Abstract
Atmospheric amino acids constitute a large fraction of water-soluble organic nitrogen compounds in aerosol particles, and have been confirmed as effective cloud condensation nuclei (CCN) materials in laboratory experiments. We present a molecular dynamics (MD) study of six amino acids with different structures and chemical properties that are relevant to the remote marine atmospheric aerosol–cloud system, with the aim of investigating the detailed mechanism of their induced changes in surface activity and surface tension, which are important properties for cloud drop activation. Distributions and orientations of the amino acid molecules are studied; these l-amino acids are serine (SER), glycine (GLY), alanine (ALA), valine (VAL), methionine (MET) and phenylalanine (PHE) and are categorised as hydrophilic and amphiphilic according to their affinities to water. The results suggest that the presence of surface-concentrated amphiphilic amino acid molecules give rise to enhanced Lennard–Jones repulsion, which in turn results in decreased surface tension of a planar interface and an increased surface tension of the spherical interface of droplets with diameters below 10 nm. The observed surface tension perturbation for the different amino acids under study not only serves as benchmark for future studies of more complex systems, but also shows that amphiphilic amino acids are surface active. The MD simulations used in this study reproduce experimental results of surface tension measurements for planar interfaces and the method is therefore applicable for spherical interfaces of nano-size for which experimental measurements are not possible to conduct.
1. Introduction
Clouds, which are collections of small water drops or ice crystals in the atmosphere, reflect and scatter incoming solar radiation and absorb outgoing heat radiation from Earth, and thus play a major role in Earth's weather and climate. The extensive stratiform cloud decks that cover the oceans of the world are poorly understood and constitute a large uncertainty for climate models (Solomon et al., Citation2007). Major improvements in our understanding of climate depend on a better understanding of these marine clouds (Wine, Citation2010). Every cloud drop starts to grow when water vapour condenses on an airborne aerosol particle, called a cloud condensation nuclei or CCN (Twomey, Citation1974). The number, size, morphology and chemical composition as well as the meteorological conditions (updraft and cooling rate) of the CCN determine the number and size of cloud droplets, and thus the microphysical properties of clouds.
Blanchard and co-workers (Blanchard, Citation1971; Blanchard and Syzdek, Citation1988) have long advocated that a significant proportion of a remote oceanic aerosol is derived from bubble bursting (film and jet drops), resulting most commonly from entrainment of air induced by wind stress at the air–water interface. In this process, bubbles scavenge not only sea salt but also microorganisms and water-soluble organic compounds such as dissolved free amino acids (DFAAs) (Kuznetsova et al., Citation2005), as they rise through the water prior to their injection into the atmosphere. In spite of these findings, it has generally been assumed that primary marine particles derived from film drops would be composed mainly of sea salt and can thus contribute a significant fraction of the marine CCN population (O'Dowd et al., Citation1999). However, studies of individual particles by Leck and Bigg (Citation2005a, Citationb, Citation2008) over the summer on Arctic pack ice have failed to find evidence of sea salt particles<200 nm in diameter. The result from these studies is supported by other remote marine studies at lower latitudes (O'Dowd et al., Citation2004; Leck and Bigg, Citation2008). O'Dowd et al. (Citation2004) showed that organic compounds of marine biogenic origin (such as lipidic and proteinaceous material, including the DFAAs and humic substances) dominate the submicrometre aerosols. The supermicrometer aerosols derived through jet drops are, on the other hand, found to be mainly composed of sea salt with a coating of organic compounds (Leck et al., Citation2002; Leck and Bigg, Citation2005b).
The process in which water vapour condenses and forms liquid cloud drops is based on equilibrium thermodynamics and was first formulated by Köhler in Citation1936 (Köhler, Citation1936). It combines the Kelvin effect, which describes the change in saturation vapour pressure of water due to a curved surface and is related to surface tension, and Raoult's law, which relates the saturation vapour pressure of water to the solutes. It has been suggested that the presence of natural atmospheric surfactants, that could decrease the surface tension of CCN, will reduce the water vapour pressure needed for droplet activation followed by growth. This could promote an increase in the population of cloud droplets with smaller sizes, which makes the cloud more reflective for incoming solar radiation (Facchini et al., Citation1999; Rodhe, Citation1999).
It has been shown by Bull and Breese (Citation1974) that some DFAAs representatives for marine areas have the ability to decrease the surface tension, which can be attributed to their surface-active surfactant properties. Furthermore, Kristensson et al. (Citation2010) confirmed by laboratory measurements that the amino acids can behave as effective CCN, and that the critical supersaturation for cloud droplet activation is slightly underestimated by the Köhler theory. Having essentially dismissed an increase or decrease in surface tension as an explanation, Kristensson et al. discussed other possible reasons for the discrepancy between the theory and measurements. To benchmark the role of amino acids as effective CCN, their inherent differences in chemical structure and properties that potentially alter the droplet distribution and surface tension have to be further addressed.
Typical levels of the dissolved amino acids found in marine rain (Mopper and Zika, Citation1987) showed enrichment by two orders of magnitude over typical seawater values. The amino acids were found predominantly in their l-optical isomers and were therefore attributed to be of biological origin. Spitzy (Citation1990) reported amino acid data from size-fractionated aerosol and rain collected in the northern Indian Ocean. He found a several-fold enrichment of amino acid nitrogen in the submicrometre size fraction over the coarse fraction. The levels found in rain samples were generally in accordance with those found by Mopper and Zika (Citation1987). Several types of l-amino acids have been identified in marine atmospheric aerosol samples (), including, proline (PRO), serine (SER), glycine (GLY), alanine (ALA), valine (VAL), methionine (MET) and phenylalanine (PHE) (Mace et al., Citation2003a, b, Citationc; Zhang and Anastasio, Citation2003; Malder et al., Citation2004; Wedyan and Preston, Citation2008). Over the central Arctic Ocean reaction products of l-methionine was suggested as being responsible for observed periods of new particle formation (Leck and Bigg, Citation1999). The laboratory experiments of Leck and Bigg (Citation1999) demonstrated that the oxidation products of l-methionine could nucleate new particles. However, a verification of this particle formation route in the Arctic is still missing. Moreover, production of amino acids from proteins and bacteria has been identified in Antarctic cloud water (Saxena, Citation1983).
