et al. Citation2011) and Barentsburg/Isfjorden (Coulson et al. Citation2013). Notably, some species responding negatively in the current study to the seabird factor (e.g., Parisotoma notabilis, Tetracanthella arctica) or totally absent from the seabird areas (Thalassaphorura duplopunctata) have previously been noted in rich (also ornithogenic), both wet and dry sites (Fjellberg Citation1998, Citation2007). This emphasizes the importance of conducting analyses of the entire springtail community inhabiting an area rather than focusing on individual species that may react differently to a given factor when additional environmental conditions are considered.
A priori, the most obvious potential link by which seabird influence might interact with collembolan communities would appear to be via the soil and vegetation developing on rich ornithogenic substrates around nesting sites, both of which clearly differ from those of non-fertilized areas (Eurola & Hakala Citation1977; Zwolicki et al. Citation2013). We have shown previously that the density of all springtails as well as that of the locally predominating species (F. quadrioculata and H. viatica) were significantly though moderately correlated (correlation coefficients ranging from 0.2 to 0.5) with individual chemical and physical soil properties (Zmudczyńska et al. Citation2012). Each of these factors was strongly correlated to seabird guano deposition (coefficients of 0.6 to 0.9, Zwolicki et al. Citation2013), but only soil conductivity significantly influenced the Collembola composition. Such evidence supports springtails not being directly dependent on a single factor but rather influenced by a range of complex environmental factors. As some studies have identified significantly distinct microarthropod assemblages associated with particular plant species/communities and/or the specific properties of soil forming beneath them (e.g. Coulson et al. Citation2003), we hypothesized that a strong relationship would exist between springtails and vegetation composition in the studied areas. However, no such correlation was apparent in our data, even when each study area was analysed separately. Plants are potentially available for springtails as food (typically during decay rather than through active grazing), as well as contributing to the modification of habitat properties such as soil moisture and temperature. However, seabirds may alter the quality and quantity of several different food resources, including algae, fungi and other microorganisms (Matulła et al. Citation2007; Wright et al. Citation2010). For instance, in Hornsund a green nitrophilous alga species, Prasiola crispa, growing abundantly below the seabird colonies was significantly associated with the collembolan community (Zmudczyńska et al. Citation2012) and might therefore be an important diet component there. Moreover, seabirds may affect distribution and abundance of different invertebrate groups, such as mites, beetles or dipteran larvae that may compete for the resources and/or prey on collembolans (Bengston et al. Citation1974; Caruso et al. Citation2013; Basset et al. Citation2014). Unfortunately, as is often the case in studies of polar terrestrial ecosystems (Worland & Lukešová Citation2000; Hogg et al. Citation2006), detailed autecological information (including diet studies) of most of the springtail species reported here is lacking.
The multivariate analyses applied here provided clear evidence of the significant role that seabird influence plays for these soil invertebrate communities in the Arctic. While the explanatory power of the seabird enrichment was relatively low, the ornithogenic effect was significant both at the scale of the entire Svalbard Archipelago and within each specific geographical location. Other factors, such as small-scale habitat conditions (enhanced by the typical patchiness of tundra habitats [e.g., Hertzberg et al. Citation1994; Ims et al. Citation2004]), population density fluctuations from year to year (Sømme & Birkemoe Citation1999), and natural tendency for aggregation (Leinaas Citation1983; Usher & Booth Citation1984, Citation1986; Benoit et al. Citation2009) contribute additional variation to the Collembola community. Given that springtails provide key ecosystem services contributing to organic matter decomposition, energy flow and nutrient cycling, and considering that these processes, together with the invertebrate communities and colonial seabirds, are expected to be strongly influenced by predicted climate warming, especially in the polar regions (Callaghan et al. Citation2005; Hodkinson Citation2013; Ims & Ehrich Citation2013), further studies of Collembola distribution, abundance and functional ecology are required, with these being planned over appropriate spatial and timescales.
Supplementary Material
Download PDF (250.9 KB)Acknowledgements
We thank Arne Fjellberg for taxonomic examination of the Collembola samples, Dorota Kidawa and Lech Iliszko for assistance in data collection and laboratory analyses during the Bjørnøya and Magdalenefjorden expeditions (respectively), and the University Centre in Svalbard and its staff, particularly Steve Coulson, for facilitating laboratory access during our work in Isfjorden. This study was supported by the Polish Ministry of Science and Higher Education (grant nos. 1883/P01/2007/32 and IPY/25/2007), the Polish–Norwegian Research Fund (PNRF-234-AI-1/07), the Faculty of Biology, University of Gdańsk (538-L120-0816-12), and the Mary & Clifford Corbridge Trust, Cambridge, UK. The study was performed under permit from the Governor of Svalbard.
