Abstract
In models of the marine carbon system, it is important to correctly represent riverine and aerial inputs of dissolved inorganic carbon (DIC) and alkalinity. We have examined the different processes contributing to this exchange. In terms of DIC, we have divided the fluxes into their internal component, constituting the carbon ultimately derived from the atmosphere, and their external component originating from rocks. We find that the only process contributing to external DIC input is carbonate and fossil carbon weathering and that erosion of organic matter ultimately constitutes a DIC sink. A number of both riverine and aerial inputs affect the alkalinity. Beside carbonate and silicate weathering, we examine processes of pyrite weathering, aerial input of sulphuric acid, and riverine and aerial inputs of various nitrogen species. Using the observation that, in the ocean, the nitrate concentration follows that of phosphate, we assume a steady state in nitrate. This leads to the surprising result that the only processes affecting the alkalinity is riverine input of nitrate, constituting an alkalinity source and input of ammonia, constituting an alkalinity sink. Furthermore, we compare the flux sizes. As expected, carbonate and silicate weathering has the largest effect on alkalinity, though we note that burial of pyrite might be of importance during periods of large-scale anoxia.
1. Introduction
This work was inspired by Chuck et al. (Citation2005). They present a simple global three-box ocean carbon model with constant riverine sources of dissolved inorganic carbon (DIC) and alkalinity. On closer inspection, we found that the riverine source of DIC seemed unrealistically large. Furthermore, we found their description of the alkalinity effect due to nitrogen input confusing. We will attempt to unravel the complexities involved in describing the fluxes of DIC and alkalinity between land and ocean.
To understand the global carbon system, it is important to understand the sources and sinks of the system. If we define the earth system to constitute the above ground realm and the ocean, we may call processes that create a flux of carbon from rocks or volcanic activity external sources to the earth system. Similarly, processes that generate a carbon flux out of the system, e.g. through sedimentation, may be called external sinks. Several difficulties arise when considering these sources and sinks. First, fluxes of both carbon and alkalinity need to be considered. Second, some of the processes that constitute carbon sources or sinks also generate fluxes between reservoirs within the earth system. These internal fluxes need to be recognised so that carbon that is in fact derived from inside the system is not considered as an external source.
The most common processes that constitute sources or sinks for DIC and alkalinity included in models are carbonate and silicate weathering (e.g. Walker and Kasting, Citation1992; Shaffer et al., Citation2008; Zeebe, Citation2012) together with sedimentation of carbonate and sometimes burial of organic carbon (e.g. Chuck et al., Citation2005; Shaffer et al., Citation2008). Weathering and burial of pyrite are but rarely considered (e.g. Goddéris et al., Citation2001; Arvidson et al., Citation2006). However, though the magnitude of pyrite weathering is small and it thus comprises only a minor alkalinity sink, the reversed process, burial of pyrite, has the potential to play an important role during periods of large-scale ocean anoxia for the global oxygen as well as for the carbon system. We note that pyrite burial besides being a source of oxygen also provides a sink for carbon dioxide much like the weathering of silicate minerals.
The riverine fluxes of DIC and alkalinity are used to estimate the transport from continents to ocean. However, the flow of DIC in river water is to a certain degree derived from the atmosphere. Knowledge of the processes that gave rise to the DIC flux is therefore necessary to distinguish between external input and internal exchange.
In an attempt to clarify the concepts and increase our understanding of the inputs to the ocean, we investigate the processes contributing to the riverine and aerial fluxes of DIC and alkalinity. How do we find what is added externally and what is really an exchange between reservoirs within the system? How large are the contributions from the different processes and how are they determined in practice?
As riverine fluxes often are expressed as fluxes of dissolved ions, we will convert these to fluxes of DIC and alkalinity. We will further divide them into their external and internal parts.
In Section 2, we investigate the specific processes contributing to the riverine flux of DIC and alkalinity. A presentation of available data is given in Section 3. We will here also review how the weathering fluxes are determined from the data. Furthermore, values for the weathering fluxes and the resulting DIC and alkalinity fluxes are presented. Discussion and conclusions are found in Section 4.
2. Processes affecting the carbon system
DIC and alkalinity are the two variables most often used to define the carbonate system in ocean water. DIC is the sum of the concentrations of the dissolved carbon species in ocean water, and as it is a conservative quantity, it is affected only by an addition or removal of dissolved carbon. Alkalinity is a more complicated concept. It is generally defined as the excess of proton acceptors over proton donors (see Wolf-Gladrow et al. (Citation2007), for a thorough analysis of the concept of alkalinity).