In the study by Mace et al. (Citation2003b), it is suggested that l-methionine, other DFAAs and peptides can react with hydroxyl radicals in the atmosphere. In particular, the surface-active properties of amphiphilic amino acid molecules are very important since they can undergo further chemical reactions with the surrounding air. lists the various chemical properties among DFAAs detected in air. The amino acids investigated in this study are marked with asterisks.
Table 1 DFAAs detected in air collected between 32.5 to 36.6°N and 132.7 and 133.0°W
As can be seen by inspection, the most common DFAAs in the sample have polar or charged side chains (apart from GLY which has only a hydrogen atom as the side chain and ALA which has only a methyl group as the side chain and therefore do not show strong hydrophobicity). The least abundant DFAAs carry hydrophobic side chains (apart from HIS). VAL, SER, PHE and MET are good representatives of marine DFAAs determined in aerosol and rain. A hypothesis for the relatively low abundance of the DFAAs with hydrophobic side chain is that they can more easily migrate to the surface and by this become relatively more available to chemical oxidation. The differences in the structural and chemical properties of the individual DFAAs lead to the differences in the molecular orientation and affinity to the surface of a droplet.
Mopper and Zika (Citation1987) observed DFAAs in marine rain water samples in concentrations up to 15 µM. The about four orders of magnitude higher amino acid concentration at activation reported by Kristensson et al. (Citation2010) was justified by the same order of magnitude higher liquid water content present in rain droplets relative to a CCN droplet. In the study by Kristensson et al. (Citation2010), particles containing amino acids were studied with a minimum diameter of 40 nm and a maximum concentration of 0.3 M. Since the number of water molecules in a droplet, which can be simulated by molecular dynamics (MD) simulations, is limited by our computer resources the applied concentration of amino acids in the droplet was set to 0.56 M. This concentration is higher than amino acid concentration at activation reported by Kristensson et al., but is assumed to be justified as higher concentrations would be probable for the relatively smaller particles under study. In order to obtain statistically reliable results with limited simulation time, we therefore increased the number of amino acids in each cluster so that the statistical error due to the limited time of simulations could be minimised. The concentrations of the DFAAs used in the modelling of the clusters were normalised to a size of about 10- nm diameter or smaller. In such small systems, the surface-to-bulk ratio is the highest and thus surface activity plays an important factor in the chemical and physical properties of the nano-sized aerosol particles and thus for their nucleating activity. As laboratory experiments cannot study systems smaller than about 40 nm in diameter, the method of MD simulation stands out as a very suitable choice. One way of overcoming the gaps between the nano-size of MD simulations and the experimental sizes of CCN, shown by Hede et al. (Citation2011), is to calculate the Langmuir–Szyszkowski parameters (Langmuir, Citation1917). These parameters will prescribe a variable value in the Köhler equation for surface tension depending on the concentration of the amino acid.
MD simulations are carried out by numerically solving Newton's equations of motion, during which the positions and velocities of the particles in a many-body system are recorded for further analysis of dynamical processes and statistical properties. Nowadays, the computational power of modern computers allows large-scale atomistic simulations and has thus promoted the applications of MD simulations in many research fields. Here, we employ the versatile MD simulation approach to investigating liquid–gas interfaces like surface tension at the molecular level. Laboratory studies of surface tension measurements concern bulk solutions of macroscopic nature, which involve a very large number of both water molecules and solutes. It is therefore appropriate to compare these measurements with MD simulations conducted in a similar way to that for a planar interface (the so-called slab system). MD simulations can thus serve as an important complement to experimental studies. Starting from the pioneer paper by Alejandre et al. (Citation1995), extensive MD simulations on surface tension have been carried out for planar liquid–gas interfaces (Yeh and Berkowitz, Citation1999; Ismail et al., Citation2006; Chen and Smith, Citation2007; Klauda et al., Citation2007; Yuet and Blankschtein, Citation2010). Meanwhile, MD studies on spherical liquid–gas interfaces mimicking ambient droplet activation are increasing (Thompson et al., Citation1984; Zakharov et al., Citation1997; Van Giessen and Blokhuis, Citation2009; Li et al., Citation2010, Citation2011; Sampayo et al., Citation2010).
As particles with sizes below 200 nm in diameter will dominate the marine CCN number population, we will assume the contribution from sea salt to be insignificant relative to the organic matter. The presence of secondary inorganic constituents, for example ammonium and sulphate ions, may alter the hydrogen-bonding network along with the amino acids and perhaps the distribution of the amino acid molecules within the droplet. However, we find it urgent to first and foremost identify the behaviour of each type of the amino acids, based on their different chemical and structural properties, in the water droplets. Therefore, inorganic ions are not covered in this study.
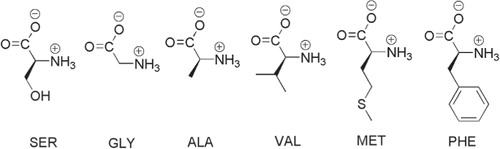
To study the cloud droplet condensational growth capacity of water-containing DFAAs representative for the remote marine atmosphere (Mopper and Zika, Citation1987; Spitzy, Citation1990; Mace et al., Citation2003a, Citationb Citationc; Zhang and Anastasio, Citation2003; Malder et al., Citation2004; Wedyan and Preston, Citation2008), the structures and chemical properties of the following six l-amino acids (see ) were investigated by the MD simulation technique: SER, GLY, ALA, VAL, MET and PHE. Furthermore, the molecular distribution and orientation were analysed for each type of amino acid, and the surface tension was computed for both planar and spherical liquid–gas interfaces and finally, a correction to the Köhler theory was suggested.
2. Computational methods
The planar approach of liquid–gas interface was first set up for investigations through MD simulations. For each type of amino acids, a cubic simulation box was constructed and filled with 10 amino acid molecules and 1000 water molecules, and then the box was elongated three times along the z-axis to create the planar liquid–gas interfaces. Later, more systems were set up to model the surface tension of spherical the liquid–gas interfaces for droplets. For each type of amino acid, three cubic boxes were set up and filled with 10 amino acid molecules + 1000 water molecules, 20 amino acid molecules + 2000 water molecules and 50 amino acid molecules + 5000 water molecules, respectively. Each box was then enlarged in three dimensions so that the length of the box was 6.0 nm longer than the diameter of the droplet, in order to minimise the interaction between the droplet and its periodic images. Here, the diameter of the droplet was estimated from the volume of the initial cubic box.
