Abstract
Unidentified DNA sequences in isolation-based or culture-free studies of conifer endophytes are a persistent problem that requires a field approach to resolve. An investigation of foliar endophytes of Picea glauca, P. mariana, P. rubens and Pinus strobus in eastern Canada, using a combined field, morphological, cultural and DNA sequencing approach, resulted in the frequent isolation of Phialocephala spp. and the first verified discovery of their mollisia-like sexual states in the field. Phialocephala scopiformis and Ph. piceae were the most frequent species isolated as endophytes from healthy conifer needles. Corresponding Mollisia or mollisioid sexual states for Ph. scopiformis, Ph. piceae and several undescribed species in a clade containing Ph. dimorphospora were collected in the sampling area and characterized by analysis of the nuc internal transcribed spacer rDNA (ITS) and gene for the largest subunit of RNA polymerase II (RPB1) loci. Four novel species and one new combination in a clade containing Ph. dimorphospora, the type of Phialocephala, are presented, accompanied by descriptions of apothecia and previously undocumented synanamorphs. An epitype culture and corresponding reference sequences for Phialocephala dimorphospora are proposed. The resulting ITS barcodes linked with robust taxonomic species concepts are an important resource for future research on forest ecosystems and endophytes.
Introduction
CitationKendrick (1961) introduced the dematiaceous hyphomycete genus Phialocephala for a group of morphologically distinct species previously classified in Leptographium. He distinguished the genera based on their modes of enteroblastic, basipetal conidiogenesis; Phialocephala species have a stationary conidiogenous locus that gradually thickens as successive conidia are produced (phialides), while Leptographium species have a progressive locus that extends percurrently after each conidium, leaving an annellated zone (annelides). The type species, Ph. dimorphospora, was collected from rotten hardwood (Ulmus sp.) in Ontario, Canada, and characterized by dark-pigmented penicillate conidiophores and phialides with deep collarettes yielding both ovoid primary conidia and globose secondary conidia that aggregate into slimy droplets. Subsequent to the inception of the genus, 37 Phialocephala species were described. Several were transferred out of Phialocephala (e.g. Sporendocladia bactrospora, S. follicola, S. ivoriensis, S. truncata) based on morphological and phylogenetic data (e.g. CitationJacobs et al. 2001, CitationDay et al. 2012). Phaeomollisia piceae recently was combined into Phialocephala based on phylogenetic placement within a major clade of Phialocephala (CitationJohnston et al. 2014), resulting in 29 currently accepted species in this genus (Index Fungorum 2015, CitationWong et al. 2015).
Phialocephala-like asexual states occur across a moderately large lineage including the core clade of Phialocephala sensu stricto (s.s., Ph. dimorphospora and allied taxa; e.g. CitationMenkis et al. 2004, CitationGrünig et al. 2009); the Ph. fortinii sensu lato (s.l.)–Acephala applanata complex (PAC) and allied taxa; Vibrissea s.s. (CitationSutton 1976, CitationHamad and Webster 1988); and several more distantly related Phialocephala species (e.g. Ph. scopiformis, Ph. sphaeroides). Phialocephala-like anamorphs are also known from several unsequenced mollisoid taxa (e.g. CitationLe Gal and Mangenot 1956, CitationAebi 1972) or aquatic synanamorphs (e.g. CitationWebster and Descals 1975, CitationDescals and Sutton 1976, CitationDescals and Webster 1982). A sterile morphotype generic concept, Acephala, was established for A. applanata, a species frequently co-isolated with Ph. fortinii s.l. (CitationGrünig and Sieber 2005). Acephala currently comprises two species and the generic name has been applied informally to a phylogenetically diverse range of sterile strains apparently congeneric with Phialocephala (CitationGrünig et al. 2009, CitationMünzenberger et al. 2009). The current taxonomic concept of Phialocephala is therefore polyphyletic but with most sequenced species within a lineage encompassing the Vibrissea-Loramyces-Mollisia clades sensu CitationWang et al. (2006a, Citationb) and CitationGrünig et al. (2009).
Phialocephala species and allied taxa often are isolated from roots and decayed wood in heathlands, forests and alpine ecosystems across north temperate and boreal regions, and as a group they display remarkable ecological plasticity (CitationAddy et al. 2000, CitationGrünig et al. 2009). The most studied belong to the PAC, which frequently are isolated from the cortex of primary roots and the periderm of lignified roots of ericaceous plants (e.g. Calluna vulgaris, Empetrum nigrum, Vaccinium myrtillus) and coniferous trees (e.g. Abies, Picea, Pinus spp.). The PAC currently comprises 10 species, collectively the most studied of the so-called dark septate endophytes (DSE), an assemblage of distantly related fungi (also including species of e.g. Cadophora, Leptodontidium, Microdochium, Periconia, Trichocladium) detected in roots of more than 600 plant species (CitationJumpponen and Trappe 1998, CitationKnapp et al. 2012). Fungi grouping with the PAC often are among the most abundant recovered from root systems using culture-dependent or culture-independent sampling methods (CitationBruzone et al. 2015, CitationLi et al. 2015, CitationPickles et al. 2015). The relationship between PAC species and their hosts is complex, with interactions ranging from pathogenic (CitationWilcox and Wang 1987, CitationStoyke and Currah 1993, CitationTellenbach et al. 2011) to neutral or mutualistic (CitationFernando and Currah 1996, CitationVohník et al. 2005, CitationPeterson et al. 2008, CitationNewsham 2011, CitationOtgonsuren and Lee 2012, CitationTellenbach and Sieber 2012).
While most Phialocephala-host interaction studies focused on host growth and biomass, emerging evidence suggests Phialocephala species may benefit the host through other complex interactions resulting in potentially beneficial outcomes, by production of antifeedant or antipathogenic secondary metabolites or other mechanisms (CitationTellenbach and Sieber 2012, CitationTellenbach et al. 2012, CitationTerhonen et al. 2014). For example, a strain of Ph. scopiformis isolated as an endophyte from Picea glauca needles produces rugulosin, an insecticidal secondary metabolite with a reported dose-dependent weight reduction in eastern spruce budworm (Choristoneura fumiferana) (CitationMiller et al. 2009). CitationMiller (2011) suggested that the reduced growth of budworms could lead to longer exposure to adverse environmental and biotic factors, perhaps disrupting reproductive synchrony. Avirulent or weakly virulent PAC species may stimulate host defenses or suppress more severe root pathogens (e.g. CitationTellenbach and Sieber 2012, CitationTellenbach et al. 2012) including more virulent PAC strains (CitationReininger et al. 2012) by space or nutrient competition or direct antagonism. Conversely Ph. bamuru recently was described as the causal agent of fairway patch, a severe emerging disease of golf course turf in Australia that appears resistant to chemical control (CitationWong et al. 2015).
Despite the lack of comparable studies of other members of Phialocephala outside the PAC, they may be similarly abundant in poorly investigated niches, such as conifer needles, deciduous leaves and living and dead wood. Several Phialocephala species (e.g. Ph. glacialis, Ph. piceae, Ph. scopiformis) and several other phylogenetically related but unnamed taxa with cystodendron-like asexual morphs were isolated repeatedly from conifer needles and may systemically infect their hosts in other organs, including roots (CitationHata and Futai 1995, Citation1996; CitationGrünig et al. 2009). Such investigations indicate that leaf colonization by Phialocephala species and allied taxa may be an important but little studied niche, particularly if similar secondary metabolites are expressed in host tissues, resulting in conditionally mutualistic benefits, as seen in root-endophyte studies.
A major obstacle to understanding the niches and functions of Phialocephala species is that morphological approaches based on phialidic asexual morphs and nuc internal transcribed spacer rDNA (ITS) sequences do not allow accurate identification or distinguishing of taxa. Identification of Phialocephala isolates with a morphotaxonomic approach is problematic because cultures often apparently are sterile and morphological variation may be insufficient for species delineation, especially among closely related species (CitationGrünig et al. 2008a). Sporulation in recalcitrant cultures has been induced by long-term incubation at low temperatures (), although sporulation in some species has never been observed (CitationGrünig and Sieber 2005, CitationMünzenberger et al. 2009). Morphological characters associated with conidiophores are taxonomically informative to an extent, but CitationGrünig et al. (2008a) noted high intraspecific variation in asexual reproductive structures of PAC species. Molecular phylogenetic approaches also face many issues, reflecting difficulties in differentiating morphologically similar taxa that appear to be phylogenetically distinct. For example, CitationHarney et al. (1997) identified a clade of three Ph. dimorphospora groups based on ITS sequences and rDNA restriction enzyme patterns, while CitationMenkis et al. (2004) isolated eight Ph. dimorphospora ITS haplotypes from decaying P. abies stumps and boles. Phylogenetically differentiating closely related taxa using the ITS alone is questionable, as is evident with the Ph. fortinii s.l. complex, which CitationGrünig et al. (2008a) determined to consist of at least six reproductively isolated species with a multilocus approach using two coding (genes for β-tubulin and translation elongation factor 1-α) and three noncoding DNA loci (pPF-018, pPF-061, pPF-076). However, ITS sequences appear to differentiate other described Phialocephala species.
Table I Incubation conditions and time required to induce sporulation in Phialocephala spp.
Molecular evidence has indicated repeatedly a close relationship among named Phialocephala species and some species of Mollisia, a teleomorph genus associated with phialocephala-like asexual states (CitationVrålstad et al. 2002, CitationWilson et al. 2004, CitationMenkis et al. 2005, CitationZijlstra et al. 2005, CitationWu and Guo 2008, CitationGrünig et al. 2009, CitationDay et al. 2012). In its current taxonomic state Mollisia is an unwieldy para- and polyphyletic genus requiring significant taxonomic revision, with at least 230 described species that are difficult or impossible to differentiate morphologically (CitationGreenleaf and Korf 1980). Currently few Mollisia sequences identified to species rank are accessioned in GenBank, and the difficulties associated with identifying species based on apothecia morphology calls these identifications into doubt. For example, sequences attributed to M. cinerea currently in GenBank probably represent several distinct and distantly related species based on their dissimilar ITS sequences. Genetic and morphological links with several other teleomorph genera also have been indicated, including Phaeomollisia (CitationJohnston et al. 2014) and Vibrissea (CitationHamad and Webster 1988, CitationWang et al. 2006b, CitationGrünig et al. 2009). Consequently molli-soid apothecia may be candidate sexual states for some endophytes known only by their asexual morphs or sterile cultures.