There are a number of different processes that contribute to the sources and sinks of DIC and alkalinity. We will start by rock weathering followed by an investigation of the effect of riverine transport of organic material. We will also look at the effect of aerial input of sulphuric acid. To conclude this section, we will discuss the different aspects of the nitrogen cycle and its effect on the alkalinity.
2.1. Weathering
A number of authors have previously considered atmospheric CO2 consumption during weathering (e.g. Amiotte Suchet and Probst, Citation1993, Citation1995; Meybeck, Citation1993a; Gaillardet et al., Citation1999; Lerman et al., Citation2007). Lerman et al. (Citation2007) address the issue of CO2 demand in carbonate and silicate weathering by introducing a weathering potential defined as the fraction of CO2 consumed to bicarbonate produced in a certain reaction (see also Mackenzie and Lerman, 2006). We chose a different approach in which we examine the direct effect of the different weathering processes on the oceanic carbon system state variables, DIC and alkalinity. We further divide the DIC produced from weathering into an internal component stemming from exchange between the atmosphere and the ocean, and an external component originating from rocks. The external component may be found by adding the effect of a certain weathering process on the atmosphere to the effect on the ocean. To clearly define the external and internal component originating from the weathering processes, we will start by reviewing the weathering reactions.
2.1.1. Carbonates.
Carbonate minerals exists mainly as calcium carbonate, in the form of calcite and the less stable form of aragonite, and dolomite as a combination of magnesium and calcium carbonate.
The weathering of calcium carbonate and dolomite occurs according to the following reactions:1
2
where CO2,atm represents carbon dioxide that is ultimately derived from the atmosphere. The produced bicarbonate is assumed to eventually reach the ocean.
For both reactions, one mole of carbon dioxide from the atmosphere results in two moles of oceanic DIC. Adding the atmosphere and ocean generates the effect on the total system, i.e. the external source, which equals one mole of DIC for every mole of calcium carbonate in reaction. We also find that, for both reactions, every mole of cation produced is accompanied by two moles of alkalinity. The effect on the system due to weathering of calcium carbonate is summarised in .
2.1.2. Weathering of silicates.
Silicate minerals are of more complicated nature than the carbonates. Silicate form minerals together with the cations Ca2+, Mg2+, Na+ and K+. We demonstrate the weathering of silicates by the prototype reaction3
We conclude that for every mole of calcium silicate in reaction, the oceanic DIC and the alkalinity increase by two moles/equivalents respectively. We also see that the concentration of CO2 is reduced by two moles. In contrast, by investigating the effect on the total system, we find that the external source of DIC is equal to zero. This can also be deduced by realising that calcium silicate does not contain carbon, and hence its dissolution does not add DIC to the system. The effects are summarised in .
Reversal of reaction (3) does not occur in the ocean. The released silicic acid is utilised by organisms without any effect on the DIC or alkalinity (Garrels and Perry, Citation1974). However, metamorphic transformation of opal to magnesium and calcium silicates occurs according to the Urey reactions (e.g. Berner et al., Citation1983).
2.1.3. Weathering of sulphides.
Sulphur exists in sediments as gypsum (CaSO4) and pyrite (FeS2). Weathering of gypsum results in the release of calcium cations and sulphate without any effect on the carbon system.
Weathering of pyrite is described by4
The absence of carbon in reaction (4) leaves the DIC of the system unchanged. The alkalinity is decreased by two equivalents for every mole of produced sulphate (). Lerman et al. (Citation2007) take this process into account by a more indirect approach in which the acid produced results in further weathering of silicates and carbonates.
The reversal of pyrite weathering described by reaction (4) occurs in anoxic sediments. Organic matter is oxidised by sulphate instead of oxygen to produce hydrogen sulphide. The hydrogen sulphide, in turn, acts to form pyrite through reaction with sedimentary iron oxides (Berner, Citation1984). The net of photosynthesis, sulphate reduction and pyrite formation is described by reaction (4) from right to left.
2.1.4. Weathering of fossil carbon.
Fossil carbon weathers through5
Reversal of reaction (5) is essentially burial of organic carbon. Weathering of fossil carbon adds DIC to the ocean or CO2 to the atmosphere while the alkalinity remains unchanged ().
2.2. Erosion of organic matter
Rivers transport large amounts of organic carbon from land to the ocean. This consists of carbon, ultimately derived from the atmosphere. A part of the transported material is dissolved upon reaching the ocean, and it thus constitutes an internal exchange between the atmosphere and the ocean. The rest is buried thereby constituting a sink for atmospheric CO2.