Notes
To access the supplementary material for this article, please see supplementary files under Article Tools online.
References
- Ávila-Jiménez M.L., Coulson S.J. A holarctic biogeographical analysis of the Collembola (Arthropoda, Hexapoda) unravels recent post-glacial colonization patterns. Insects. 2011; 2: 273–296.
- Babenko A. Collembolan assemblages of polar desert and Subarctic nival communities. Pedobiologia. 2000; 44: 421–429.
- Babenko A.B. Golovatch S.I., etal. Are there many tundra species among Collembola of the tundra belt?. Species and communities in extreme environments. 2009; Sofia: Pensoft Publishers. 113–130.
- Bardgett R.D., Chan K.F. Experimental evidence that soil fauna enhance nutrient mineralization and plant uptake in montane grassland ecosystem. Soil Biology and Biochemistry. 1999; 31: 1007–1014.
- Barton T.R. A modified technique for extracting live ticks from small soil and litter samples. Experimental and Applied Acarology. 1995; 19: 357–360.
- Basset I.E., Elliot G.P., Walker K.J., Thorpe S., Beggs J.R. Are nesting seabirds important determinants of invertebrate community composition on Subantarctic Adams Island. Polar Biology. 2014; 37: 531–540.
- Bengston S.-A., Fjellberg A., Solhöy T. Abundance of tundra arthropods in Spitsbergen. Entomologica Scandinavica. 1974; 5: 137–142.
- Benoit J.B., Elnitsky M.A., Schulte G.G., Lee R.E. Jr., Denlinger D.L. Antarctic collembolans use chemical signals to promote aggregation and egg laying. Journal of Insect Behavior. 2009; 22: 121–133.
- Birkemoe T., Leinaas H.P. Effects of temperature on the development of an Arctic Collembola (Hypogatrura tullbergi). Functional Ecology. 2000; 14: 693–700.
- Block W., Convey P. The biology, life cycle and ecophysiology of the Antarctic mite Alaskozetes antarcticus (Michael). Journal of Zoology. 1995; 236: 431–449.
- Bokhorst S., Huiskes A., Convey P., Aerts R. External nutrient inputs into terrestrial ecosystems of the Falkland Islands and the maritime Antarctic. Polar Biology. 2007; 30: 1315–1321.
- Bokhorst S., Huiskes A., Convey P., van Bodegom P.M., Aert R. Climate change effects on soil arthropod communities from the Falkland Islands and the maritime Antarctic. Soil Biology & Biochemistry. 2008; 40: 1547–1556.
- Byzova J.B., Uvarov A.V., Petrova A.D. Seasonal changes in communities of soil invertebrates in tundra ecosystem of Hornsund, Spitsbergen. Polish Polar Research. 1995; 16: 245–266.
- Callaghan T.V., Björn L.O., Chapin F.S. III, Chernov Y., Christensen T.R., Huntley B., Ims R., Johansson M., Riedlinger D.J., Jonasson S., Matveyeva N., Oechel W., Panikov N., Shaver G. Symon C., etal. Arctic tundra and polar desert ecosystems. Arctic climate impact assessment—scientific report. 2005; Cambridge: Cambridge University Press. 243–352.
- Caruso T., Bargagli R. Assessing abundance and diversity patterns of soil microarthropod assemblages in northern Victoria Land (Antarctica). Polar Biology. 2007; 30: 895–902.
- Caruso T., Borghini F., Bucci C., Colacevich A., Bargagli R. Modelling local-scale determinants and the probability of microarthropod species occurrence in Antarctic soils. Soil Biology and Biochemistry. 2007; 39: 2949–2956.
- Caruso T., Trokhymets V., Bargagli R., Convey P. Biotic interactions as a structuring force in soil communities: evidence from the micro-arthropods of an Antarctic moss model system. Oecologia. 2013; 172: 495–503.
- Chown S.L., Convey P. Spatial and temporal variability across life's hierarchies in the terrestrial Antarctic. Philosophical Transactions of the Royal Society B. 2007; 362: 2307–2331.
- Coulson S., Hodkinson I.D., Strathdee A., Bale J.S., Block W., Worland M.R., Webb N.R. Simulated climate change: the interaction between vegetation type and microhabitat temperatures at Ny Ålesund, Svalbard. Polar Biology. 1993; 13: 67–70.