Another important issue is that knowledge of Phialocephala spp. in the environment is limited to their endophytic or saprotrophic life stages, ignoring their broader ecology. This at least partly reflects the lack of data on life cycles and dispersal mechanisms (e.g. CitationGrünig 2008b, CitationZaffarano 2011). Despite genetic and morphological data suggestive of the existence of sexual states, named Phialocephala spp. currently are unconnected to sexual states, probably because most studies include only cultures or environmental DNA sequences. Such links perhaps could be most readily investigated by combined studies involving isolation of endophytes, morphological characterization of the resulting cultures and DNA sequencing of field-collected apothecia from the same forest, niche, biome or environment.
In this study a survey of endophytes associated with conifers in eastern Canada, with a focus on Picea rubens, was conducted to provide baseline biodiversity data and to identify sterile cultures of unidentifiable species by making connections with sporulating field specimens. Based on ITS sequences and morphology many strains of described and unidentified Phialocephala spp. were isolated as endophytes of healthy needles. Endophytes identified as Phialocephala spp. were of particular interest because of their potential as a forest management tool against economically important forest pests (CitationMiller et al. 2008). A combined lab- and field-based approach facilitated the discovery of the sexual states of several described Phialocephala species, which were confirmed with DNA phylogenetic analyses of the ITS barcode sequences and the gene for the largest subunit of RNA polymerase II (RPB1). Detailed morphological observations of these undescribed sexual morphs and their conidial states are provided.
Materials and methods
Sampling and isolation of fungi
Specimens were collected at several sites in New Brunswick, Canada, and two sites in Aylmer, Quebec, Canada. Branches collected from understory and overstory conifers were stored in plastic bags at 4 C, and subsequent isolations occurred within 24 h or up to 10 d following collection. For endophyte isolations needles were removed with forceps and surface-sterilized by serial passage in 70% ethanol (1 min), 3% NaClO (7.5 min) and 70% ethanol (30 s). Needles then were rinsed in sterile distilled H2O, blotted on sterile Kimwipes (Kimberly Clark, Mississauga, Canada) and cut in half longitudinally. Needle segments were placed on 2% malt extract agar (MEA; 20 g Bacto malt extract, Difco Laboratories, Sparks, Maryland; 15 g agar, EMD Chemicals Inc., New Jersey; 1 L distilled water) in 9 cm Petri dishes and incubated in the dark at 16 C. Mycelia emerging from needle tissue were excised axenically and subcultured on 6 cm Petri dishes containing MEA.
Isolations from apothecial specimens were made by suspending sporulating ascomata from the lid of a Petri dish with petroleum jelly or drops of water, allowing ascospores to eject onto MEA. When possible monospore cultures were obtained from individual ascospores transferred to 6 cm Petri dishes containing MEA. All cultures were incubated in the dark at 16 C. Attempts to induce sporulation in culture involved prolonged incubation in the light or dark at 5, 10, and 15 C for up to 18 mo and inoculation of isolates on various media: cornmeal agar (CMA; Acumedia Manufacturers Inc., Lansing, Michigan), oatmeal agar (OA; CitationCrous et al. 2009), oatmeal tomato paste agar (OA amended with 10 g tomato paste, 1 g MgSO4· 7H2O, 1 g KH2PO4, 1 g NaNO3), pine needle potato agar (PNPA; CitationSu et al. 2012), and MEA and 1.5% water agar (WA, with 1 mL trace metal solution; CitationCrous et al. 2009) with or without the addition of sterile Picea needles on the agar surface.
Morphological studies
Vertical sections of fresh apothecia were cut by hand and mounted in either water, 85% lactic acid, Melzer’s reagent, 5% KOH, or Lugol’s solution, with or without 5% KOH pretreatment. Colony colors were described with the alphanumeric codes of CitationKornerup and Wanscher (1978). Microscopic measurements were taken from material mounted in water and are presented as ranges calculated from the mean ± standard deviation of each measured value, with outliers in brackets. Observations were made with an Olympus BX50 light microscope and micrographs were captured using an Evolution MP color camera (Media Cybernetics, Silver Spring, California) and Image-Pro Plus 6.0 (Media Cybernetics) or InfinityX-32 camera (Lumenera Corp., Ottawa, Canada) and Infinity Analyze (Lumenera Corp.) software. Colony macrophotographs were captured with a Nikon Coolpix P5000 (Nikon Inc., Tokyo, Japan) and photographic plates were assembled with Adobe Photoshop 5.5 (Adobe Systems, San Jose, California).
To assess germination, conidia from two Ph. dimorphospora isolates (DAOMC 87232, DAOMC 250111) were collected with a fine tungsten inoculation loop, suspended in sterile Millipore water, and streaked onto plates containing MEA or CMA and incubated in the dark at 5, 10, 15 and 20 C. Each treatment was conducted in triplicate and germination of conidia assessed visually every 24 h for 7 d.
Phylogenetic studies
Total genomic DNA was extracted from 4–12 wk old cultures or dried cultures (herbarium specimens) with the Ultraclean Microbial DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, California) or NucleoSpin Plant II Kit (Macherey-Nagel, Düren, Germany) following the manufacturer’s protocol. The primers ITS1 and ITS4 (CitationWhite et al. 1990) or ITS4A and ITS5 (CitationLarena et al. 1999) were used to amplify and sequence the ITS region. The largest subunit of RNA polymerase II (RPB1) was amplified and sequenced with RPB1-Af and RPB1-6Rlasc (CitationStiller and Hall 1997, CitationHofstetter et al. 2007). DNA was amplified with a PCR master mix consisting of 0.5 μm 2 mM dNTPs, 0.04 μm 20 μM forward primer, 0.04 μm 20 μM reverse primer, 1 μL 10× Titanium Taq buffer (Clontech, Mountain View, California), 0.1 μL 50× Titanium Taq enzyme (Clontech, Mountain View, California) and 1 μL DNA template and 7.32 μL sterile purified water per reaction. All loci were amplified with the following PCR profile: 95 C for 3 min, then 35 cycles at 95 C for 1 min, 56 C for 45 s, and 72 C for 1.5 min, followed by a final extension at 72 C for 10 min. PCR products were verified by agarose gel electrophoresis and sequenced with Big Dye Terminator (Applied Biosystems, Foster City, California).
Sequence contigs were assembled and trimmed with Geneious R6 6.1.8 (Biomatters, Auckland, New Zealand). The sequence of each locus was aligned with MAFFT (CitationKatoh et al. 2005) and the resulting alignments trimmed and manually checked using BioEdit (CitationHall 1999). All sequences in this study are accessioned in GenBank (Supplementary Table I) and the alignments and phylogenetic trees in TreeBASE (study No. 17404).
Phylogenetic analyses of single-gene datasets were performed with Bayesian inference. The most suitable sequence evolution models for each gene (sym+i+g for ITS, sym+g for RPB1) were determined based on the optimal Akaike information criterion scores in MrModeltest 2.2.6 (CitationNylander 2004). Bayesian analysis was performed with MrBayes 3.2 (CitationRonquist and Huelsenbeck 2003) with Loramyces macrosporus as outgroup. For each locus three independent Markov chain Monte Carlo (MCMC) runs were run simultaneously for 3.0 × 106 generations (standard deviation of split frequencies < 0.01), sampling every 500 generations. The first 25% trees were discarded as burn-in and the remaining 6746 trees were kept and combined into one consensus tree with 50% majority rule (). Consensus trees were imported into FigTree (CitationRambaut 2014) and exported as SVG vector graphics for assembly in Adobe Illustrator (Adobe System, San Jose, California) and Adobe Photoshop.
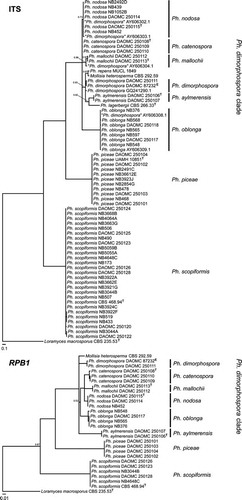
Fig. 1 Bayesian 50% majority rule ITS and RPB1 consensus trees containing representative Mollisia and Phialocephala isolates: culture collection, GenBank or JB Tanney personal collection accession numbers are followed by the species name (T = ex-type; E = ex-epitype). All branches have Bayesian posterior probability values of 1.0; values lower than 1.0 are presented at nodes. The trees were rooted to Loramyces macrosporus and bars indicate expected changes per site per branch.
Results
Isolations from surface-sterilized needle segments yielded 1010 isolates, including 79 isolates of Phialocephala spp. based on initial identifications with ITS barcodes. Phialocephala scopiformis was the most abundant species, represented by 53 isolates, with Ph. piceae the second most abundant, represented by 14 isolates. Two isolates belonging to a well-defined clade including Ph. dimorphospora (henceforth referred to as the Ph. dimorphospora clade) were recovered (NB249-2D, NB105-2B) and the remaining 10 isolates represented seven unnamed ITS phylotypes phylogenetically related to Phialocephala s.l., to be considered in subsequent studies.
A total of 128 mollisioid apothecial specimens were collected from a variety of woody substrates in the same forest stands sampled for endophytes. Colony morphologies of isolates resulting from ascospore isolations generally resembled those of Phialocephala endophyte isolates. Comparison of ITS sequences of Phialocephala endophytes and isolates derived from mollisioid ascomata indicated the discovery of apothecia corresponding to Ph. scopiformis, Ph. nodosa sp. nov. and Ph. piceae (). Connections between endophyte strains and corresponding apothecia field collections were further confirmed with RPB1 sequences ().
Apothecia for most species could not be differentiated unambiguously in the field, although apothecia of Ph. scopiformis had a characteristic blue hymenium. Several species in the Ph. dimorphospora clade were readily distinguished based on the morphology of asexual states on MEA, while others were clearly delineated by ITS sequences. These species include Ph. nodosa, characterized by darkly pigmented microsclerotia consisting of irregularly coiled 1–2-septate cells; Ph. catenospora, which produces two synanamorphs consisting of typical penicillate conidiophores and pigmented 1–4-septate diplococcium-like conidia in simple or branched chains; and Ph. aylmerensis, which produces two types of conidiophores producing minute conidia in slimy heads, hyaline reduced conidiophores and pigmented 1–2 branching conidiophores. One isolate was not observed to form a conidial state but was con-specific with the synnematous fungus Paradidymobotryum oblongum (99% identity based on ITS sequences; K. Seifert unpubl) and is therefore named Ph. oblonga due to priority. Conidial states were not observed for Ph. mallochii.
The type specimen of Ph. dimorphospora (DAOM 71465[c]) consists of a herbarium sheet with four herbarium packets. The holotype comprises four small pieces of Ulmus sp. (probably U. americana) wood with sparse Ph. dimorphospora conidiophores. Two packets contain slides and micrographs of the type specimen and one packet contains five dried ex-type cultures. Living ex-type cultures of Ph. dimorphospora no longer exist (C. Babcock pers. comm) and attempts to sequence ITS and RPB1 from the dried cultures associated with the type specimen of Ph. dimorphospora failed. There were insufficient quantities of DNA or the resulting sequences were of Malassezia globosa, presumably a contaminant on the herbarium specimens. A Ph. dimorphospora culture isolated from paper mill slime in Nova Scotia, Canada, (DAOMC 87232 = CBS 300.62) and authenticated by W.B. Kendrick is selected below as an epitype. This culture is consistent morphologically with the type specimen and produces abundant conidiophores on MEA.