The GROMACS program package (version 4.5.2) (Van der Spoel et al., Citation2005; Hess et al., Citation2008) was used to carry out the MD simulations. The SPC/E (extended simple point charge) water model (Berendsen et al., Citation1987) was employed, with the two O–H bond lengths constrained at 1.0 Å by the LINCS (linear constraint solver) algorithm (Hess et al., Citation1997; Hess, Citation2008) and the H–O–H angle kept flexible. The OPLS (optimised potentials for liquid simulations) all-atom force field developed by Jorgensen et al. (Citation1996) was used to model the amino acid molecules, with the bonds containing hydrogen atoms constrained by the LINCS algorithm (Hess et al., Citation1997; Hess, Citation2008). All simulations were performed in canonical (constant-NVT) ensembles, with the temperature maintained at 298 K by the Nosé–Hoover thermostat (Nosé, Citation1984; Hoover, Citation1985). Periodic boundary conditions were applied during the simulations. The van der Waals interaction was modelled by the Lennard–Jones potential truncated at the cut-off radius of 10 Å. The Coulomb interaction was evaluated by the Ewald summation, where the real part of the potential is truncated at 10 Å and the reciprocal interaction is calculated by the SPME (smoothed particle mesh Ewald) (Darden et al., Citation1993; Essmann et al., Citation1995) method with a mesh of reciprocal vectors of 1.2 Å in every direction and a spline of the order of four. The time step of the simulations was set to 2 fs. Each system was subject to energy minimisation and then a 3-ns simulation to reach equilibrium, after which another 2-ns simulation was carried out with trajectories saved every 0.2 ps.
With the MD simulations completed and the trajectories recorded, molecular distributions and orientations of the amino acids were analysed, and surface tension for liquid–gas interface was computed as described in the previous study (Li et al., Citation2011).
3. Results and discussion
3.1. Planar interfaces
In order to verify that the MD simulation technique is valid, we first examine planar interfaces for which experimental measurements are available for comparison. The effect of one chemical compound on the interfacial tension between a bulk solution and the surrounding air is directly dependent on the surface-active properties of the compound, and these properties are in turn dependent on the chemical structure and orientation of the compound. Even if the effect on surface activity is reduced, the orientation of the compound at the surface could facilitate chemical reactions with the surrounding air as well as interaction with other compounds inside the aerosol droplet.
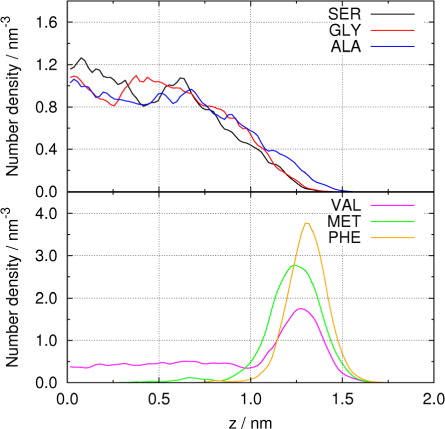
In the simulations of planar interfaces, surface tension is obtained from Cartesian components of the pressure tensor and length of the simulation box in the z-direction (Li et al., Citation2011); in this case surface tension is averaged over two liquid–gas interfaces of the slice of water. We here present the axial number densities of amino acids along the z-direction, where the origin is put at the centre of the water slab and the density at +z and −z are averaged, as shown in . Distinct density distributions are found among the six amino acids; SER, GLY and ALA show maximal densities in bulk phase at small z, while VAL, MET and PHE are concentrated at the surface region as reflected by the peaks in the density curves at relatively large z. Although ALA contains a hydrophobic methyl group as the side chain, the whole molecule shows hydrophilicity due to the presence of the and the −COO− groups. Therefore, we here designate SER, GLY and ALA as hydrophilic amino acids and VAL, MET and PHE as amphiphilic ones. For the three hydrophilic amino acids, it is found that in bulk phase (0.0 nm<z<0.5 nm) the average density follows the order SER>GLY>ALA, and that in the region close to surface (1.0 nm<z< 1.5 nm) the magnitude of density follows reverse order SER<GLY<ALA, indicating the intrinsic differences in hydrophilicity due to different side chains. The three amphiphilic amino acids also show different density profiles. VAL with smaller side chain than MET and PHE is less concentrated on the surface, while PHE with bulky and non-polar phenyl side chain shows the highest density peak at the surface. MET has a long alkyl side chain and its density peak appears at smaller z than VAL and PHE.
Distinct axial distributions of the amino acid densities are expected to result in a different impact on surface tension of planar interface. lists a series of physical quantities related to surface tension of planar interfaces. By comparing the Cartesian components of pressure tensor, we see that P zz is hardly affected by the presence of amino acids and that (P xx +P yy )/2 is the major source of surface tension perturbation, leading to the inference that the interacting forces parallel to the surface are affected by the presence of amino acid molecules. From , it can be seen that SER, GLY and ALA are able to increase surface tension of planar liquid–gas interface, while VAL, MET and PHE can decrease surface tension, in qualitative agreement with experimental observations (Pappenheimer et al., Citation1936; Bull and Breese, Citation1974). We would like to point out that the measurements reported by Bull and Breese (Citation1974) were carried out in salt solution, in which Na+ and Cl- ions could influence the surface tension and result in the discrepancy between computed dσ/dC and experimental results.
Table 2 Cartesian components of pressure tensor (in bar), length of simulation box in z-direction (in nm), surface tension σ (in mJ m−2), and surface tension-concentration slope dσ/dC for planar liquid-gas interface together with reference data (in mJ m−2 L mol−1)
3.2. Spherical interfaces
3.2.1. Molecular distributions and orientations.
As mentioned above simulations of spherical water clusters, with a high degree of curvature, is an approach that holds a more realistic representation of atmospheric cloud droplet activation processes compared to simulations of a planar interface. Here, we show the snapshots of water droplets containing 5000 water molecules and different amino acids in a. It can be seen that VAL, MET and PHE are more concentrated on the surface, while SER, GLY and ALA are not. Noteworthy in particular is that PHE molecules are able to form ordered structures on droplet surfaces, which are very stable during the simulation. A close view is shown in b, in which the stable hydrogen bond network among the PHE molecules is shown.