- Coulson S.J., Convey P., Aakra K., Aarvik L., Ávila-Jiménez M.L., Babenko A., Biersma E., Boström S., Brittain J., Carlsson A., Christoffersen K.S., De Smet W.H., Ekrem T., Fjellberg A., Fuereder L., Gustafsson D., Gwiazdowicz D.J., Hågvar S., Hansen L.O., Kaczmarek L., Kolicka M., Kuklin V., Lakka H.-K., Lebedeva N., Makarova O., Maraldo K., Melekhina E., Ødegaard F., Pilskog H.E., Simon J.C., Sohlenius B., Solhøy T., Søli G., Stur E., Tanasevitch A., Taskaeva A., Velle G., Zmudczynska-Skarbek K. The terrestrial and freshwater invertebrate biodiversity of the archipelagoes of the Barents Sea; Svalbard, Franz Josef Land and Novaya Zemlya. Soil Biology and Biochemistry. 2014; 68: 440–470.
- Coulson S.J., Fjellberg A., Gwiazdowicz D.J., Lebedeva N.V., Melekhina E.N., Solhøy T., Erséus C., Maraldo K., Miko L., Schatz H., Schmelz R.M., Søli G., Stur E. The invertebrate fauna of anthropogenic soils in the High-Arctic settlement of Barentsburg, Svalbard. Polar Research. 2013; 32: 19273. doi: http://dx.doi.org/10.3402/polar.v32i0.19273.
- Coulson S.J., Fjellberg A., Snazell R., Gwiazdowicz D.J., Ávila-Jiménez M.L. On the Collembola, Araneae and Gamasida from the Kinnvika region of Nordaustlandet, Svalbard. Geografiska Annaler. 2011; 93: 253–257.
- Coulson S.J., Hodkinson I.D., Webb N.R. Microscale distribution patterns in High Arctic soil microarthropod communities: the influence of plant species within the vegetation mosaic. Ecography. 2003; 26: 801–809.
- Coulson S.J., Leinaas H.P., Ims R.A., Søvik G. Experimental manipulation of the winter surface ice layer: the effects on a High Arctic soil microarthropod community. Ecography. 2000; 23: 299–306.
- Coulson S.J., Midgley N.G. The role of glacier mice in the invertebrate colonisation of glacial surfaces: the moss balls of the Falljökull, Iceland. Polar Biology. 2012; 35: 1651–1658.
- Croll D.A., Maron J.L., Estes J.A., Danner E.M., Byrd G.V. Introduced predators transform Subarctic islands from grassland to tundra. Science. 2005; 307: 1959–1961.
- De Smet W.H., Van Rompu E.A. Rotifera and Tardigrada from some cryoconite holes on a Spitsbergen (Svalbard) glacier. Belgian Journal of Zoology. 1994; 124: 27–37.
- Dollery R., Hodkinson I.D., Jónsdóttir I.S. Impact of warming and timing of snow melt on soil microarthropod assemblages associated with Dryas-dominated plant communities on Svalbard. Ecography. 2006; 29: 111–119.
- eKlima. Monthly normal values. 2014. Norwegian Meteorological Institute. Accessed on the internet at http://sharki.oslo.dnmi.no on 14 July 2014..
- Elvebakk A. A survey of plant associations and alliances from Svalbard. Journal of Vegetation Science. 1994; 5: 791–802.
- Eurola S., Hakala A.V.K. The bird cliff vegetation of Svalbard. Aquilo Series Botanics. 1977; 15: 1–18.
- Fjellberg A. The Collembola of the Norwegian Arctic islands. 1994; Oslo: Norwegian Polar Institute. Meddelelser 133.
- Fjellberg A. Collembola from Nordaustlandet, Svalbard. Fauna Norvegica B. 1997; 44: 71–75.
- Fjellberg A. The Collembola of Fennoscandia and Denmark. Part 1: Poduromorpha. 1998; Leiden: Brill. Fauna Entomologica Scandinavica 35.
- Fjellberg A. The Collembola of Fennoscandia and Denmark. Part 2: Entomobryomorpha and Symphypleona. 2007; Leiden: Brill. Fauna Entomologica Scandinavica 42.
- Filser J. The role of Collembola in carbon and nitrogen cycling in soil. Pedobiologia. 2002; 46: 234–245.