In 2014 a Ph. dimorphospora specimen (DAOMC 250111) was collected from decaying hardwood (Populus sp.) ca. 30 km northeast of the type locale of Ph. dimorphospora. Based on morphology and ITS and RPB1 sequences, this specimen is conspecific with the selected Ph. dimorphospora epitype (DAOMC 87232). Conidia from DAOMC 87232 and DAOMC 250111 streaked on CMA and MEA and incubated at 10, 15 and 20 C germinated within 72 h, confirming their function as propagules and not exclusively as spermatia.
Phialocephala scopiformis, Ph. piceae and members of the Ph. dimorphospora clade were consistently delineated in both ITS and RPB1 phylogenies. The Ph. dimorphospora clade formed a strongly supported (posterior probability [PP] = 1.0) polytomous clade containing Ph. aylmerensis, Ph. catenospora, Ph. dimorphospora, Ph. lagerbergii, Ph. mallochii, Ph. nodosa, Ph. oblonga, Ph. repens and Mollisia heterosperma.
Taxonomy
All species described below, except Phialocephala scopiformis and Ph. piceae, are part of what we interpret as the core clade of Phialocephala s.s. following single-name nomenclature (CitationHawksworth et al. 2011), which includes species with different combinations of mollisioid sexual states, phialidic asexual states that may or may not have penicillate conidiophores, companion asexual states with acropetal chains of septate conidia (here called diplococcium-like), similar diplococcium-like asexual states producing synnemata or microsclerotia. Phialocephala scopiformis and Ph. piceae are treated as Phialocephala s.l. and their final classification requires resolution of other taxonomic issues not addressed here. Mollisia heterosperma is treated as a member of the core Phialocephala s.s. clade, but its reclassification awaits a broader treatment of Mollisia and the phylogenetic placement of an epitype for M. cinerea. We (i) propose an epitype specimen for Ph. dimorphospora, the type species of the genus; (ii) add descriptions of sexual morphs to the previously asexual concept of Ph. scopiformis; (iii) make the first report of Ph. piceae apothecia collected in the field and expand on the protologue; (iv) describe the new species Ph. nodosa with apothecia and a microsclerotial asexual state; (v) describe Ph. catenospora with apothecia, a penicillate anamorph and diplococcium-like anamorph; (vi) describe Ph. mallochii with apothecia only and (vii) describe Ph. aylmerensis with apothecia and a phialidic asexual state, and make a new combination Ph. oblonga for a species with apothecia and a synnematous diplococcium-like asexual state previously described as the type of the hyphomycete genus Paradidymobotryum. For all descriptions, details of additional specimens, cultures and GenBank accession numbers for DNA sequences are provided (Supplementary Table I). Details of additional specimens and cultures examined in the personal collection of JB Tanney are provided (Supplementary Table II).
Phialocephala dimorphospora W.B. Kendrick, Can J Bot 39:1080. 1961.
Typification: CANADA. ONTARIO: Manotick, on rotten wood of Ulmus sp., 4 Nov 1960, S.J. Hughes (holotype, DAOM 71465[c]). NEW BRUNSWICK: a dried culture originally isolated from slime in pulp mill, 1961, W.B. Kendrick (epitype designated here, DAOM 574894. Ex-epitype culture DAOMC 87232 [= CBS 300.62 = CMW 665 = UPSC 2186]).
Phialocephala scopiformis T. Kowalski & R.D. Kehr, Can J Bot 73:27. 1995. ,
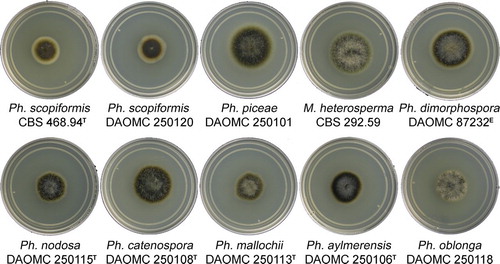
Fig. 2 Colony morphologies of representative isolates, 2 wk after inoculation on MEA at 20 C in the dark (T = ex-type, E = ex-epitype).
Colonies 19–21 mm diam after 14 d in the dark at 20 C on MEA; flat to slightly convex in center, sparse grayish brown (8F3) funiculose hyphae in center, margin diffuse, flat, wide, yellowish brown (5E5) to white; surface grayish brown to dark brown (8F3–8F4) with wide (2–3 mm), yellowish brown (5E5) outer concentric circle; reverse dark brown (7F4) with yellowish brown to white margin (5E5). Exudates and soluble pigments absent in younger cultures, older (> 4 mo) cultures exuding small yellowish orange (4B4) droplets on colony surface and surrounding agar turning grayish yellow (3B5), yellowish orange (4B4) plate-like crystals sometimes present on surface of colony or below agar surface.
Apothecia scattered to gregarious or caespitose; sessile; subiculum not evident; urceolate to cup-shaped when young, disk plain to concave at maturity; outline entire, sometimes sinuate; grayish blue (21E7), outer surface slightly darker; 0.3–1.0 mm diam, 0.2–0.3 mm high; margin sometimes paler because of refractive contents in subhyaline marginal cells, smooth. Ectal excipulum at base and mid flanks textura globulosa to angularis, 25–105 μm thick near base, 15–40 μm thick toward margin, composed of globose to isodiametric cells with thin to slightly thickened walls, (12–)12.5–18(–21) × (8–)8.5–11.5(–12) μm; at upper flank and margin textura angularis to prismatica, composed of globose to elongated clavate cells with +/− thin walls, 8–12(–15) × (6–)6.5–8(–9) μm; marginal cells organized in distinctive parallel rows, obovoid to clavate or spathulate, 9–12(–12.5) long, maximum width toward apex 4–5.5 (–7) μm, minimum width at base 2–3 μm; brownish orange to brown (5D4–6E4) around margin and becoming dark brown (5F8) toward base, not gelatinized, crystals or exudates absent; tissue becoming olive to dark green (1E6–27F5) when mounted in 5% KOH. Subicular hyphae sparse to moderately abundant, sometimes gelatinized, 2.5–3.5(–4) μm, thick-walled (< 0.5–1 μm), dark brown (5F8). Medullary excipulum hyaline, textura intricata, 20–35 μm thick. Paraphyses cylindrical with rounded apices, septate, simple, thin-walled, 3–3.5(–4) μm wide, containing large highly refractive vacuole bodies; not exceeding mature asci. KOH reaction: negative. Asci arising from croziers, cylindrical-clavate, eight-spored, 35–45(–55) × 3–5.5 μm, pars sporifera 20–25 μm, pore amyloid in Melzer’s reagent or Lugol’s solution with 5% KOH pretreatment (hemiamyloid), protoplasm turning brick red (7D7) in Lugol’s solution. Ascospores biseriate to obliquely uniseriate, (6.5–)8–9.5(–11) × (2–)2.5–3 μm, ellipsoidal to oblong-ellipsoidal or fusoid-clavate, apices rounded with one apex often conspicuously acute, 0(–1)-septate, thin-walled, frequently guttulate with 2–3(–4) large guttules (1–2.5 μm diam) or several small guttules (< 0.5 μm diam) aggregated at both poles.
See CitationKowalski and Kehr (1995) for description of the Ph. scopiformis asexual state.
Cardinal temperatures: 5–30 C, optimum 20–25 C, minimum slightly < 5 C, maximum slightly > 30 C.
Host range: Associated with decaying wood and living foliage and branches of conifers, including Picea abies, P. glauca, P. mariana and P. rubens.
Distribution: Canada (New Brunswick), Germany, United Kingdom.
Additional specimens and cultures examined: CBS 468.94 (see Supplementary Table II).
Notes: Phialocephala scopiformis is frequently isolated as an endophyte of living Picea spp. needles. In this study apothecia were found only on decaying Picea rubens branches. Apothecia are distinguished by marginal cells that are organized in distinctive parallel rows, a thin medullary excipulum, heavily melanized ectal excipulum and occasionally sinuate outline. The characteristic blue hymenium is observable in the field.
Phialocephala piceae (T.N. Sieber & C.R. Grünig) Rossman, IMA Fungus 5:104. 2014. ,
≡ Phaeomollisia piceae T.N. Sieber & C.R. Grünig, Mycol. Res. 113:215. 2009 (Basionym)
Colonies 32–36 mm diam after 14 d in the dark at 20 C on MEA; flat to slightly convex in center, woolly olive brown (4E3) aerial mycelia in center, margin diffuse, flat, and white; surface olive brown (4E5–4F4); reverse olive gray (3F2). Exudates and soluble pigments absent.
Apothecia scattered to gregarious; sessile; subiculum not evident; urceolate to cup-shaped when young, becoming plain to slightly concave at maturity; outline entire; grayish brown to yellowish brown (5D3–5D5), outer surface slightly darker; 0.5–2.0 mm diam, 0.2–0.4 mm high; margin lighter, smooth. Ectal excipulum at base and mid flanks textura globulosa to angularis, 50–140 μm thick near base, 15–40 μm thick toward margin, composed of globose to isodiametric cells with thin to slightly thickened walls, 13–16.5(–19) × (8–)9.5–12(–12.5) μm; at upper flank and margin textura angularis to prismatica, composed of globose to elongate cylindrical to slightly clavate cells with +/− thin walls, 6–8.5(–10) × (5–)5.5–7.5(–9) μm; marginal cells cylindrical to clavate, (9–)11–18(–20) μm long, maximum width toward apex 5–8 μm, minimum width at base 3–5 μm; hyaline to grayish yellow (4B4) around margin and becoming light brown (6D7) toward base, not gelatinized, crystals or exudates absent; tissue becoming olive (3E5) when mounted in 5% KOH. Subicular hyphae sparse, sometimes gelatinized, 2–3(–3.5) μm, thick-walled (0.5–1 μm), dark brown (5F8). Medullary excipulum hyaline, textura intricata, 32–48(–55) μm thick. Paraphyses cylindrical with rounded apices, septate, simple, thin-walled, 2–2.5(–3) μm wide, containing large highly refractive vacuole bodies; not exceeding the length of mature asci. KOH reaction: negative. Asci arising from croziers, cylindrical-clavate, eight-spored, (33–)37–49(–53) × 4–7 μm, pars sporifera 20–30 μm, pore amyloid in Melzer’s reagent or Lugol’s solution with 5% KOH pretreatment (hemiamyloid), protoplasm turning brick red (7D7) in Lugol’s solution. Ascospores biseriate to obliquely uniseriate, (7.5–)9–12(–15) × (2–)2.5–3(–3.5) μm, ellipsoidal to ellipsoidal-fusiform, apices rounded, 0(–1)-septate, thin-walled, several < 0.5–1(–2) μm diam guttules usually aggregated at both poles.