Fig. 3 (a) Snapshots for water droplets containing different amino acids. (b) Hydrogen bond network in phenylalanine molecules on droplet surface.
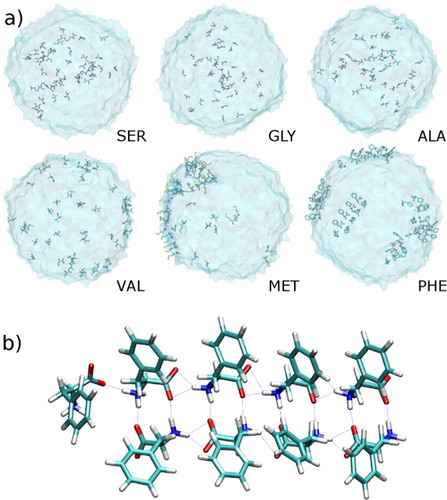
Such ordered structures of PHE as well as surface-concentrated VAL and MET are expected to affect the interfacial properties of water droplets. It can be assumed that the surface-active behaviour may have effects on larger molecules containing these amphiphilic amino acid residues. Peptides may change the way they are naturally folded due to the interaction with the surface. This can in turn affect or enhance the probability for aggregation with other organic and inorganic compounds. The state of mixture in the aerosol particles can be affected leading to changes in the process of cloud droplet activation.
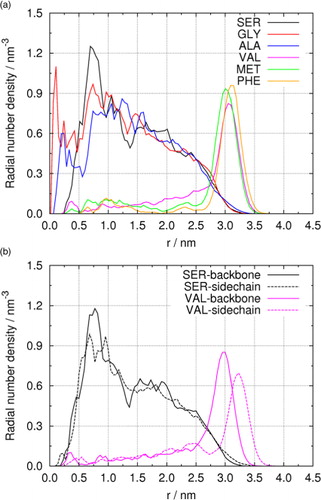
Further insight can be obtained from radial number densities of amino acids shown in a. In general, SER, GLY and ALA have notable radial densities inside the droplet, while peak densities for VAL, MET and PHE appear on the droplet surface. Different from axial number densities (), the radial densities of SER, GLY, and ALA suffer large oscillations at small radius, since the spherical volume with a small radius r is relatively small and the statistics therefore less reliable. VAL, MET and PHE are again concentrated on the droplet surface, with peak densities (PHE>MET>VAL) and corresponding peak positions on r-axis (MET<VAL<PHE) similar to those observed in . Beyond radial distribution, it is also of interest to examine the orientation of amino acid molecules in the droplets. b illustrates radial number densities of backbone and side-chain parts of two amino acids, SER and VAL. For the hydrophilic SER, no significant preference of molecular orientation is found. At surface region (3.0 nm<r<3.5 nm), the density of side chain slightly exceeds that of backbone, reflecting the fact that the backbone containing and −COO− ions has stronger hydrophilicity than the hydroxyl side chain. On the contrary, distinct radial densities are observed for backbone and side chain of amphiphilic VAL at the surface region (r ~3.0 nm and r ~3.3 nm), indicating that the zwitterionic backbone is immersed on droplet surface with the hydrophobic isopropyl side chain pointing outward.
Fig. 4 (a) Radial number densities of different amino acids in droplets containing 5000 water molecules. (b) Molecular orientation of SER and VAL in droplets.
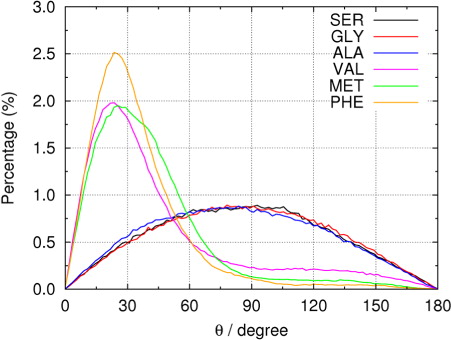
The orientation of amino acid molecules can also be reflected by the order parameter which characterises the degree of order of a system. The order parameter is frequently used in different applications to describe the molecular properties of orientation. In our case, the order parameter gives insights to the behaviour of the amino acids, whether they appear randomly in the bulk of the nanoaerosol droplet, or if they assemble on the surface in an ordered manner. The latter proposing a surface-active property which may have effects on the cloud-forming processes. The order parameter is described by <3 cos2 θ–1>/2. Here, θ is defined as the angle between the two vectors, O→Cα and Cα→Cβ, where O denotes the geometrical centre of the droplet, and Cα and Cβ are the α-carbon and β-carbon atoms in the amino acid molecule, respectively. For GLY, Cβ is represented by the corresponding hydrogen atom on Cα. We computed the distribution of angle θ over the simulation trajectory, as shown in . Again, the six amino acids fall into two distinct groups, the amphiphilic ones with strong preference in small θ (around 25°) and the hydrophilic ones with broader and almost symmetric preference on θ with respect to 90°. The latter situation will result in an order parameter of around zero. Shown in are the computed order parameters; not surprisingly, the order parameters of both SER and GLY are very close to zero, indicating that there is little alignment between the radial vector O→Cα and the direction of amino acid side chain. The order parameter of ALA is slightly larger than those of SER and GLY, reflecting the weak preference of orientation caused by the hydrophobic effect of the methyl group. The amphiphilic VAL, MET and PHE have much larger order parameters, the largest value of 0.5209 suggesting that PHE has the strongest preference of orientation and therefore the most hydrophobic side chain. These findings are related with the fact that surface-active components can undergo further chemical reactions with the surrounding air and that amphiphilic DFAAs may be lower in abundance.