There is also the option that dissolved organic carbon (DOC) reaching the ocean may accumulate in the water column. Due to the short time-scale characterising this reservoir this option is not considered important (Smith and Hollibaugh, Citation1993).
2.3. Aerial input of sulphuric acid
Deposition of the sulphuric acid over the ocean results in a reduction of alkalinity according to6
For every mole of H2SO4 deposited, the alkalinity is reduced by two equivalents.
2.4. The nitrogen cycle
There are a number of processes within the nitrogen cycle that affect the alkalinity which complicates the modelling. In an attempt to clarify this difficulty, we will review the nitrogen cycle processes one-by-one and discuss the direct alkalinity effects, and the implications of these effects assuming a steady state oceanic nitrate concentration. A schematic of the processes involved in the nitrogen cycle and the associated alkalinity effects is shown in .
Fig. 1 Reservoirs and processes within the marine nitrogen cycle. The arrows represent exchange processes and the numbers are the associated alkalinity effects.
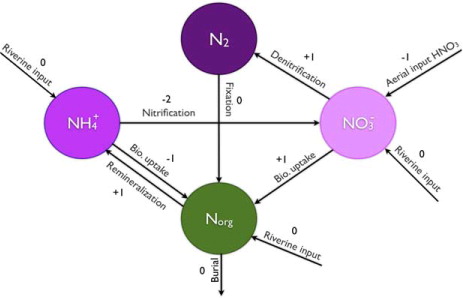
2.4.1. Nitrate uptake and remineralization.
Production of organic nitrogen using nitrate as the nutrient source may be described as7
where NH3,org is ammonia incorporated into organic material.
Eventually the organic material is remineralized according to8
Remineralization returns the nitrogen taken up through photosynthesis in the form of ammonia, which at seawater pH reacts with hydrogen to form ammonium, regardless of which form of reactive nitrogen was taken up originally.
Wolf-Gladrow et al. (Citation2007) introduce the term ‘nutrient-H + -compensation principle’ to describe the organic production. To maintain neutral charge, the uptake of a negatively charged nitrate ion needs to be accompanied by an uptake of a hydrogen ion. The alkalinity is increased by one equivalent for every mole of nitrate uptake and further increased by one equivalent after remineralization. This is represented in by an arrow pointing from the nitrate reservoir towards the organic nitrogen and an arrow representing remineralization pointing from the organic nitrogen towards the ammonium reservoir.
2.4.2. Nitrogen fixation.
Through nitrogen fixation, nitrogen gas is converted into organic material according to9
Hence, nitrogen fixation has no effect on the alkalinity until the organic material is remineralized.
2.4.3. Nitrification.
Ammonium is converted to nitrate through nitrification, which is described by10
The alkalinity is thus reduced by two equivalents for every mole of ammonium in reaction.
2.4.4. Denitrification.
In anoxic sediments, nitrate is used as a reduction agent in a process termed denitrification. Nitrate is thereby converted into nitrogen gas.11
The corresponding change in alkalinity is a one equivalent increase for every mole of nitrate in reaction.
2.4.5 Aerial and riverine inputs.
Reactive nitrogen is also supplied to the ocean through aerial input of ammonia and nitrous acid, and through riverine input of nitrate, ammonium and organic nitrogen.
Nitrous acid dissolves in water through the reaction12
The produced hydrogen ions result in a one equivalent decrease of alkalinity for every mole of HNO3.
Riverine input of nitrate is not accompanied by hydrogen ions and thus the alkalinity is unchanged. The same argument holds for riverine input of ammonium. Also shown in is riverine input of organic nitrogen which has no immediate effect on alkalinity.
2.4.6 Reduced system.
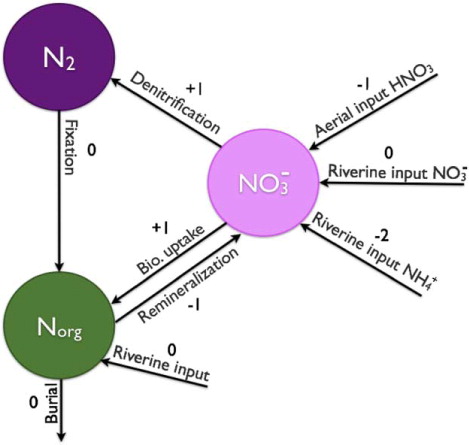
By a couple of simplifying assumptions it is possible to reduce the nitrogen system shown in . First, we assume that the ammonium is rapidly nitrified. This is a good and often implemented simplification (Doney et al., Citation2007). Ammonium is the preferred form of reactive nitrogen. It is used up fast and exists in much smaller concentrations in the water than nitrate.