See CitationGrünig et al. (2009) for description of the phialocephala-like and cadophora-like asexual states of Ph. piceae.
Cardinal temperatures: 5–30 C, optimum 20 C, minimum slightly < 5 C, maximum slightly > 30 C.
Host range: Associated with living foliage of Picea abies, P. glauca, P. mariana, P. rubens and Pinus strobus and decomposing branches or wood of Acer saccharum.
Distribution: Canada (New Brunswick), Lithuania, Sweden, Switzerland.
Additional specimens and cultures examined: see Supplementary Table II.
Notes: Phialocephala piceae apothecia are distinguished by yellowish brown pigment and by often being erumpent from bark of host branches (Acer saccharum).
Phialocephala nodosa J.B. Tanney & B. Douglas, sp. nov. , ,
Fig. 3 A. Mature Phialocephala catenospora apothecia on 4 mo old culture (DAOMC 250108) on MEA. B–D. Synnemata of Ph. oblonga. E–G. Ph. dimorphospora conidiophores. H–N. Ph. catenospora synanamorphs. O–S. Ph. nodosa microsclerotia. T–X. Ph. aylmerensis conidiophores. Bars = 10 μm.

Fig. 4 Phialocephala scopiformis. A–C. Apothecia on fallen decaying Picea rubens branches; D. Vertical section of apothecium. E–F. Marginal cells. G. Vertical section displaying ectal and medullary excipulum. H–I. Paraphyses displaying refractive vacuole bodies. J. Asci with hemiamyloid tips in Lugol’s solution after KOH pretreatment. K. Discharged and germinating ascospores. Bars: D = 500 μm, E = 20 μm, F–K = 10 μm.

Fig. 5 Phialocephala piceae. A–C. Apothecia erumpent on fallen decaying Acer saccharum branches. D. Vertical section of apothecium. E–F. Marginal cells. G. Vertical section showing ectal and medullary excipulum. H. Ectal excipulum cells. I–J. Paraphyses displaying refractive vacuole bodies. K. Immature asci. L. Ascus with hemiamyloid tip in Lugol’s solution after KOH pretreatment. M. Ascospores. Bars: D = 500 μm, E–M = 10 μm.
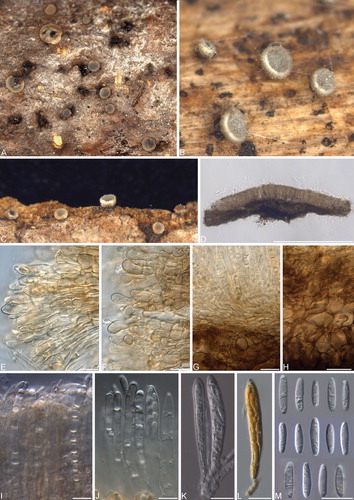
Fig. 6 Phialocephala nodosa. A–D. Apothecia on fallen decaying Acer saccharum branches. E. Vertical section showing margin and paraphyses with refractive vacuole bodies. F. Ectal excipulum. G–H. Marginal cells. I. Ectal excipulum cells. J. Subicular hyphae with thick gelatinous sheath. K. Paraphyses with refractive vacuole bodies. L–M. Asci with hemiamyloid tips in Lugol’s solution after KOH pretreatment. N. Ascospores under phase contrast. O. Ascospores under DIC. Bars = 10 μm.

MycoBank MB811718
Typification: CANADA. NEW BRUNSWICK: Alma, Fundy National Park, Maple Grove trail, 45.58178 −64.98633, from decaying Acer saccharum branch, 17 Jul 2014, J.B. Tanney NB-475 (holotype DAOM 628553). Ex-type culture DAOMC 250115.
Etymology: nodosa (Latin), knotty, referring to the appearance of microsclerotia produced in culture.
Colonies 20–22 mm diam after 14 d in the dark at 20 C on MEA; flat to convex in center, woolly olive gray (3E2) aerial mycelia in center, margin diffuse, flat, and white; surface brownish gray to olive brown (4F2–4F5); reverse olive gray (3E2); shiny black microsclerotia sparsely to moderately abundant in aerial mycelia. Exudates and soluble pigments absent. Mycelium consisting of subhyaline to dematiaceous, smooth, septate, branched, hyphae 2–3.5 μm diam, sometimes covered with gelatinous sheaths 1–3.5 μm diam. Helicoid initials proliferate into dense microsclerotia comprising darkly pigmented, moniliform cells, 3–6 × 1.5–3.5 μm. Microsclerotia abundant in aerial mycelia and later forming crust on colony surface on MEA, OA and CMA.
Apothecia scattered to gregarious; sessile; subiculum not evident; urceolate to cup-shaped when young, disk plain to concave at maturity; outline entire; orange gray to grayish brown (5B2–5E3), outer surface darker; 0.6–2 mm diam, 0.2–0.4 mm high; margin frequently paler color, smooth. Ectal excipulum at base and mid flanks textura globulosa to angularis, 32–125 μm thick near base, 27–36 μm thick toward margin, composed of globose to isodiametric cells with thin to slightly thickened walls, (14–)16–19.5(–22) × (8–)9–12 μm; at upper flank and margin textura angularis to prismatica, composed of globose to elongated clavate cells or cylindrical with +/− thin walls, 7–9.5(–11.5) × (5.5–)6–7 μm; marginal cells cylindrical to obovoid or clavate, (8–)11–19(–20.5) long, maximum width toward apex 6–7.5(–8) μm, minimum width at base (4.5–)5–6(–6.5) μm; brownish orange to brown (5D4–6E4) around margin and becoming dark brown (6F7) toward base, not gelatinized, crystals or exudates absent; tissue becoming olive to dark green (1E6–27F5) when mounted in 5% KOH. Subicular hyphae sparse to moderately abundant, sometimes gelatinized with conspicuously thick (2–3.5 μm) layer, 2.5–3.5(–4) μm, thick-walled (0.5–1 μm), dark brown (5F8). Medullary excipulum hyaline, textura intricata, 28–36 (–45) μm thick. Paraphyses cylindrical with rounded apices, septate, simple, thin-walled, 3–3.5(–4) μm wide, containing large, highly refractive vacuole bodies; not exceeding mature asci. KOH reaction: negative. Asci arising from croziers, cylindrical-clavate, eight-spored, (49–)51.5–62(–65) × 5–6.5 μm, pars sporifera 19–30 μm, pore amyloid in Melzer’s reagent or Lugol’s solution with 5% KOH pretreatment (hemiamyloid), protoplasm turning brick red (7D7) in Lugol’s solution. Ascospores biseriate to obliquely uniseriate, (7–)8–10.5(–12) × (2–)2.5–3(–4) μm, ellipsoidal-fusiform to oblong, apices rounded, aseptate, thin-walled, frequently guttulate with 3–7 guttules (< 0.5–1 μm) aggregated toward both poles.
Cardinal temperatures: 5–35 C, optimum 25 C, minimum < 5 C, maximum slightly > 35 C.
Host range: Associated with living foliage of Picea mariana and Pinus strobus and decomposing branches or wood of Acer rubrum, A. saccharum, Betula cordifolia and B. papyrifera.
Distribution: Canada (New Brunswick).
Additional specimens and cultures examined: see Supplementary Table II.
Notes: Phialocephala nodosa is distinguished by the abundant microsclerotia produced in aerial mycelium, later forming a crust on the colony surface.
Phialocephala catenospora J.B. Tanney & B. Douglas, sp. nov. ; ;
Fig. 7 Phialocephala catenospora. A–C. Apothecia on fallen decaying Betula papyrifera branch. D. Vertical section of apothecium. E. Vertical section of apothecial margin. F, G. Ectal and medullary excipulum. H. Ectal excipulum cells. I. Vertical section of apothecium with subicular hyphae in host tissue. J. Refractive vacuole bodies of paraphyses under DIC. K. Refractive vacuole bodies of paraphyses under phase contrast. L. Ectal excipulum cells mounted in H2O. M. Ectal excipulum cells in KOH. N. Asci. O–P. Asci with hemiamyloid tips in Lugol’s solution after KOH pretreatment. Q. Ascospores. Bars = 10 μm.

MycoBank MB811719
Typification: CANADA. NEW BRUNSWICK: Charlotte County, Little Lepreau, 45.136830 –66.481550, from decaying Betula papyrifera branch, 13 Jul 2014, J.B. Tanney NB-432 (holotype DAOM 628547). Ex-type culture DAOMC 250108.
Etymology: catena (Latin), chained together, to describe the conidial chains.
Colonies 25–30 mm diam after 14 d in the dark at 20 C on MEA; flat with fascicular aerial hyphae aggregated in center; margin wide, diffuse, and white; surface grayish brown to olive brown (5F3–4F8); reverse brownish gray (4E2). Exudates and soluble pigments absent. Mycelium consisting of subhyaline to dematiaceous, smooth, septate, branched hyphae, 2–3.5 μm diam, sometimes covered with gelatinous sheaths 1–3.5 μm diam. Diplococcium-like and Phialocephala synanamorphs both observed. Diplococcium-like synanamorph: Conidiophores macronematous, erect, brown, smooth, cylindrical, thick-walled, unbranched to once or twice branched, length indeterminate, 3.5–5 μm wide, pluriseptate, occurring singly or caespitose; conidiogenous cells monoblastic or polyblastic, integrated, terminal or intercalary, extensions sympodial, with 1–2 conidiogenous loci, subdenticulate. Conidia (10–)12–22.5(–28.5) × (4.5–)5–6 μm, 1–3(–5)-septate, dry, catenate, acropetal, in unbranched or branched chains from ramoconidia, subcylindrical, rounded to subtruncate base, sometimes slightly constricted at septa, brown, often versicolorous with one cell (usually basal) paler, smooth, basal cell with a lighter, refractive, flattened to protruding hilum, 1 μm wide; Phialocephala synanamorph: Conidiophores micronematous to macronematous, erect, subhyaline to pale brown, cylindrical, thin-walled, solitary to divaricate with 2–4 metulae, metulae smooth, subhyaline to pale brown, thin-walled, (5–)6–8(–9) × (2.5–)3–4 μm, 2–3(–4) conidiogenous cells arising from metula, conidiophores becoming more septate, melanized, and densely branched with maturity. Conidiogenous cells phialidic, terminal or intercalary, (7–)9–13 × (2.5–) 3–3.5 μm, collarettes cylindrical to flaring, 2–3(–4) × 2–2.5 μm, hyaline to pale brown. Mature conidiogenous cells sometimes developing into diplococcium-like synanamorph. Conidia 2–3.5(–4.5) × (1.5–)2 μm, aseptate, hyaline, subglobose to obovoid or oblong, occurring in slimy heads.