Table 3 Order parameter of amino acids in the spherical clusters
3.2.2. Surface tension.
In this work, the approximate surface tension of spherical droplets is obtained from the work of formation of the droplet, which is computed from normal component of Irving–Kirkwood pressure tensor, P N(r). According to Thompson et al. (Citation1984), P N(r) consists of two contributions, that is the kinetic term P K(r) and the conformational term P U(r). The kinetic term is dependent on radial density profile ρ(r) and temperature T, while the conformational term arises from intermolecular forces. To shed light on the origin of surface tension, we here decompose the work of formation of the droplet consisting of 5000 water molecules and different amino acid molecules into kinetic and conformational contributions, that is W=W K+W U=W K+W LJ+W QQ, where W LJ and W QQ are conformational contributions arising from intermolecular Lennard–Jones interaction and Coulomb interaction, respectively. The individual contributions W K and W U are computed from integration of the kinetic and the conformational term, respectively. The results are listed in . Clearly, the different effects of these amino acids on droplet surface tension mainly arise from the conformational contribution W U. For water droplets containing SER, GLY and ALA, the conformational contributions are very close to that of pure water droplets, while the conformational contributions of droplets containing VAL, MET and PHE are less negative, thus contributing to larger work of formation and hence surface tension. In we also list ΔW LJ and ΔW QQ which are the differences between droplets containing amino acids and pure water droplets. For SER, GLY and ALA, ΔW LJ and ΔW QQ are of similar magnitude but opposite in sign, resulting in similar W U to that of pure water droplets. For VAL, MET and PHE, the positive ΔW LJ slightly exceeds the negative ΔW QQ, thus resulting in a less negative W U and a larger surface tension.
Table 4 Density of liquid phase ρ α (in nm−3), radius of equimolar dividing surface R e (in nm), surface tension σ (in mJ m−2), and individual contributions to work of formation W (in 10−19 J) for spherical droplets consisting of 5000 water molecules and different amino acids
3.3. Curvature dependence of surface tension
We have demonstrated the effects of amino acids on the surface tension of planar and spherical interfaces, and in this section we will compare the planar and spherical cases and outline the curvature dependence of surface tension. From it can be seen that the presence of VAL, MET and PHE results in larger positive perturbation in the tangential component of pressure tensor (P xx +P yy )/2 than SER, GLY and ALA, therefore giving rise to decreased surface tension. From this point, we infer that the amphiphilic VAL, MET and PHE, which are concentrated on the surface, provide strong interacting forces that contribute to the pressure tensor. According to Alejandre et al. (Citation1995), a pair of repulsive force contributes positively to the pressure tensor, while a pair of attractive force contributes negatively. Therefore, we conclude that VAL, MET and PHE on the surface affect the surface tension through enhanced repulsive interaction. This is also shown by the data presented in , where the increase in droplet surface tension induced by the presence of amphiphilic amino acid arises from positive Lennard–Jones contribution W LJ, that is, Lennard–Jones repulsion.
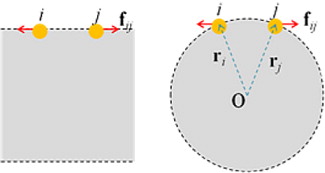
The enhanced repulsive effect contributes differently to the surface tension of planar and spherical interfaces. A pair of repulsive forces parallel to planar surface contributes positively to (P xx +P yy )/2, that is, the tangential component of pressure tensor P T; however, there is no contribution to P zz , namely the normal component of pressure tensor P N, as shown in . Therefore, the surface tension of planar interface is diminished. Differently, the same repulsive interaction is divided into two contributions to both normal component P N(r) and tangential component P T(r), since the interacting force f ij crosses the spherical slice near surface with a certain intersecting angle, as seen in . The balance between P N(r) and P T(r), which is closely dependent on the curvature of the droplet surface, determines the variation of surface tension. This explains why the presence of amphiphilic amino acids such as VAL, MET and PHE diminishes surface tension of a planar interface while it enhances the surface tension of spherical interfaces with a diameter of 6–7 nm (). Naturally, such an effect is closely dependent on the curvature of the surface; it can be inferred that the effects on P N(r) and P T(r) could be balanced at some larger diameter so that the influence of amphiphilic amino acids on the surface tension vanishes.
Fig. 6 Different effects of a pair of repulsive force on the surface tension of planar and spherical interfaces. Here, i and j denotes the two interacting particles, f ij is the force exerted on particle j, O denotes centre of mass of droplet, and r i and r j are the O→i and O→j vectors, respectively.
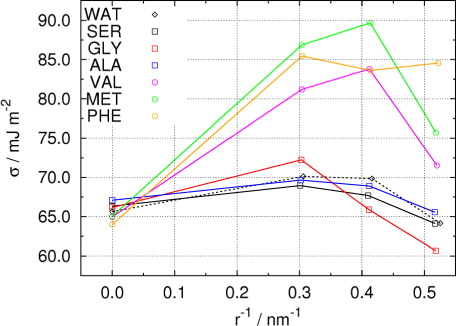
If we regard the planar interface as the limiting case of a spherical interface with infinite radius (or zero curvature), shows the dependence of surface tension on the inverse of droplet radius in water droplets containing different amino acid molecules. For all droplets, the surface tension shows non-monotonic curvature dependence that could be fitted by a quadratic polynomial function of the inverse of droplet radius, R e. In Li et al. (Citation2011) we have performed the fitting for pure water droplets and droplets containing GLY molecules, and the resultant expression for the surface tension slightly improved the supersaturation of droplet activation predicted by the Köhler theory (Kristensson et al., Citation2010). Still, such correction is relatively small in magnitude and cannot fully explain the discrepancy between experimental measurements and the Köhler theory. Nonetheless, the quadratic polynomial fitting is a reasonable approximation that could provide insight into curvature dependence of the surface tension, especially for large droplets which are not yet accessible by molecular simulations. By applying a quadratic polynomial fitting, we found that the diameter of the droplet at which the surface tension perturbation from amphiphilic amino acid vanishes is between 80 and 400 nm. Therefore, at typical activation point at the critical water supersaturation where the diameter of droplet is on the order of several hundred nanometres, the influence of amphiphilic amino acids on surface tension is expected to be very small, and it might be reasonable to neglect such effects. Still, it should be noted that the surface tension of a droplet with a diameter of several hundred nanometres is slightly enhanced compared with that of the planar interface, due to the previously mentioned quadratic dependence on droplet curvature. Beyond amino acids, the total contribution from a more complex amino acid-containing cluster, mixed either with different inorganic ions or with mixtures of different amino acids, or both, should be addressed.