Using this assumption, we may short-circuit the ammonium reservoir and arrive at the simplified representation shown in . Remineralization is now represented by an arrow pointing directly towards the nitrate reservoir. The alkalinity effect associated with remineralization is now the net of remineralization and nitrification and it is the reverse of the effect due to biological production. Furthermore, the riverine input of ammonium now points towards the nitrate reservoir and the associated alkalinity change is equal to the effect due to nitrification.
Fig. 2 Simplified representation of the marine nitrogen cycle. The ammonium is assumed instantly nitrified. The numbers coupled to the arrows are the alkalinity effect associated with each exchange process.
Finally, we make one last simplification. The oceanic pool of organic nitrogen is small compared to the pool of nitrate. In the same manner as for the ammonium reservoir, we short-circuit the organic nitrogen reservoir and end up with a system of only two reservoirs: the oceanic nitrate and the atmospheric nitrogen gas. This simplified system is illustrated in . Burial of organic matter now constitutes a source for alkalinity. This is because the organic matter burial represents the net biological production – the difference between what is produced and what is remineralized. Riverine input of organic nitrogen must now be considered as it is assumed to be rapidly remineralized and nitrified.
Fig. 3 Simplified representation of the marine nitrogen cycle. The ammonium is assumed to be nitrified as in . Furthermore, the organic nitrogen reservoir is assumed small compared to the nitrate reservoir.
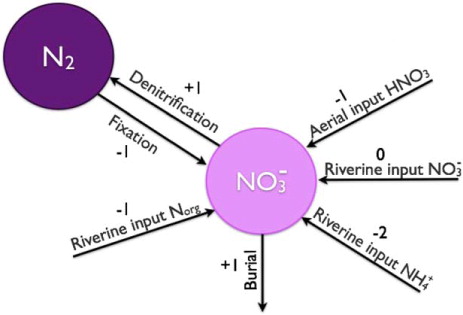
The final system can be described in terms of four reactions. Nitrogen fixation may now be expressed as the opposite of denitrification [reaction (11)]. Aerial input of nitrous acid is still represented by reaction (12) and riverine input of ammonium is now described by the reaction for nitrification [reaction (10)]. Riverine input of organic nitrogen is described by the net of remineralization [reaction (8)] and nitrification [reaction (10)], i.e.13
Hence, the alkalinity is reduced by one equivalent for every mole of riverine organic matter that reaches the ocean.
2.4.7. Steady state.
It has long been observed that the concentration of nitrate follows that of phosphate. Broecker and Peng (Citation1982) discuss that nitrogen fixation and denitrification are mechanisms that control the concentration of nitrate. If the nitrate concentration is lower relative to phosphate than what is expected from the Redfield ratios, nitrogen fixing bacteria will gain an advantage and the nitrate concentration will increase. If, on the other hand, the concentration of nitrate is high relative to that given by the Redfield ratios, nitrogen fixers will be outrivaled and denitrification will eventually restore the nitrate concentration. Broecker and Peng do, however, state that the time-scale of this restoration mechanism is highly uncertain.
A modelling experiment of the nitrate controls was carried out by Tyrrell (Citation1999). He confirmed that phosphate is the ultimate limiting nutrient and that the nitrate concentration is kept close to the Redfield ratios due to nitrogen fixation. However, the steady state solution generates a concentration of nitrate slightly lower than expected. This explains the observation that an addition of nitrate will, on short time-scales, generate an increased production.
We may conclude from the above discussion that if the phosphate concentration is held constant, on some time-scale, the controls of the nitrate concentration will result in a continuous steady state, i.e. the sources for nitrate to the ocean are always balanced by the sinks.
We use the arrows in to define our sources and sinks of oceanic nitrate. For steady state we obtain14
But what does this imply for the alkalinity? We write the time rate of change of alkalinity due to the nitrate fluxes as (cf. )15
By using reaction (14) we can rewrite reaction (15) as16
In conclusion, nitrate conservation leads to the result that the processes within the nitrogen cycle affecting the alkalinity are the riverine nitrate and ammonium flux. Furthermore, riverine nitrate input leads to an increased alkalinity while the ammonium flux, on the other hand, leads to an alkalinity decrease. This is in conflict with Chuck et al. (Citation2005) as they use riverine nitrate input as an alkalinity sink.