- Gwiazdowicz D.J., Coulson S.J. High-Arctic gamasid mites (Acari, Mesostigmata): community composition on Spitsbergen, Svalbard. Polar Research 30. 2011; 8311. doi: http://dx.doi.org/10.3402/polar.v30i0.8311.
- Hertzberg K., Leinaas H.P., Ims R.A. Patterns of abundance and demography: Collembola in habitat patch gradient. Ecography. 1994; 17: 349–359.
- Hertzberg K., Yoccoz N.G., Ims R.A., Leinaas H.P. The effects of spatial habitat configuration on recruitment, growth and population structure in Arctic Collembola. Oecologia. 2000; 124: 381–390.
- Hodkinson I.D. Meltofte H. Terrestrial and freshwater invertebrates. Arctic biodiversity assessment. Status and trends in Arctic biodiversity. 2013; Akureyri: Conservation of Arctic Flora and Fauna. 247–275.
- Hodkinson I.D., Coulson S.J. Are High Arctic terrestrial food chains really that simple?—Bear Island food web revisited. Oikos. 2004; 106: 427–431.
- Hodkinson I.D., Coulson S., Webb N.R, Block W., Strathdee A.T., Bale J.S. Feeding studies on Onychiurus arcticus (Tullberg) (Collembola: Onychiuridae) on west Spitsbergen. Polar Biology. 1994; 14: 17–19.
- Hogg I.D., Cary S.C., Convey P., Newsham K., O'Donnell T., Adams B.J., Aislabie J., Frati F.F., Stevens M.I., Wall D.H. Biotic interactions in Antarctic terrestrial ecosystems: are they a factor?. Soil Biology and Biochemistry. 2006; 38: 3035–3040.
- Hopkin S.P. Biology of the springtails. Insecta: Collembola. 1997; Oxford: Oxford University Press.
- Hugo E.A., McGeoch M.A., Marshall D.J., Chown S.L. Fine scale variation in microarthropod communities inhabiting the keystone species Azorella selago on Marion Island. Polar Biology. 2004; 27: 466–473.
- Ims R.A., Ehrich D. Meltofte H. Terrestrial ecosystems. Arctic biodiversity assessment. Status and trends in Arctic biodiversity. 2013; Akureyri: Conservation of Arctic Flora and Fauna. 385–440.
- Ims R.A., Leinaas H.P., Coulson S. Spatial and temporal variation in patch occupancy and population density in a model system of an Arctic Collembola species assemblage. Oikos. 2004; 105: 89–104.
- Jakubas D., Zmudczynska K., Wojczulanis-Jakubas K., Stempniewicz L. Faeces deposition and numbers of vertebrate herbivores in the vicinity of planktivorous and piscivorous seabird colonies in Hornsund, Spitsbergen. Polish Polar Research. 2008; 29: 45–58.
- Kolb G.S., Jerling L., Essenberg C., Palmborg C., Hambäck P.A. The impact of nesting cormorants on plant and arthropod diversity. Ecography. 2012; 35: 726–740.
- Kolb G.S., Young H.S., Anderson W.B. Mulder C.P.H., etal. Effects of seabirds on islands consumers. Seabird islands. Ecology, invasion and restoration. 2011; New York: Oxford University Press. 212–241.
- Leinaas H.P. Synchronized moulting controlled by communication in group living Collembola. Science. 1983; 219: 193–195.
- Lindeboom H.J. The nitrogen pathway in a penguin rookery. Ecology. 1984; 65: 269–277.
- Loeng H., Drinkwater K. An overview of the ecosystems of the Barents and Norwegian seas and their response to climate variability. Deep-Sea Research Part II. 2007; 54: 2478–2500.
- Matula J., Pietryka M., Richter D., Wojtun B. Cyanoprokaryota and algae of Arctic terrestrial ecosystems in the Hornsund area, Spitsbergen. Polish Polar Research. 2007; 28: 283–315.
- Mulder C.P.H., Anderson W.B., Towns D.R., Bellingham P.J. Seabird islands. Ecology, invasion and restoration. 2011; New York: Oxford University Press.
- Odasz A.M. Nitrate reductase activity in vegetation below an Arctic bird cliff, Svalbard, Norway. Journal of Vegetation Science. 1994; 5: 913–920.
- Orgeas J., Vidal E., Ponel P. Colonial seabirds change beetle assemblages on a Mediterranean island. Ecoscience. 2003; 10: 38–44.
- Payer D.C., Josefson A.B., Fjeldså J. Meltofte H. Species diversity in the Arctic. Arctic biodiversity assessment. Status and trends in Arctic biodiversity. 2013; Akureyri: Conservation of Arctic Flora and Fauna. 67–77.