3.4. Solubility and growth factor
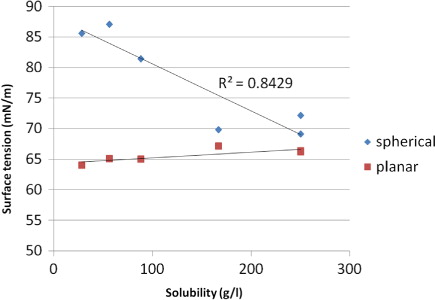
In addition to spatial distribution of amino acids in droplets and surface tension, the solubility and growth factor are important properties that affect atmospheric physicochemical processes and hence the climate. The solubility of a compound in water is determined by the ability to form hydrogen bonds. A typically hydrophilic compound is small in size and similar in chemical properties to water, that is, it contains polar functional groups. An amino acid has per definition two polar parts, namely the amine part and the carboxylic part. In some cases, these functional groups can carry a net charge and thereby become even more polar, while in this study all DFAAs are neutral. The presence of these two functional groups makes all the 20 existing DFAAs soluble in water. However, amino acids have a side chain, and this side chain can introduce different chemical properties. In this study, we have focused on calculations of surface tensions for the DFAAs. As can be seen in , there is a weak relationship between the surface tension and the solubility of the DFAAs in this study for the planar interface. However, the surface tension is strongly related to the solubility when studying a nano-sized cluster (). This implies two conclusions; first, the DFAAs with a hydrophobic side chain are less soluble and therefore more surface active. They accumulate on the surface and increase the surface tension. Second, the partitioning between the surface and bulk, where the DFAAs with hydrophobic side chain prefer the surface and the DFAAs with hydrophilic side chain prefer the bulk phase, has an impact on the Rault term of the Köhler equation as both the water activity and the amount of solute are affected. These two effects are not strongly influencing the predicted supersaturation from the Köhler equation, but still non-negligible especially for nano-sized droplets.
Table 5 Solubility and surface tensions for the DFAAs used in this study
Growth factors (GF) are defined for particles below water vapour saturation [relative humidity (RH)<100%]. A GF is the ratio between the diameter of the particle for a certain RH and the diameter for the dry particle:1
In the MD study by Harmon et al. (Citation2010), GF of between 1.6 for RH=80% up to 2.0 for RH=95% are reported for sodium chloride particles. Higher GFs are expected and depends on the better ability for salt particles to absorb water molecules (hygroscopicity) than for the DFAAs. Hämeri et al. (Citation2001) determined experimentally the GF for as small as 8-nm sodium chloride particles, and the results differ only to some extent by reporting slightly higher values. Biskos et al. (Citation2006) report the GF for sodium chloride particles determined experimentally, and there is a decrease of GF with decreasing dry particle size, and for a dry particle of 6 nm in diameter (similar in size with the clusters simulated in this study) the GF varies between RH=75% at 1.2 to RH=100% at 1.6 which is very close to our predictions for the most hydrophilic DFAAs.
If a unity growth factor at RH=0% can be assumed, the RH dependence can be described by (Zhou, Citation2001):2
Here, γ is a constant for each typical type of each CCN particle. We have determined an empirical relation that corresponds well with the calculated GF of each type of DFAA:3
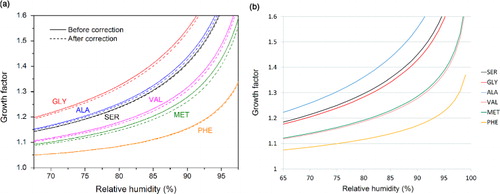
The values for the surface tension of the DFAAs are the planar surface data, since it best corresponds to the activated particles with low curvature. The relationship is not applicable to the data of surface tension for spherical interfaces. This is not surprising since the conditions for hygroscopic growth for nano-sized particles differs in so many aspects from the activated ones. A comparison between the calculated GF according to Biskos et al. (Citation2006) and the empirical relation is made in . Here the empirical GF–RH curves are based on surface tension of planar interfaces, while the formula of Biskos et al. (Citation2006) is fed with surface tension for spherical droplets to take into account the Kelvin effect. Nonetheless, the general trends proposed by these two methods are almost the same, except that the curves for GLY and ALA interchanged their position. On one hand, the empirical relation suggests that a lower surface tension leads to a smaller growth factor at a certain relative humidity, that is a lower activity as CCN. This is usually related to hydrophobic/amphiphilic species which is expected to reduce both surface tension and growth factor. On the other hand, growth factor calculations based on the formula of Biskos et al. (Citation2006) and surface tension of spherical droplets give almost the same results, thus confirming the rationality and reliability of our calculations. Furthermore, the results from the formula of Biskos et al. (Citation2006) are considered more reasonable as it takes into account the Kelvin effect and predict a larger growth factor of GLY over ALA, in line with experimentally measured surface tensions of aqueous amino acids ().
Fig. 9 Comparison between (a) calculated GF according to Biskos et al. (Citation2006) and (b) the empirical relation. In (a) the GF–RH curves with surface tension correction taken into account were shown as dashed lines.
4. Summary and conclusions
In order to shed light on the role of water-soluble organic nitrogen compounds in cloud droplet activation, we have conducted MD simulations. We have investigated the mechanism of surface tension perturbation and surface activity in atmospheric droplets induced by the presence of DFAA molecules representative for the remote marine areas. Six types of l-amino acids were studied, either hydrophilic or amphiphilic in character: serine, glycine, alanine, valine, methionine and phenylalanine. In the simulations of both planar and spherical liquid–gas interfaces, the first three amino acids are found to stay in the bulk phase owing to their strong hydrophilicity arising from the zwitterionic backbone and smaller side chain, while the three latter amino acids are concentrated on the surface due to an amphiphilic effect dependent on their bulky and non-polar side chains. For planar interfaces, the hydrophilic amino acids are able to slightly increase the surface tension, while the amphiphilic ones were found to have the ability to decrease the surface tension. For spherical droplets, the perturbations on surface tension induced by hydrophilic amino acids were again relatively small (within 3%), while the amphiphilic amino acids were able to largely increase the surface tension (up to 24%) of water droplets with a diameter of 6.6 nm. It is concluded that the presence of amphiphilic amino acid molecules give rise to a larger enhancement in Lennard–Jones repulsion than that in Coulomb attraction, and that such enhanced Lennard–Jones repulsion results in different effects on surface tension of planar and spherical interfaces. The mechanism presented here suggests that the surface tension perturbation in atmospheric droplets depends closely on the curvature of droplet surface. The solubility and growth factor of amino acid were also discussed in connection with the surface tension of aerosol droplets, and it is found that GLY gives rise to the largest growth factor and that PHE leads to the lowest, in line with their affinity to water (hydrophilic or amphiphilic).