3. Data
3.1. Data of dissolved species in river water
Data of dissolved constituents in river water based on 60 of the world's largest rivers have been given by Meybeck (Citation1979). The main species found are Na+, K+, Ca2+, Mg2+, , Cl−,
and SiO2. The riverine fluxes to the ocean of these species are given in . The concentration data are for natural (unpolluted) world average river water and are here multiplied by a global runoff of 3.74 · 1013 m3 yr−1 to generate the fluxes.
Seitzinger et al. (Citation2010) used a global model together with individual river and basin data to determine the particulate organic carbon (POC) and the DOC fluxes. Results for the year 2000 are presented in .
Data of nitrate and ammonium concentrations are obtained from Meybeck (Citation1993b). The data is based on the nitrate and ammonium concentrations most commonly found in unpolluted river water.
The flux of total dissolved nitrogen (TDN), which is the sum of dissolved inorganic nitrogen (DIN) and dissolved organic nitrogen (DON), is also given in the table. DIN is essentially the sum of nitrate and ammonium. The concentration of nitrite in river water is low and never larger than 7% of DIN (Meybeck, Citation1982).
3.2. Determination of the riverine input of DIC and alkalinity
The alkalinity flux is possible to derive directly from the above given data. The DIC flux, however, needs to be separated into an external component derived from rocks and an internal component derived from the atmosphere.
Numerous authors have, with different objectives, considered the issue of determining the weathering fluxes and consumption of atmospheric CO2 from available data (e.g. Berner et al., Citation1983; Meybeck, Citation1987; Amiotte Suchet and Probst, Citation1993; Gaillardet et al., Citation1999; Lerman et al., Citation2007). Basically, two methods have crystallised. The first is a direct method where observed riverine dissolved species of the world's largest rivers are traced back to their origin, e.g. carbonate minerals, silicate minerals or evaporites. Such a method was first developed by Garrels and Mackenzie (Citation1971). The other method is a more indirect method by which lithological maps are used together with a model for chemical weathering (Meybeck, Citation1987). Riverine data is then used to constrain the model.
In , data of ion fluxes due to different processes are presented. The data presented for carbonate and silicate weathering have been determined with the direct (Berner et al., Citation1983) and the indirect method (Ludwig et al., Citation1996).
Berner et al. (Citation1983) use a direct method where data of the concentration of magnesium and calcium ions are used to determine the weathering of carbonate and silicate. In addition, a series of assumptions are implemented to differentiate between the calcium and magnesium flux derived from carbonate weathering and that derived from silicate weathering. Weathering of silicates consisting of cations other than calcium and magnesium is ignored.
By use of a direct modelling approach, Gaillardet et al. (Citation1999) found the CO2 consumption rate due to carbonate and silicate weathering. In contrast to Berner et al. (Citation1983) they do not ignore the contribution from sodium and potassium silicate species. The results they obtain are, however, remarkably close to those obtained by Berner et al. (Citation1983) and they discuss that this is due to overestimation of Ca2+ and Mg2+ fluxes in Berner et al. (Citation1983). Perhaps this is due to an underestimation of the evaporate contribution to the Ca2+ and Mg2+ flux which has been carefully accounted for in Gaillardet et al. (Citation1999).
Amiotte Suchet and Probst (Citation1993) describe an indirect method by which the amount of atmospheric carbon dioxide consumed by silicate and carbonate weathering is determined by the riverine concentration of bicarbonate together with the rock type of the drainage area. If the area is dominated by silicic rock, it follows from reaction (3) that all of the bicarbonate found in the water originates from atmospheric CO2. If, on the other hand, the area is dominated by carbonate rock, only half of the bicarbonate originates from the atmosphere, as is seen in reaction (1). Translating to DIC fluxes, reaction (3) implies that there is no external source of DIC in a catchment dominated by silicate rock. If the area is dominated by carbonate rock, reaction (1) implies that the flux of DIC derived from rocks equals half the bicarbonate flux. The alkalinity flux, in both cases, is given by the entire flux of bicarbonate.