Apothecia scattered to gregarious; sessile; subiculum not evident; urceolate to cup-shaped when young, disk plain to shallowly concave at maturity; outline entire; hymenial surface smooth to finely pruinose because of adherence of ejected ascospores, bluish gray to grayish blue (20C3–21C4), outer surface slightly darker; 0.4–1.2 mm diam, 0.2–0.3 mm high; margin not differentiated, smooth. Ectal excipulum at base and mid flanks textura globulosa to angularis, 40–110 μm thick near base, 15–30 μm thick toward margin, composed of globose to isodiametric cells with thin to slightly thickened walls, (11–)12.5–16(–18) × (6–)8–10.5(–11) μm; at upper flank and margin textura angularis to prismatica, composed of globose to elongated clavate cells with +/− thin walls, 6–8.5(–10) × (5–)5.5–7.5(–9) μm; marginal cells cylindrical-clavate to obovoid, (9–)11–18(–25) μm long, maximum width toward apex 2.5–5 μm, minimum width at base 2–3 μm; hyaline to brownish orange (5C3) around margin and becoming dark brown (5F8) toward base, not gelatinized, crystals or exudates absent; tissue becoming olive to olive gray (3F4 to 3F2) when mounted in 5% KOH. Subicular hyphae sparse to abundant, sometimes thinly gelatinized, 2–3.5(–4) μm, thick-walled (0.3–1 μm), dark brown (5F8). Medullary excipulum hyaline, textura intricata, 30–60 μm thick. Paraphyses cylindrical with rounded apices, septate, simple, thinwalled, (2.=–)3–3.=(–4) μm wide, containing large, highly refractive vacuole bodies; not exceeding mature asci. KOH reaction: negative, rarely weak pale yellow. Asci arising from croziers, cylindrical-clavate, eightspored, biseriate, (=3–)=4–67(–69.=) × 6–8. μm, pars sporifera 21–26 μm, pore amyloid in Melzer’s reagent or Lugol’s solution with 5% KOH pretreatment (hemiamyloid), protoplasm turning brick red (7D7) in Lugol’s solution. Ascospores (6.5–)7–8.5(–10) × (2–)2.5–3 μm, oblong to fusiform or clavate, straight to allantoid or slightly sigmoid, aseptate, thin-walled, frequently guttulate with 1–6 guttules (0.5 μm diam) aggregated toward poles.
Cardinal temperatures: 5–35 C, optimum 25 C, minimum slightly < 5 C, maximum slightly > 35 C.
Host range: Associated with decomposing wood of Acer rubrum, Betula alleghaniensis and B. papyrifera.
Distribution: Canada (New Brunswick).
Additional specimens and cultures examined: see Supplementary Table II.
Notes: Phialocephala catenospora is distinguished by the diplococcium-like asexual state observed in culture.
Phialocephala mallochii J.B. Tanney & B. Douglas, sp. nov. ,
Fig. 8 Phialocephala mallochii. A–D. Apothecia on decaying Betula alleghaniensis log. E, F. Vertical sections of apothecia. G. Marginal cells. H. Ectal excipulum cells. I. Marginal cells with refractive vacuole bodies. J. Ectal and medullary excipulum. K. Refractive vacuole bodies of paraphyses. M. Ectal and medullary excipulum. N. Refractive vacuole bodies of paraphyses. O. Ascus. P. Ascus with hemiamyloid tip in Lugol’s solution after KOH pretreatment. Q. Ascospores. Bars: E = 500 μm, F–Q = 10 μm.

MycoBank MB811720
Typification: CANADA. NEW BRUNSWICK: Charlotte County, Little Lepreau, 45.140744 –66.479359, from decaying Alnus viridis stem, 12 Jul 2014, B. Malloch NB-430, (holotype DAOM 628552). Ex-type culture DAOMC 250113.
Etymology: Named for its initial collector, Bruce Malloch.
Colonies 20–23 mm diam after 14 d in the dark at 20 C on MEA; convex, densely woolly, olive brown (4E3) aerial mycelia; diffuse white margin; surface olive (4E5–4F4); reverse dark gray to brownish gray (1F1–4F2). Exudates and soluble pigments absent. Asexual state not observed.
Apothecia scattered to gregarious; sessile; subiculum not evident; urceolate to cup-shaped when young, disk plain to concave at maturity; outline entire; bluish gray (23E3), outer surface darker; 0.5–2 mm diam, 0.2–0.4 mm high; margin frequently lighter because of cells containing refractive vacuole bodies, smooth. Ectal excipulum at base and mid flanks textura globulosa to angularis, 35–128 μm thick near base, 23–38 μm thick toward margin, composed of globose to isodiametric cells with thin to slightly thickened walls, 7.5– 11(–15) × (5–)6–7.5(–9) μm; at upper flank and margin textura angularis to prismatica, composed of globose to elongated clavate cells or cylindrical with +/− thin walls, 10–16(–21) × (5.5–)7–9(–10) μm; marginal cells obovoid to clavate or spathulate, 9–16(–17) μm long, maximum width toward apex 5.5–7(–7.5) μm, minimum width at base 2–3.5 μm, frequently containing refractive vacuole bodies; brownish orange to brown (5D4–6E4) around margin and becoming dark brown (6F7) toward base, not gelatinized, crystals or exudates absent; tissue becoming olive to dark green (1E6–27F5) when mounted in 5% KOH. Subicular hyphae sparse to moderately abundant, sometimes gelatinized, 2–3(–4) μm, thick-walled (0.5–1 μm), dark brown (5F8). Medullary excipulum hyaline, textura intricata, 23–43 μm thick. Paraphyses cylindrical with rounded apices, septate, simple, thin-walled, 3–4 (–4.5) μm wide, containing large highly refractive vacuole bodies; not exceeding mature asci. KOH reaction: negative. Asci arising from croziers, cylindrical-clavate, eight-spored, (34–)37–46(–52) × 3.5–5(–6.5) μm, pars sporifera 18–26 μm, pore amyloid in Melzer’s reagent or Lugol’s solution with 5% KOH pretreatment (hemiamyloid), protoplasm turning brick red (7D7) in Lugol’s solution. Ascospores biseriate to obliquely uniseriate, (7–)8–11.5(–14) × (2.5–)3–4(–4.5) μm, ellipsoidal to fusoid-clavate or oblong, apices rounded, 0–1(–3) septate, thin-walled, frequently guttulate with many 0.5–2 μm diam guttules aggregated toward poles.
Cardinal temperatures: 5–30 C, optimum 25 C, minimum < 5 C, maximum slightly > 30 C.
Host range: Associated with decomposing wood of Acer saccharum and Alnus viridis.
Distribution: Canada (New Brunswick)
Additional specimens and cultures examined: see Supplementary Table II.
Notes: Phialocephala mallochii is distinguished by ascospores containing up to three septa.
Phialocephala oblonga (C.J.K. Wang & B. Sutton) J.B. Tanney, Seifert & B. Douglas, comb. nov. , ,
Fig. 9 Phialocephala oblonga. A–D. Apothecia on decayed Acer saccharum log. E, F. Vertical sections of apothecia. G. Vertical section of apothecial margin. H. Marginal cells with refractive vacuole bodies. I. Vertical section showing subicular hyphae in host tissue. J. Asci. K, L. Asci and paraphyses with refractive vacuole bodies. M, N. Asci with hemiamyloid tips in Lugol’s solution after KOH pretreatment. O. Ascospores under DIC (top three rows) and light microscopy (bottom row). Bars: E, F = 500 μm; G–O = 10 μm.

MycoBank MB811722
≡ Paradidymobotryum oblongum C.J.K. Wang & B. Sutton, Mycologia 76:572. 1984 (Basionym).
Colonies 24–27 mm diam after 14 d in the dark at 20 C on MEA; convex, abundant woolly, olive gray (3E2) aerial mycelia; diffuse white margin; surface brownish gray to olive brown (4F2–4F5); reverse dark gray to brownish gray (1F1–4E2). Exudates and soluble pigments absent.
Apothecia scattered to gregarious; sessile; subiculum not evident; urceolate to cup-shaped when young, disk plain to concave at maturity; outline entire; grayish blue to deep blue (21D6–22E8), outer surface darker; 1.5–2.5 mm diam, 0.2–0.3 mm high; margin frequently lighter due to cells containing refractive vacuole bodies, smooth. Ectal excipulum at base and mid flanks textura globulosa to angularis, 85–130 μm thick near base, 21–53 μm thick toward margin; at base and lower flank composed of globose to isodiametric cells with thin to slightly thickened walls, (17.5–) 18.5–24.0(–27.5) × (8–)10.5–15.5(–17) μm; at upper flank and margin textura angularis to prismatica, composed of globose to elongated clavate cells or cylindrical with +/− thin walls, 9–11(–12) × (5.5–)6–8(–9.5) μm; marginal cells obovoid to clavate or spathulate, (9–) 10.5–15.5(–16) long, maximum width toward apex (5.5–)6–8(–8.5) μm, minimum width at base (2.5–) 3.5–5(–5.5) μm, frequently containing refractive vacuole bodies; brownish orange to brown (5D4–6E4) around margin and becoming dark brown (6F7) toward base, not gelatinized, crystals or exudates absent; tissue becoming olive to dark green (1E6–27F5) when mounted in 5% KOH. Subicular hyphae usually sparse, 2–3 μm, thick-walled (0.5 μm), dark brown (5F8). Medullary excipulum hyaline, textura intricata, 22–42 μm thick. Paraphyses cylindrical with rounded apices, septate, simple, thin-walled, 2.5–3.5 μm wide, containing large highly refractive vacuole bodies; not exceeding mature asci. KOH reaction: negative. Asci cylindrical-clavate, eight-spored, (49–)58–76 × (4–)4.5–6(–7) μm, pars sporifera 20–25 μm, pore amyloid in Melzer’s reagent or Lugol’s solution with 5% KOH pretreatment (hemiamyloid), protoplasm turning brick red (7D7) in Lugol’s solution; arising from croziers. Ascospores biseriate to obliquely uniseriate, (8–)9–11.5(–17) × (2.5–)3–3.5(–4) μm, elliptic-fusiform to oblong, sometimes appearing bent, apices rounded, aseptate, thin-walled, frequently guttulate with guttules 0.5–1 μm diam aggregated toward both poles and sometimes bearing two larger guttules 2–2.5 μm diam.
See CitationWang and Sutton (1984) for description of the asexual state (as Paradidymobotryum oblongum).