- Peres-Neto P.R., Legendre P., Dray S., Borcard D. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology. 2006; 87: 2614–2625.
- Rusek J. Biodiversity of Collembola and their functional role in the ecosystem. Biodiversity and Conservation. 1998; 7: 1207–1219.
- Saskaug E., Johnsen G.H., Kovacs K.M. Ecosystem Barents Sea. 2009; Trondheim: Tapir Academic Press.
- Sinclair B.J., Sjursen H. Terrestrial invertebrate abundance across a habitat transect in Keble Valley, Ross Island, Antarctica. Pedobiologia. 2001; 45: 134–145.
- Sømme L., Birkemoe T. Demography and population densities of Folsomia quadrioculata (Collembola, Isotomidae) on Spitsbergen. Norwegian Journal of Entomology. 1999; 46: 35–45.
- StatSoft, Inc. Team. STATISTICA, version 10. 2011; Tulsa, OK: StatSoft, Inc.
- Stempniewicz L., Błachowiak-Samołyk K., Wesławski J.M. Impact of climate change on zooplankton communities, seabird populations and arctic terrestrial ecosystem—a scenario. Deep-Sea Research Part II. 2007; 54: 2934–2945.
- Summerhayes V.S., Elton C.S. Contributions to the ecology of Spitsbergen and Bear Island. Journal of Ecology. 1923; 11: 214–286.
- ter Braak C.J.F., Šmilauer P. CANOCO reference manual and user's guide: software for ordination (version 5.0). 2012; Ithaca, NY: Microcomputer Power.
- Towns D.R., Wardle D.A., Mulder C.P.H., Yeates G.W., Fitzgerald B.M., Parrish G.M., Bellingham P.J., Bonner K.I. Predation of seabirds by invasive rats: multiple indirect consequences for invertebrate communities. Oikos. 2009; 118: 420–430.
- Usher M.B., Booth R.G. Arthropod communities in a maritime Antarctic moss–turf habitat: three-dimensional distribution of mites and Collembola. Journal of Animal Ecology. 1984; 53: 427–441.
- Usher M.B., Booth R.G. Arthropod communities in a maritime Antarctic moss–turf habitat: multiple scales of pattern in the mites and Collembola. Journal of Animal Ecology. 1986; 55: 155–170.
- Uvarov A.V., Byzova J.B. Species diversity and distribution of Collembola in the vicinity of Polish Polar Station, Hornsund area, Spitsbergen. Polish Polar Research. 1995; 16: 233–243.
- Worland M.R., Lukešová A. The effect of feeding on specific soil algae on the cold-hardiness of two Antarctic microarthropods (Alaskozetes antarcticus and Cryptopygus antarcticus). Polar Biology. 2000; 23: 766–774.
- Wright D.G., van der Wal R., Wanless S., Bardgett R.D. The influence of seabird nutrient enrichment and grazing on the structure and function of island soil food webs. Soil Biology & Biochemistry. 2010; 42: 592–600.
- Zmudczyńska K., Olejniczak I., Zwolicki A., Iliszko L., Convey P., Stempniewicz L. Influence of allochtonous nutrients delivered by colonial seabirds on soil collembolan communities on Spitsbergen. Polar Biology. 2012; 35: 1233–1245.
- Zmudczyńska K., Zwolicki A., Barcikowski M., Barcikowski A., Stempniewicz L. Spectral characteristics of the Arctic ornithogenic tundra vegetation in Hornsund area, SW Spitsbergen. Polish Polar Research. 2009; 30: 249–262.
- Zmudczyńska K., Zwolicki A., Barcikowski M., Iliszko L., Stempniewicz L. Variability of individual biomass and leaf size of Saxifraga nivalis L. along transect between seabirds colony and seashore in Hornsund, Spitsbergen. Ecological Questions. 2008; 9: 37–44.
- Zmudczyńska-Skarbek K., Barcikowski M., Zwolicki A., Iliszko L., Stempniewicz L. Variability of polar scurvygrass Cochlearia groenlandica along a seabird influenced gradient across Spitsbergen tundra. Polar Biology. 2013; 36: 1659–1669.
- Zwolicki A., Zmudczyńska-Skarbek K., Iliszko L., Stempniewicz L. Guano deposition and nutrient enrichment in the vicinity of planktivorous and piscivorous seabird colonies in Spitsbergen. Polar Biology. 2013; 36: 363–372.