The method of Amiotte Suchet and Probst was applied by Ludwig et al. (Citation1996) to determine the global consumption of CO2 due to rock weathering. They found that the global riverine flux of bicarbonate is 0.32 Gton C yr−1 of which 0.23 Gton C yr−1 is derived from the atmosphere. Since the entire flux of bicarbonate due to silicate weathering stems from the atmosphere, the carbon that is not atmospheric must originate from calcium carbonate rock. Hence, 0.18 Gton of the carbon flux is the result of carbonate weathering, and 0.14 Gton of silicate weathering. To compare these numbers with those obtained by Berner et al. (Citation1983), we recalculate in units of moles of calcium plus magnesium per year. We thus assume that the bicarbonate flux due to carbonate weathering is associated with either a calcium or magnesium flux half the size of the flux of bicarbonate. We make the same assumption for the weathering of silicates. The obtained values are presented in .
A caveat should be kept in mind when considering the above given data. Using improved lithological maps, Hartmann et al. (Citation2009) obtained a higher silicate to carbonate weathering ratio than previous studies. The higher resolution data included also weathering in hyper active regions and hotspots which they showed could be an important factor.
further includes data of pyrite weathering and burial on the ocean floor, oceanic burial of organic carbon, aerial input of and riverine inputs of nitrate and ammonium. The oceanic burial fluxes have been included for the sake of comparison. The rate of pyrite weathering is determined by the use of a ‘Pyrite derived fraction’ of
in river water (Lerman et al., Citation2007). The value for burial of organic carbon and pyrite is obtained from Berner (Citation1982).
The data in are recalculated into fluxes of DIC and alkalinity with the help of . The results of the external input of DIC together with the total riverine DIC flux and the internal exchange are presented in . The riverine and aerial fluxes of alkalinity are presented in . The value given for the weathering rate of fossil carbon is based on the somewhat arbitrary assumption that the weathering flux is equal to the organic carbon burial. This assumption has been used before by, for example, Walker and Kasting (Citation1992). However, they assume that all of the carbon dioxide from fossil carbon weathering is released to the atmosphere while we have given this as a riverine flux in .
Table 1. Effect of the different processes on DIC and alkalinity. Units of moles for DIC and equivalents for alkalinity
From the data given by Seitzinger et al. (Citation2010) () we can calculate the total organic carbon (TOC) flux by adding the fluxes of POC and DOC. A part of the organic riverine carbon is remineralized in the ocean and will therefore constitute an internal exchange. Berner (Citation1982) used data of the flux of suspended material to the ocean together with the fraction of carbon present in sediments associated with large rivers to determine how much carbon is buried in sediments near river mouths. We take this to be equal to the amount of land-derived carbon that is buried upon reaching the ocean. Subtracting the burial rate from the total riverine carbon flux generates the rate of remineralization of terrigenous organic carbon as long as DOC is not accumulating in the ocean. Taking the riverine flux of TOC to be 25.4 Tmol yr−1, as calculated from the data of Seitzinger et al. (Citation2010), and the burial rate to be 8.7 Tmol yr−1as given by Berner (Citation1982) (corrected for a 20% loss due to early diagenesis) we obtain the oxidation rate through17
where F TOC is the riverine flux of TOC and F Bur is the burial rate of land derived organic carbon. We obtain an oxidation rate, and thus an internal exchange, of 16.7 Tmol yr−1. In we also give the external component of the riverine TOC flux (flux derived from erosion of organic matter), which is equal to the rate of burial of land derived organic carbon.
Table 2. Riverine fluxes of different ions, Particulate and Dissolved Organic Carbon (POC, DOC) and Total Dissolved Nitrogen (TDN)
Table 3. Riverine fluxes of dissolved species due to different processes. Also included are ocean floor burial of pyrite and organic carbon
We present values only for riverine input of nitrate and ammonium since these are the only fluxes that change the integrated alkalinity according to reaction (16). The other nitrogen additions have only the potential of redistributing alkalinity within the ocean.
We also provide, in Table and 5, references to models that include a given process.
Table 4. Total river fluxes of DIC derived from different processes together with the internal and external components. Also shown are examples of models that include a certain process
4. Discussion and conclusions
Carbonate weathering is the dominating process contributing to both alkalinity and DIC. From we can establish that weathering of pyrite constitutes a small sink for alkalinity and riverine nitrate an even smaller source. It seems natural to exclude those contributions in defining the riverine alkalinity source, which is also done in most models. However, burial of pyrite is a process that has the potential of becoming an important alkalinity source during periods of widespread ocean anoxia.