Cardinal temperatures: 5–35 C, optimum 25 C, minimum slightly < 5 C, maximum slightly > 35 C.
Host range: Associated with decomposing wood of Acer saccharum, Betula alleghaniensis and Ulmus americana.
Distribution: Canada (New Brunswick, Ontario) and USA (New York).
Additional specimens and cultures examined: DAOMC 250119, see Supplementary Table II.
Notes: Phialocephala oblonga is characterized by long ascospores and its synnematous asexual state.
Phialocephala aylmerensis J.B. Tanney & B. Douglas, sp. nov. , ,
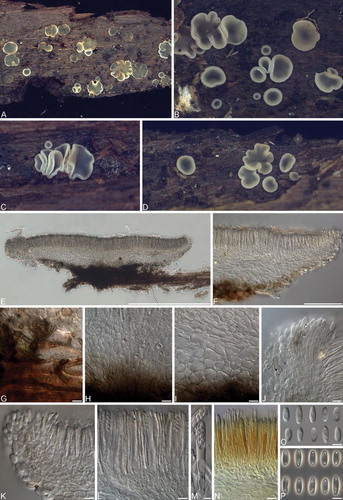
Fig. 10 Phialocephala aylmerensis. A–D. Apothecia on decaying Betula papyrifera wood. E, F. Vertical sections of apothecia. G. Subicular hyphae. H, I. Ectal excipulum cells near base (H) and toward flanks (I). J, K Marginal cells. L. Asci and paraphyses. M. Mature ascus containing ascospores. N. Asci with hemiamyloid tips in Lugol’s solution after KOH pretreatment. O. Ascospores under DIC. P. Ascospores under phase contrast. Bars: E = 500 μm, F = 100 μm, G–L, N = 10 μm, M, O, P = 5 μm.
MycoBank MB811721
Typification: CANADA. QUEBEC: Aylmer, Jardin Lavigne Park, 45.418969 –75.834870, from decaying Betula papyrifera log, 16 Sep 2014, J.B. Tanney/B. Tanney NB-543, (holotype DAOM 628548). Ex-type culture DAOMC 250106.
Etymology: Named for the type locale, Aylmer, Quebec.
Colonies 22–27 mm diam after 14 d in the dark at 20 C on MEA; flat, sparse fascicular olive gray (3E2) aerial mycelia mostly aggregated in center; diffuse white margin; surface brownish gray to olive brown (4F2–4F5); reverse dark gray to brownish gray (1F1–4F2). Exudates and soluble pigments absent. Mycelium consisting of subhyaline to dematiaceous, smooth, septate, branched, hyphae 2.0–3(–3.5) μm diam, sometimes covered with gelatinous sheath 1–3.5 μm diam. Conidiophores micronematous to macronematous, erect, pale to dark brown, smooth, cylindrical, thin- or thick-walled, unbranched or occasionally singly branched, up to 17.5 × 3.5 μm, pluriseptate. Conidiogenous cells phialidic, terminal, sometimes intercalary, ampuliform, (7–)8.5–13(–17) × (2–)3–4(–4.5) μm, collarettes cylindrical to flaring, 2.5–4(–5) × 2–2.5 μm, hyaline to pale brown. Conidia occurring in slimy heads, dimorphic, primary conidia 3–5(–6) × 2 mm, aseptate, hyaline, obovoid to oblong, secondary conidia (2–)2.5–3 × 1.5–2 mm, aseptate, hyaline, globose to subglobose, base often subtruncate.
Apothecia scattered to gregarious; sessile; subiculum not evident; urceolate to cup-shaped when young, disk plain to concave at maturity; outline entire to sinuate; pale gray (1B1) to bluish gray (23E3), outer surface darker; 1–3 mm diam, 0.2–0.4 mm high; margin frequently lighter because of cells containing refractive vacuole bodies, smooth. Ectal excipulum at base and mid flanks textura globulosa to angularis, 49–105 μm thick near base, 19–38 μm thick towards margin, composed of globose to isodiametric cells with thin to slightly thickened walls, 14–19(–25) × (7.5–) 10–13(–14) μm; at upper flank and margin textura angu-laris to prismatica, composed of globose to elongated clavate cells or cylindrical with +/− thin walls, (14–)15–20(–23) × (8–)11–13(–14) μm; marginal cells cylindrical to obovoid to clavate or spathulate, (13–)14–18(–20.5) μm long, maximum width toward apex 5.5–7.5(–9) μm, minimum width at base 2–3.5 μm, frequently containing refractive vacuole bodies; hyaline to brownish gray (5D3) around margin and becoming grayish brown (6F3) toward base, not gelatinized, crystals or exudates absent; tissue becoming olive to dark green (1E6–27F5) when mounted in 5% KOH. Subicular hyphae sparse to moderately abundant, 2–4(– 4.5) μm, thick-walled (0.5–1 μm), dark brown (5F8). Medullary excipulum hyaline, textura intricata, 40–58 μm thick. Paraphyses cylindrical with rounded apices, septate, simple, thin-walled, 2.5–3(–4) μm wide, containing large highly refractive vacuole bodies; not exceeding mature asci. KOH reaction: negative. Asci arising from croziers, cylindrical-clavate, eight-spored, (50–)52–60(–62) × 6–7 μm, pars sporifera 18–23 μm, pore amyloid in Melzer’s reagent or Lugol’s solution with 5% KOH pretreatment (hemiamyloid), protoplasm turning brick red (7D7) in Lugol’s solution. Ascospores biseriate to obliquely uniseriate, 7–9(–10.5) × 3–3.5(–4) μm, oblong to fusiform or clavate, straight to allantoid, aseptate, thin-walled, frequently guttulate with 2–7 guttules (0.5–1 μm diam) aggregated toward poles.
Cardinal temperatures: 5–35 C, optimum 25 C, minimum slightly < 5 C, maximum slightly > 35 C.
Host range: Associated with decomposing wood of Betula papyrifera.
Distribution: Canada (Quebec).
Additional specimens and cultures examined: DAOMC 250107, DAOM 675857, DAOM 675868 (Supplementary Table II).
Notes: Phialocephala aylmerensis is characterized by reduced phialides borne directly on hyphae or on short conidiophores.
Discussion
The detection of Phialocephala sexual states and corresponding endophytic and anamorphic states within the same forest stands provides evidence of life cycles involving vegetative endophytic stages, saprotrophic stages with phialidic states on non-foliar substrates and the formation of mollisioid sexual morphs enabling widespread ascospore dispersal. Although we isolated Ph. nodosa, Ph. piceae and Ph. scopiformis as foliar endophytes of conifers, sexual states were not observed on fresh, senescent or abscised needles. During summer and fall in the sampling areas, mollisioid sexual states of these species were encountered commonly on woody substrates ranging from intact fallen branches to old coarse woody debris, usually originating from angiosperm trees. These observations suggest the ability of those Phialocephala species to occupy several niches as either specialists or generalists, indicating complex and diverse life-history strategies.
Phialocephala piceae was described from a Picea abies needle endophyte isolate in Switzerland that produced apothecia with dark ascospores in vitro (CitationGrünig et al. 2009). It also was detected in a stump of Piceae abies and a dead stem of Betula pendula (CitationMenkis et al. 2004). In this study Ph. piceae was isolated as an endophyte from Picea glauca, P. mariana, P. rubens and Pinus strobus, and its sexual state was collected only on fallen branches of Acer saccharum. Our specimen appears to be the first collection of Ph. piceae apothecia from nature. Pigmented ascospores were not observed in our collections, possibly a result of apothecial immaturity, cultural artifacts in the original study or differences in geographic races. Some collections had apothecia erumpent through the bark of the host branch, a distinctive character that suggests its presence as an endophyte in woody tissue before branch drop.
Members of the Ph. dimorphospora clade have been isolated most commonly as DSE from coniferous tree roots or from decomposing wood (CitationMenkis et al. 2004), although several other ITS sequences and distinct ITS phylotypes present in GenBank have been reported from other substrates (e.g. EU434847 Ph. repens from Populus and JF340261 Phialocephala sp. M49 from Alnus). In this study apothecia assignable to the Ph. dimorphospora clade were collected from deciduous wood (Acer saccharum, Alnus viridis, Betula alleghaniensis, B. cordifolia, B. papyrifera, Fagus grandifolia) that usually was decorticated and in advanced states of decomposition. Phialocephala dimorphospora s.l. causes soft rot and significant weight loss in deciduous wood (CitationNilsson 1973, CitationMorrell and Zabel 1985, CitationWang and Zabel 1990, CitationHeld 2013) and is implicated in wood staining (CitationKowalski 1991). CitationMenkis et al. (2004) hypothesized that Ph. dimorphospora may latently infect healthy trees, causing wood staining when the health of the host declines and decomposing wood following host death. Latent infection of living branches also may play a role in natural pruning (CitationButin and Kowalski 1986, CitationKowalski and Kehr 1992, CitationBarklund and Kowalski 1996, CitationKowalski and Zych 2002). Despite members of this clade being among the most commonly field-collected apothecia in this study, only two isolates of the Ph. dimorphospora clade were recovered as endophytes from Picea mariana and Pinus strobus needles (all Ph. nodosa), suggesting needle colonization may be infrequent and opportunistic.
CitationMenkis et al. (2004) isolated a cluster of eight Ph. dimorphospora-affiliated ITS-phylotypes from P. abies stumps and boles, which they classified as one species because of the lack of differentiating characters or available sequences of other Phialocephala spp. in this clade. Our results confirm that the Ph. dimorphospora clade is composed of genetically distinct species, many of which produce distinctive asexual states. A well-defined Ph. dimorphospora was required to precisely delineate distinct species in this clade and establish Phialocephala s.s. as a generic concept. No existing ex-type culture exists, and attempts to sequence DNA from dried cultures associated with the type specimen failed. We therefore selected an authenticated Ph. dimorphospora isolate (DAOMC 87232 = CBS 300.62) as epitype based on its congruency with the species concept. In Taxonomy the core Phialocephala s.s. clade is defined to include Ph. aylmerensis, Ph. catenospora, Ph. dimorphospora, Ph. lagerbergii, Ph. mallochii, Ph. nodosa, Ph. oblonga, Ph. repens and Mollisia heterosperma.