As one of the state-of-the-art simulation techniques, an MD simulation is also able to provide microscopic information and detailed understanding on the atomistic scale, and we show here that it is possible to elucidate the solute–solvent interaction in droplets and the impact on surface tension mechanism using this technique. The insight on molecular level showed for the most abundantly observed amino acids in primary marine particles that the distribution between surface and bulk differs between hydrophilic and amphiphilic molecules. This is a very important conclusion that shows the need to reconsider the state of mixture in aerosol particles in the process of cloud droplet activation. There are other more subtle effects found in this study, like the ordering properties of phenylalanine related to surface activity, which implies the ability for facilitated chemical reactions for amphiphilic compounds at the surface with other constitutes in the surrounding air and at the droplet surface. Beyond this study, we recommend that more complex amino acid-containing clusters, mixed either with different inorganic ions or with mixtures of different amino acids, or both, should be addressed.
The result of our detailed MD simulations confirmed the experimental results by Kristensson et al. (Citation2010) that intermediately surface-active substances, like the DFAAs, have a minor effect on surface tension for planar or low curvature interfaces. Even though the effect on the surface tension is not prominent for activated cloud droplets, there is a significant effect, as reported in this study, for spherical droplets of nano-sizes. These new findings imply that the Köhler theory has some disadvantages when describing the earliest stage of condensational droplet growth; a more detailed description of the surface-active processes would favour the total understanding of droplet growth from new particle formation to activation. The use of MD simulations to gain molecular level insight in the field of aerosol–cloud interactions is still in its cradle and we are on the brink of a breakthrough in the possibilities of future atmospheric science.
Acknowledgments
The authors thank the Swedish Infrastructure Committee (SNIC) for providing computational resources for the project ‘Multiphysics modeling of molecular materials’, SNIC 022/09-25 and the Swedish Research Council for their financial support.
References
- Alejandre J , Tildesley D. J , Chapela G. A . Molecular dynamics simulation of the orthobaric densities and surface tension of water. J. Chem. Phys. 1995; 102: 4574–4583.
- Berendsen H. J. C , Grigera J. R , Straatsma T. P . The missing term in effective pair potentials. J. Phys. Chem. 1987; 91: 6269–6271.
- Biskos G , Russell L. M , Buseck P. R , Martin S. T . Nanosize effect on the hygroscopic growth factor of aerosol particles. Geophys. Res. Lett. 2006; 33: 07801.
- Blanchard D. C . The oceanic production of volatile cloud nuclei. J. Atmos. Sci. 1971; 28: 811–812.
- Blanchard D. C , Syzdek L. D . Film drop production as a function of bubble size. J. Geophys. Res. 1988; 93: 3649–3654.
- Bull H. B , Breese K . A hydrophobicity scale of the amino acid residues. Arch. Biochem. Biophys. 1974; 161: 665–670.
- Chen F , Smith P. E . Simulated surface tensions of common water models. J. Chem. Phys. 2007; 126: 221101-1–221101-3.
- Darden T , York D , Pedersen L . Particle mesh Ewald: an N-log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993; 98: 10089–10092.
- Essmann U , Perera L , Berkowitz M. L , Darden T , Lee H , co-authors . A smooth particle mesh Ewald method. J. Chem. Phys. 1995; 103: 8577–8592.
- Facchini M. C , Mircea M , Fuzzi S , Charlson R. J . Cloud albedo enhancement by surface-active organic solutes in growing droplets. Nature. 1999; 401: 257–259.
- Hämeri K , Laaksonen A , Väkevä M , Suni T . Hygroscopic growth of ultrafine sodium chloride particles. J. Geophys. Res. 2001; 106(D18): 20749–20757.
- Harmon C. W , Grimm R. L , McIntire T. M , Peterson M. D , Njegic B , co-authors . Hygroscopic growth and deliquence of NaCl nanoparticles mixed with surfactant SDS. J. Phys. Chem. B. 2010; 114: 2435–2449.
- Hede T , Li X , Leck C , Tu Y , Ågren H . Model HULIS compounds in nanoaerosol clusters – investigations of surface tension and aggregate formation using molecular dynamics simulations. Atmos. Chem. Phys. 2011; 11: 6549–6557.
- Hess B . P-LINCS: a parallel linear constraint solver for molecular simulation. J. Chem. Theory Comput. 2008; 4: 116–122.
- Hess B , Bekker H , Berendsen H. J. C , Fraaije J. G. E. M . LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 1997; 18: 1463–1472.
- Hess B , Kutzner C , Van der Spoel D , Lindahl E . GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008; 4: 435–447.
- Hoover W. G . Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A. 1985; 31: 1695–1697.
- Ismail A. E , Grest G. S , Stevens M. J . Capillary waves at the liquid–vapor interface and the surface tension of water. J. Chem. Phys. 2006; 125: 014702.
- Jorgensen W. L , Maxwell D. S , Tirado-Rives J . Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996; 118: 11225–11236.
- Klauda J. B , Wu X , Pastor R. W , Brooks B. R . Long-range Lennard–Jones and electrostatic interactions in interfaces: application of the isotropic periodic sum method. J. Phys. Chem. B. 2007; 111: 4393–4400.
- Köhler H . The nucleus in and the growth of hygroscopic droplets. Trans. Faraday Soc. 1936; 32: 1152–1161.
- Kristensson A , Rosenørn T , Bilde M . Cloud droplet activation of amino acid aerosol particles. J. Phys. Chem. A. 2010; 114: 379–386.
- Kuznetsova M , Lee C , Aller J . Characterization of the proteinaceous matter in marine aerosols. Marine Chem. 2005; 96: 359–377.
- Langmuir I . The constitution and fundamental properties of solids and liquids. II. Liquids. J. Am. Chem. Soc. 1917; 39: 1848–1906.