Table 5. Alkalinity fluxes due to different processes together with models that include a certain process
Some models specifically consider carbonate and silicate weathering (e.g. Shaffer et al., Citation2008; Zeebe, Citation2012). The internal exchange that arises through the formation of carbonic acid that enhances the dissolution of rock material is therefore accounted for by a flux term from the atmosphere to the ocean. Other models prescribe a constant DIC and alkalinity flux directly to the ocean to account for weathering processes (e.g. Chuck et al., Citation2005). In such a case, only the external part of the total river DIC flux should be considered.
According to their references, Chuck et al. (Citation2005) seem to have used the total river flux of organic carbon as the external input of DIC. This is in conflict with our analysis showing that the only part of the terrigenous TOC input that generates an external exchange is the part that is buried on the ocean floor, representing a carbon sink.
Through an investigation of the nitrogen cycle we found that if we assume a steady state nitrate concentration, the only processes within the nitrogen cycle that affect the alkalinity are riverine input of nitrate, constituting an alkalinity source, and riverine input of ammonium, constituting an alkalinity sink. This effect arises because the hydrogen ions added to the ocean through aerial input of nitrous acid, nitrogen fixation, or riverine input of organic nitrogen, are consumed during denitrification. Denitrification and burial should be balanced by nitrogen fixation and riverine and aerial inputs as long as nitrate is not accumulating in the ocean. In contrast, Chuck et al. (Citation2005) used riverine nitrate input as an alkalinity sink (although their references suggest that they may have used data for the flux of TDN). When modelling the alkalinity effect due to the nitrogen cycle it is thus important to consider which processes are modelled. If nitrogen fixation and denitrification are not explicitly taken into account, the observation that the nitrate concentration follows the phosphate needs to be considered. Such an approach is taken by Shaffer et al. (Citation2008). They choose to couple the nitrate input to the riverine source of phosphorus through the Redfield ratios. If the phosphorus source increases, nitrogen fixation will increase also the nitrate concentration. The result is a corresponding alkalinity decrease. Of course, it has to be assumed that all of the nitrogen input comes either through nitrogen fixation, aerial inputs or riverine input of organic nitrogen.
To conclude, the difficulty in modelling DIC fluxes from land to the ocean is that they are dependent on the processes that gave rise to the flux. We have separated the DIC fluxes into its internal and external components. A different difficulty is exposed when trying to find the sources and sinks of alkalinity to the ocean. First, the sources and sinks of alkalinity are diverse, and a motivation must follow the omitted processes. Second, great care must be taken when modelling the alkalinity effect due to the different processes within the nitrogen cycle, as it is dependent on which of the processes are modelled. Hopefully, our investigation will have shed some light on these difficulties.
5. Acknowledgments
Many thanks to Jonas Nycander for numerous read-throughs, comments, and the idea for the nitrogen cycle figures. Great thanks also to Toby Tyrrell for his several readings and comments on the manuscript and for providing the idea for the layout of Table and 5. Finally, we would like to thank Bo Gustafsson and Magnus Mörth for their comments on the manuscript. The Swedish Foundation for Strategic Environmental Research (Mistra) is acknowledged for financial support.
References
- Amiotte Suchet P. , Probst J.-L . Modelling of atmospheric CO2 consumption by chemical weathering of rocks: application to the Garonne, Congo and Amazon basins. Chem. Geol. 1993; 107: 205–210.
- Amiotte Suchet P. , Probst J.-L . A global model for present day atmospheric/soil CO2 consumption by chemical erosion of continental rocks (GEMCO2). Tellus B. 1995; 47: 273–280.
- Arvidson R. S. , Mackenzie F. T. , Guidry M . MAGic: a Phanerozoic model for the geochemical cycling of major rock-forming components. Am. J. Sci. 2006; 306(3): 135–190.
- Berner E. K. , Berner R. A . Global Environment; Water, Air, and Geochemical Cycles. 2012; Princeton, New Jersey: Princeton University Press.
- Berner R . Burial of organic carbon and pyrite sulfur in the modern ocean: its geochemical and environmental significance. Am. J. Sci. 1982; 282: 451–473.
- Berner R . Sedimentary pyrite formation: an update. Geochim. Cosmochim. Acta. 1984; 48: 605–615.
- Berner R. , Lasaga A. C. , Garrels R. M . The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am. J. Sci. 1983; 283: 641–683.
- Broecker W. , Peng T.-H . Tracers in the Sea. 1982; Columbia University, New York: Lamont-Doherty Geological Observatory.
- Chuck A. , Tyrrell T. , Totterdell I. , Holligan P . The oceanic response to carbon emissions over the next century: investigation using three ocean carbon cycle models. Tellus B. 2005; 57: 70–86.