Our observations alter the monomorphic generic concept of Phialocephala to include sexual states and additional synanamorphs. Some species in the Ph. dimorphospora clade produced distinct conidial states previously attributable to other poorly sampled hyphomycete genera (e.g. Bispora, Diplococcium, Paradidymobotryum, Septonema). While such conidial states have not been formally associated with Phialocephala, Diplococcium spicatum (CBS 162.47, CBS 852.73), Trimmatostroma betulinum (CBS 282.74) and T. salicis (CPC 13571), sequences in GenBank are closely related to Phialocephala and Mollisia (CitationCrous et al. 2007, CitationShenoy et al. 2010, CitationLin et al. 2011). Trimmatostroma, a phylogenetically heterogeneous asexual genus, is characterized by arthric chains of brown phragmo- or dictyoconidia while Diplococcium, also phylogenetically heterogeneous, is characterized by brown, didymo- or phragmoconidia in branched or unbranched acropetal chains from polytretic conidiogenous cells (CitationSeifert et al. 2011). Phialocephala catenospora produced a diplococcium-like conidial state with acropetal chains of 1–4-septate, blastic conidia. This probably explains why accessioned sequences of fungi collected from wood and identified as Trimmatostroma or Diploccium sometimes are associated in the phylogenetic and morphotaxonomic literature with Phialocephala or Mollisia states (CitationCrous et al. 2007, CitationShenoy et al. 2010). Notably CitationLe Gal and Mangenot (1956) described a Mollisia sp. culture (“Mollisia sp. 1 from Betula”) with two synanamorphs that are similar to those observed in our cultures of Ph. nodosa.
Phialocephala oblonga is based on Paradidymobotryum oblongum, the type species of a monotypic hyphomycete genus described from rotten Ulmus americana wood collected in New York state (CitationWang and Sutton 1984). This connection was confirmed by comparing ITS and RPB1 sequences of two cultures derived from single-spore isolations of independently collected Ph. oblonga specimens. This synanamorph connection was unexpected because of the striking differences in morphology and conidiogenesis of Pa. oblongum and Phialocephala. However, there are morphological similarities between Pa. oblongum and the diplococcium-like synanamorph of Ph. catenospora.
Authenticated cultures of Mollisia heterosperma (CBS 292.59), Ph. lagerbergii (ex-type culture; CBS 266.33) and Ph. repens (purported ex-type culture fide CitationGrunig et al. 2009; MUCL 1849) also belong to the Ph. dimorphospora clade. The known asexual state of M. heterosperma consists of penicillate conidiophores with deep collarettes bearing both subglobose and ovoid conidia, reminiscent of Ph. dimorphospora. Although morphologically similar, Kendrick (1963) distinguished Ph. repens from Ph. dimorphospora because the former species lacked the characteristic elongated primary conidium and consequently had shorter collarettes. CitationMelin and Nannfeldt (1934) described Ph. lagerbergii from blue-stained Pinus sylvestris wood in Sweden. This species is characterized by ellipsoidal or reniform conidia produced in slimy drops from the deep collarettes of pigmented phialides, which occur singly or in clusters. In this study species in the Ph. dimorphospora clade were readily differentiated using ITS and RPB1 sequences, and several closely related species can be distinguished by morphological characters of asexual states.
Phialocephala scopiformis was the most encountered Phialocephala endophyte in our study and the only species found as both an endophyte and saprotroph on tissues of a single host (i.e. needles and fallen decorticated branches of Picea rubens). More sampling is required to determine the host and substrate preference of the sexual morph, although it is evident that Ph. scopiformis is an endophyte on other conifer host species. White spruce seedlings were successfully colonized by Ph. scopiformis after wound inoculation or aerial dispersal of a mycelial suspension, indicating infection may become systemic and not require ascospores (CitationSumarah et al. 2005). Future work should explore the infection process (e.g. via stomatal invasion) and the growth of infecting hyphae from the needles through the petiole to the vascular system or vice versa. Phialocephala scopiformis could provide a good model for such a study because it first was described from the periderm of living Picea abies branches, is routinely isolated as an endophyte of Picea needles and its sexual state was found many times exclusively on fallen Picea branches, usually decorticated and exhibiting extensive decomposition.
The ability of many Phialocephala species to grow and sporulate at low temperatures indicates that infection of foliage in the early stages of leaf emergence following bud break should be investigated. We selected two Ph. dimorphospora strains (DAOMC 87232, DAOMC 250111) to test germination of conidia, because other authors suggested that conidia of some Phialocephala spp. are spermatia (CitationDay et al. 2012). Germination of conidia from both strains was observed after 48 h at 15 and 20 C on both MEA and CMA, indicating that they can function effectively as propagules and are not strictly spermatia, although a spermatial role cannot be ruled out. The production of conidia in slimy droplets suggests dispersal by insects or rain splash.
Members of the PAC were not isolated from needles nor were their corresponding sexual morphs observed in our study, supporting work demonstrating the below-ground niche of this group. Our connections between other Phialocephala species and mollisioid apothecia suggests that the collection, description and sequencing of mollisioid discomycetes in appropriate environments should lead to the discovery of PAC sexual states, the existence of which are suggested by the observations of abortive apothecia by CitationCurrah et al. (1993) and mating type loci work by CitationZaffarano et al. (2010).
The connection between Phialocephala and Mollisia or mollisioid sexual states was expected because of evidence from phylogenetic studies (Gminder pers comm, CitationVrålstad et al. 2002, CitationGrünig et al. 2009, CitationDouglas 2013), and morphological observations by CitationLe Gal and Mangenot (1956, Citation1960, Citation1961, Citation1966) made detailed observations of both apothecia and cultural characters of Mollisia species and described several conidial states that are consistent with the present generic concept of Phialocephala s.l. For example, they described a phialidic morph with deep collarettes (“a transitional form between Phialophora and Cystodendron”) that took at least one year to develop in cultures of M. coerulans (CitationLe Gal and Mangenot 1966) and another delayed cystodendron-like state in M. palustris cultures. CitationBubák (1914) described Cystodendron, characterized by conidia aggregated in slimy masses from phialides with deep collarettes and morphologically differentiated from Phialocephala by the aggregation of conidiophores into sporodochia. Older (6–8 mo) cultures of M. discolor var. longispora formed sporodochia composed of densely aggregated phialides with deep collarettes and considered to be cystodendron-like (CitationLe Gal and Mangenot 1956, Citation1958). CitationAebi (1972) also described Cystodendron states in pure cultures of Belonopsis ericae, Tapesia (= Mollisia) cinerella, T. fusca, T. hydrophila and T. villosa and considered Phialocephala to be morphologically identical to (and therefore synonymous with) Cystodendron.
Phialocephala s.l. is still currently polyphyletic, both by including distantly related species and by occurrence in numerous closely related clades intermixed with species named in other genera, some of which have nomenclatural priority (e.g. Cystodendron, Mollisia, Trimmatostroma). Based on ITS sequences CitationGrünig et al. (2002) showed that Ph. fusca, Ph. humicola (= Ph. xalapensis) and Ph. virens are outside the main Phialocephala clade. ITS BLAST queries suggest the placement of Ph. fusca and Ph. humicola in the Chaetosphaeriaceae. Morphological and phylogenetic evidence also reveal that Ph. trigonospora is not congeneric with Ph. dimorphospora and most likely belongs to Verticicladiella (CitationGrünig et al. 2009). Acephala, a genus with two described species belonging to the PAC, is congeneric with Phialocephala and differentiated only by the lack of observed sporulation in culture (CitationGrünig and Sieber 2005), a taxonomic choice enabled by some interpretations of dual nomenclature but now unacceptable after recent changes to the Code. Although the core clade of Phialocephala s.s. is clearly defined as the Phialocephala dimorphospora clade, the presence of Mollisia cinerea-like teleomorphs in this clade cast at least some doubt on its nomenclatural priority over Mollisia until this species and the genus are epitypified (an ongoing project; A. Gminder pers comm). The apparent phylogenetic affinity of the PAC and allied taxa to Vibrissea in most phylogenies is also a major taxonomic issue that requires resolution. It is further evident that Phialocephala s.s. remains to be comprehensively circumscribed, awaiting reassignment of phylogenetically closely related and unrelated Phialocephala species, and the formal transfer of species currently congeneric with Phialocephala s.s. (e.g. in Acephala and Mollisia), to stable and monophyletic genus concepts.
If Ph. dimorphospora is indeed congeneric with M. cinerea, then nomenclatural decisions will have to consider the interests of users of these names and the independent taxonomic histories of these genera. A principle of the Code is priority of publication. Mollisia, described by CitationKarsten in 1871, clearly has priority over Phialocephala (1961). Unfortunately Mollisia is not yet robustly circumscribed and the prevailing concept is broad, polyphyletic and paraphyletic (CitationCrous et al. 2003, CitationGrünig et al. 2009). A definitive nomenclatural conclusion requires at least a preliminary phylogenetic investigation of Mollisia. Based on Index Fungorum, 603 names have been applied to Mollisia. The number of accepted Mollisia species after excluding synonymized taxa and subspecific identifiers is 231, excluding species of other genera that are probably congeneric or that already appear to overlap phylogenetically, such as Belonopsis, Haglundia, Nimbomollisia, Pyrenopeziza and Tapesia. Only seven Mollisia species are represented in GenBank, including only one representing an ex-type strain, M. dextrinospora (better placed in Pyrenopeziza, B. Douglas unpubl).
Sequencing directly from type specimens whenever possible is the preferred course of action. The uncertain location of many types (e.g. the type specimen for M. cinerea is lost; A. Gminder pers comm) or their condition or age significantly tempers optimism for this approach. Species descriptions are often vague by contemporary standards and based on exsiccatae lacking vital characters important to some discomycete taxonomists (CitationBaral 1992). The delineation of Mollisia, along with its relationship to Phialocephala and the subsequent nomenclatural decisions, are the subject of a concurrent study that should ameliorate some of these important issues.
The issue of unidentified endophytes resulting from culture-based or amplicon-based metagenomic studies can be resolved at least partly by employing an approach using morphology, anatomy and molecular phylogenetic methods combined with studies of the habitats of these fungi. Endophytes of woody plants are frequently horizontally transferred and may not be constrained to colonized host tissue, therefore looking beyond the host or senescent colonized tissue for reproductive morphs should prove fruitful. For example, the discovery of Dwayaangam colodena, an aquatic hyphomycete and foliar endophyte of Picea mariana and P. rubens, provides evidence of a complex aquatic-terrestrial lifecycle that would have remained undiscovered if endophyte researchers restricted their sampling to living host tissue (CitationSokolski et al. 2006, CitationSumarah et al. 2010). Cultural studies of endophytes frequently yield sterile isolates that may be unidentifiable using morphological characters or DNA barcodes or conversely can produce novel teleomorphs. For instance CitationKnapp et al. (2015) isolated three novel pleosporalean DSE genera from grass roots and attempted to induce in vitro sporulation with various methods, including culturing on different media or sterilized plant material, mechanically damaging mycelia, exposure to near-UV light and slowly drying out cultures. Isolates of Darksidea produced ascomata on the surface of stinging nettle stems on synthetic nutrient-poor agar 3–4 wk after inoculation at room temperature.