- Leck C , Bigg E. K . Aerosol production over remote marine areas – a new route. J. Geophys. Res. 1999; 26: 3577–3580.
- Leck C , Bigg E. K . Biogenic particles in the surface microlayer and overlaying atmosphere in the central Arctic Ocean during summer. Tellus B. 2005a; 57: 305–316.
- Leck C , Bigg E. K . Source and evolution of the marine aerosol – a new perspective. Geophys. Res. Lett. 2005b; 32: 19803.
- Leck C , Bigg E. K . Comparison of sources and nature of the tropical aerosol with the summer high Arctic aerosol. Tellus B. 2008; 60(1): 118–126.
- Leck C , Norman M , Bigg E. K , Hillamo R . Chemical composition and sources of the high Arctic aerosol relevant for cloud formation. J. Geophys. Res. 2002; 107: 4135.
- Li X , Hede T , Tu Y , Leck C , Ågren H . Surface-active cis-pinonic acid in atmospheric droplets: a molecular dynamics study. J. Phys. Chem. Lett. 2010; 1: 769–773.
- Li X , Hede T , Tu Y , Leck C , Ågren H . Glycine in aerosol water droplets: a critical assessment of Köhler theory by predicting surface tension from molecular dynamics simulations. Atmos. Chem. Phys. 2011; 111: 519–527.
- Lide D. R. (ed.) . CRC Handbook of Chemistry and Physics. 2005; 86th ed, Boca Raton, FL: CRC Press.
- Mace K. A , Artaxo P , Duce R. A . Water-soluble organic nitrogen in Amazon Basin aerosols during the dry (biomass burning) and wet seasons. J. Geophys. Res. 2003a; 108: 4512.
- Mace K. A , Duce R. A , Tindale N. W . Organic nitrogen in rain and aerosol at Cape Grim, Tasmania, Australia. J. Geophys. Res. 2003b; 108: 4338.
- Mace K. A , Kubilay N , Duce R. A . Organic nitrogen in rain and aerosol in the eastern Mediterranean atmosphere: an association with atmospheric dust. J. Geophys. Res. 2003c; 108: 4320.
- Malder B. T , Yu J. Z , Xu J. H , Li Q. F , Wu W. S , co-authors . Molecular composition of the water-soluble fraction of atmospheric carbonaceous aerosols collected during ACE-Asia. J. Geophys. Res. 2004; 109: 06206.
- Mopper K , Zika R. G . Free amino acids in marine rains: evidence for oxidation and potential role in nitrogen cycling. Nature. 1987; 325: 246–249.
- Nosé S . A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984; 52: 255–268.
- O'Dowd C. D , Facchini M. C , Cavalli F , Ceburnis D , Mircea M , co-authors . Biogenically-driven organic contribution to marine aerosol. Nature. 2004. 431, 676–680..
- O'Dowd C. D , Lowe J. A , Smith M. H , Kaye A. D . The relative importance of non-sea-salt sulphate and sea-salt aerosol to the marine cloud condensation nuclei population: an improved multi-component aerosol-cloud droplet parametrization. Q. J. Roy. Meteorol. Soc. 1999; 125(556): 1295–1313.
- Pappenheimer J. R , Lepie M. P , Wyman J Jr . The surface tension of aqueous solutions of dipolar ions. J. Am. Chem. Soc. 1936; 58: 1851–1855.
- Rodhe H . Clouds and climate. Nature. 1999; 401: 223–225.
- Sampayo J. G , Malijevský A , [Macute]úller E. A , De Miguel E , Jackson G . Communications: evidence for the role of fluctuations in the thermodynamics of nanoscale drops and the implications in computations of the surface tension. J. Chem. Phys. 2010; 132: 141101.
- Saxena V. K . Evidence of the biogenic nuclei involvement in Antarctic coastal clouds. J. Phys. Chem. 1983; 87: 4130–4134.
- Solomon S , Qin D , Manning M , Chen Z , Marquis M , co-authors . Climate Change 2007 The Physical Science Basis Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. 2007. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA..
- Spitzy A , Ittekot V , Kempe S . Facets of Modern Biogeochemistry. 1990; Berlin: Springer-Verlag. 313–317. Amino acids in marine aerosol and rain..
- Thompson S. M , Gubbins K. E , Walton J. P. R. B , Chantry R. A. R , Rowlinson J. S . A molecular dynamics study of liquid drops. J. Chem. Phys. 1984; 81: 530.
- Twomey S . Pollution and the planetary albedo. Atmos. Environ. 1974; 8: 1251–1256.
- Van der Spoel D , Lindahl E , Hess B , Van Buuren A. R , Apol E , co-authors . Gromacs User Manual version 4.0. 2005. Online at: http://www.gromacs.org .
- Van Giessen A. E , Blokhuis E. M . Direct determination of the Tolman length from the bulk pressures of liquid drops via molecular dynamics simulations. J. Chem. Phys. 2009; 131: 164705.
- Wedyan M. A , Preston M. R . The coupling of surface seawater organic nitrogen and the marine aerosol as inferred from enantiomer-specific amino acid analysis. Atmos. Environ. 2008; 42: 8698–8705.
- Wine P. H . Atmospheric and environmental physical chemistry: pollutants without borders. J. Phys. Chem. Lett. 2010; 1: 1749–1751.
- Yeh I.-C , Berkowitz M. L . Ewald summation for systems with slab geometry. J. Chem. Phys. 1999; 111: 3155–3162.
- Yuet P. K , Blankschtein D . Molecular dynamics simulation study of water surfaces: comparison of flexible water models. J. Phys. Chem. B. 2010; 114: 13786–13795.
- Zakharov V. V , Brodskaya E. N , Laaksonen A . Surface tension of water droplets: a molecular dynamics study of model and size dependencies. J. Chem. Phys. 1997; 107: 10675–10683.
- Zhang Q , Anastasio C . Free and combined amino compounds in atmospheric fine particles (PM2.5) and fog waters from Northern California. Atmos. Environ. 2003; 37: 2247–2258.
- Zhou J . Hygroscopic Properties of Atmospheric Aerosol Particles in Various Environments. 2001; Lund University, Lund, Sweden. Doctoral Thesis.