- Doney S. C. , Mahowald N. , Lima I. , Feely R. A. , Mackenzie F. T. , co-authors . Impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system. Proc. Natl. Acad. Sci. 2007; 104(37): 14580–14585.
- Gaillardet J. , Dupr B. , Louvat P. , Allgre C. J . Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999; 159: 3–30.
- Garrels R. M. , Mackenzie F. T . Evolution of Sedimentary Rocks. 1971; New York: Norton.
- Garrels R. M. , Perry E. A . Goldberg E. D . Cycling of carbon, sulfur, and oxygen through geologic time. The Sea. 1974; New York: Wiley-Interscience. 303–336.
- Goddéris Y. , François L. M. , Veizer J . The early Paleozoic carbon cycle. Earth. Planet. Sci. Lett. 2001; 190: 181–196.
- Hartmann J. , Jansen N , Dürr H. H. , Kempe S. , Köhler P . Global CO2-consumption by chemical weathering: what is the contribution of highly active weathering regions?. Glob. Planet. Change. 2009; 69(4): 185–194.
- Kump L. R. , Arthur M. A . Interpreting carbon-isotope excursions: carbonates and organic matter. Chem. Geol. 1999; 161(1): 181–198.
- Lerman A. , Wu L. , Mackenzie F . CO2 and H2SO4 consumption in weathering and material transport to the ocean, and their role in the global carbon balance. Mar. Chem. 2007; 106: 326–350.
- Ludwig W. , Amiotte-Suchet P. , Probst J.-L . River discharges of carbon to the world oceans: determining local inputs of alkalinity and of dissolved and particulate organic carbon. C. R. Acad. Sci. Ser. IIa. 1996; 323: 1007–1014.
- Mackenzie F. T. , Lerman A . Carbon in the Geobiosphere: Earth's Outer Shell. 2006; Dordrecht: Springer. 225–254.
- Mackenzie F. T. , Lerman A . Wollast R. , Mackenzie F. T. , Chou L . C, N, P and S global biogeochemical cycles and modeling of global change. Interactions of C, N, P and S Biogeochemical Cycles and Global Change. 1993; Berlin: Springer. 1–61.
- Meybeck M . Concentration des eaux fluviales en lments majeurs et apports en solution aux ocans. Rev. Geol. Dyn. 1979; 21(3): 215–246.
- Meybeck M . Carbon, nitrogen, and phosphorus transport by world rivers. Am. J. Sci. 1982; 282: 401–450.
- Meybeck M . Global chemical weathering of surficial rocks estimated from river dissolved loads. Am. J. Sci. 1987; 287(5): 401–428.
- Meybeck M . Riverine transport of atmospheric carbon: sources, global typology and budget. Water. Air. Soil. Pollut. 1993a; 70(1): 443–463.
- Meybeck M . Wollast R. , Mackenzie F. T. , Chou L . C, N, P and S in rivers: from sources to global inputs. Interactions of C, N, P and S Biogeochemical Cycles and Global Change. 1993b; Berlin: Springer. 163–195.
- Seitzinger S. P. , Mayorga E. , Bouwman A. F. , Kroeze C. , Beusen A. H. W. , co-authors . Global river nutrient export: a scenario analysis of past and future trends. Global Biogeochem. Cycles. 2010; 24 GB0A08.
- Shaffer G. , Malskær Olsen S. , Pepke Pedersen J. O . Presentation, calibration and validation of the low-order, DCESS Earth system model (Version 1). Geosci. Model Dev. 2008; 1: 17–51.
- Smith S. V. , Hollibaugh J. T . Coastal metabolism and the oceanic organic carbon balance. Rev. Geophys. 1993; 31(1): 75–89.
- Tyrrell T . The relative influences of nitrogen and phosphorus on oceanic primary production. Nature. 1999; 400(6744): 525–531.
- Walker J. C. G. , Kasting J. F . Effects of fuel and forest conservation on future levels of atmospheric carbon dioxide. Glob. Planet. Change. 1992; 5(3): 151–189.
- Wolf-Gladrow D. , Zeebe R. , Klaas C. , Körtzinger A. , Dickson A . Total alkalinity: the explicit conservative expression and its application to biogeochemical processes. Mar. Chem. 2007; 106: 287–300.
- Zeebe R. E . LOSCAR: Long-term Ocean-atmosphere-Sediment CArbon cycle Reservoir Model v2.0.4. Geosci. Model. Dev. 2012; 5(1): 149–166.