We recommend a pragmatic strategy to enable confident species identification of taxa combining field mycology, culture isolations and molecular phylogenetic methods to bridge the gap between the present dominance of unnamed DNA sequences and the historical literature. This requires epitypification of well-described and distinct species but also the more laborious but accurate and complete morphological description of novel or imprecisely characterized species, all associated with accessioned DNA sequence data. A concerted effort to sequence types and vouchered specimens of species unrepresented in sequence databases will connect unidentifiable sequences of endophytes with named or describable morphotaxonomic concepts, significantly increase the utility of environmental sequencing and metagenomics tools and facilitate the development and communication of taxonomic and ecological knowledge (CitationBrock et al. 2009, CitationNagy et al. 2011).
umyc_a_11831522_sm0001.xls
Download MS Excel (43.5 KB)umyc_a_11831522_sm0002.xls
Download MS Excel (37.5 KB)Acknowledgments
This study was supported by the NSERC PGSD2-459312-2014 to JB Tanney and the NSERC CRDPJ 421782-11 to JD Miller, KA Seifert and DW Malloch. Jonathan Mack provided specimens of Ph. oblonga and Ph. dimorphospora. Anna Tomczak assisted with DNA extraction and sequencing, and Shona Miller collected conifer needles for study. Stephen Clayden and Donald McAlpine (New Brunswick Museum) facilitated collecting in Fundy National Park (Permit No. FNP-2013-14973). We thank the CBS Fungal Biodiversity Centre for kindly providing cultures of Mollisia heterosperma and Loramyces macrosporus used in this study.
Literature cited
- AddyHDHambletonSCurrahRS. 2000. Distribution and molecular characterization of the root endophyte Phialocephala fortinii along an environmental gradient in the boreal forest of Alberta. Mycol Res 104:1213–1221, doi:10.1017/S0953756200002896
- AebiB. 1972. Untersuchungen über Discomyceten aus der Gruppe Tapesia-Trichobelonium. Nova Hedwigia 23: 49–112.
- BaralHO. 1992. Vital versus herbarium taxonomy: morphological differences between living and dead cells of ascomycetes, and their taxonomic implications. Mycotaxon 44:333–390.
- BarklundPKowalskiT. 1996. Endophytic fungi in branches of Norway spruce with particular reference to Tryblidiopsis pinastri. Can J Bot 74:673–678, doi:10.1139/b96-085
- BrockPMDöringHBidartondoM. 2009. How to know unknown fungi: the role of a herbarium. New Phytol 181:719–724, doi:10.1111/j.1469-8137.2008.02703.x
- BruzoneMCFontenlaSBVohníkM. 2015. Is the prominent ericoid mycorrhizal fungus Rhizoscyphus ericae absent in the southern hemisphere’s Ericaceae? A case study on the diversity of root mycobionts in Gaultheria spp. from northwest Patagonia, Argentina. Mycorrhiza 25:25–40, doi:10.1007/s00572-014-0586-3
- BubákF. 1914. Ein Beitrag zur Pilzflora von Tirol und Istrien. Ann Mycol 12:205–220.
- ButinHKowalskiT. 1986. The natural pruning of branches and their biological preconditioning III. The fungus flora of common maple, gray alder, silver birch, hornbeam and common ash. Eur J Forest Pathol 16:129–138, doi:10.1111/j.1439-0329.1986.tb01053.x
- CrousPWBraunUGroenewaldJZ. 2007. Mycosphaerella is polyphyletic. Stud Mycol 58:1–32, doi:10.3114/sim.2007.58.01
- CrousPWGroenewaldJEGamsW. 2003. Eyespot of cereals revisited: ITS phylogeny reveals new species relationships. Eur J Plant Pathol 109:841–850, doi:10.1023/A:1026111030426
- CrousPWVerkleyGJGroenewaldJZSamsonR. 2009. Fungal biodiversity. Utrecht, the Netherlands: CBS Laboratory Manual Series, Centraalbureau voor Schimmelcultures. 269 p.
- CurrahRSTsunedaAMurakamiS. 1993. Morphology and ecology of Phialocephala fortinii in roots of Rhododendron brachycarpum. Can J Bot 71:1639–1644, doi:10.1139/b93-199
- DayMJHallJCCurrahRS. 2012. Phialide arrangement and character evolution in the helotialean anamorph genera Cadophora and Phialocephala. Mycologia 104:371–381.
- DescalsCESuttonBC. 1976. Anavirga dendromorpha and its Phialocephala phialidic state. Trans Br Mycol Soc 67:269–274, doi:10.1016/S0007-1536(76)80133-7
- DescalsCEWebsterJ. 1982. Taxonomic studies on aquatic hyphomycetes III. Some new species and a new combination. Trans Br Mycol Soc 78:405–437, doi:10.1016/S0007-1536(82)80149-6
- DouglasB. 2013. The taxonomy, phylogenetics and ecology of fungal plant-endosymbionts assignable to the genera Mollisia, Pyrenopeziza and Hyaloscypha (Helotiales, Leotiomycetes, Ascomycota) [doctoral thesis]. Wales, United Kingdom: Aberystwyth University Press.
- FernandoAACurrahRS. 1996. A comparative study of the effects of the root endophytes Leptodontidium orchidicola and Phialocephala fortinii (Fungi Imperfecti) on the growth of some subalpine plants in culture. Can J Bot 74:1071–1078, doi:10.1139/b96-131
- GreenleafMAKorfRP. 1980. Mollisia in Macaronesia: an exercise in frustration. Mycotaxon 10:459–472.
- GrünigCRDuòASieberTNHoldenriederO. 2008a. Assignment of species rank to six reproductively isolated cryptic species of the Phialocephala fortinii s.l.-Acephala applanata species complex. Mycologia 100:47–67.
- GrünigCRQuelozVDuòASieberTN. 2009. Phylogeny of Phaeomollisia piceae gen. sp. nov.: a dark, septate, conifer-needle endophyte and its relationships to Phialocephala and Acephala. Mycol Res 113:207–221.
- GrünigCRQuelozVSieberTNHoldenriederO. 2008b. Dark septate endophytes (DSE) of the Phialocephala fortinii s. l.-Acephala applanata species complex in tree roots: classification, population biology and ecology. Botany 86:1355–1369.
- GrünigCRSieberTN. 2005. Molecular and phenotypic description of the widespread root symbiont Acephala applanata gen. et sp. nov., formerly known as dark-septate endophyte Type 1. Mycologia 97:628–640.
- GrünigCRSieberTNRogersSOHoldenriederO. 2002. Genetic variability among strains of Phialocephala fortinii and phylogenetic analysis of the genus Phialocephala based on rDNA ITS sequence comparisons. Can J Bot 80:1239–1249.
- HallTA. 1999. BioEdit: a user-friendly biological sequence alignment editor andanalysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98.
- HamadSWebsterJ. 1988. Anavirga dendromorpha, anamorph of Apostemidium torrenticola. Sydowia 40:60–64.
- HarneySRogersSWangC. 1997. Molecular characterization of dematiaceous root endophytes. Mycol Res 101:1397–1404, doi:10.1017/S095375629700419X
- HataK. 1996. Variation in fungal endophyte populations in needles of the genus Pinus. Can J Bot 74:103–114.
- HataKFutaiK. 1995. Endophytic fungi associated with healthy pine needles and needles infested by the pine needle gall midge, Thecodiplosis japonensis. Can J Bot 73:384–390, doi:10.1139/b96-015
- HawksworthDLCrousPWRedheadSAReynoldsDRSamsonRASeifertKATaylorJWWingfieldMJAbaciOAimeCet al. 2011. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2:105–112, doi:10.5598/imafungus.2011.02.01.14
- HeldBW. 2013. Diversity and characterization of wood-decay fungi from historic wood in Antarctica [doctoral dissertation]. Retrieved from the University. Minnesota Digital Conservancy. 99 p.
- HofstetterVMiadlikowskaJKauffFLutzoniF. 2007. Phylogenetic comparison of protein-coding versus ribosomal RNA-coding sequence data: a case study of the Lecanoromycetes (Ascomycota). Mol Phylogenet Evol 44: 412–426, doi:10.1016/j.ympev.2006.10.016
- JacobsKWingfieldMJJacobsAWingfieldBD. 2001. A taxonomic re-evaluation of Phialocephala phycomyces. Can J Bot 79:110–117.
- JohnstonPRSeifertKAStoneJKRossmanAYMarvanováL. 2014. Recommendations on generic names competing for use in Leotiomycetes (Ascomycota). IMA Fungus 5:91–120, doi:10.5598/imafungus.2014.05.01.11
- JumpponenARITrappeJM. 1998. Dark-septate endophytes: a review of facultative biotrophic rootcolonizing fungi. New Phytol 140:295–310, doi:10.1046/j.1469-8137.1998.00265.x
- KarstenPA. 1871. Mycologia Fennica. Pars prima. Discomycetes. Bidr Känn Finl Nat Folk 19:189–264.
- KatohKKumaKTohHMiyataT. 2005. MAFFT 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33:511–518, doi:10.1093/nar/gki198
- KendrickWB. 1961. The Leptographium complex. Phialocephala gen. nov. Can J Bot 39:1079–1085.
- KnappDKovácsGZajtaEGroenewaldJCrousP. 2015. Dark-septate endophytic pleosporalean genera from semiarid areas. Persoonia 35:87–100.
- KnappDGPintyeAKovácsGM. 2012. The dark side is not fastidious dark septate endophytic fungi of native and invasive plants of semiarid sandy areas. PLoS One 7: e32570.
- KornerupAWanscherJH. 1978. Methuen handbook of colour. London, United Kingdom: Eyre Methuen. 252 p.
- KowalskiT. 1991. Oak decline I. Fungi associated with various disease symptoms on overground portions of middleaged and old oak (Quercus robur L.). Eur J For Pathol 21:136–151, doi:10.1371/journal.pone.0032570.s007
- KowalskiTKehrR. 1992. Endophytic fungal colonization of branch bases in several forest tree species. Sydowia 44:137–168.
- KowalskiTKehrRD. 1995. Two new species of Phialocephala occurring on Picea and Alnus. Can J Bot 73:26–32, doi:10.1139/b95-004
- KowalskiTZychP. 2002. Fungi isolated from living symptomless shoots of Pinus nigra growing in different site conditions. Osterr Z Pilzkd 11:107–116.
- LarenaISalazarOGonzálezVJuliánMaCRubioV. 1999. Design of a primer for ribosomal DNA internal transcribed spacer with enhanced specificity for ascomycetes. J Biotechnol 75:187–194.
- Le GalM. 1958. Contribution à l’étude des Mollisiodées II. Rev Mycol 23:28–86.
- Le GalM. 1960. Contribution à l’étude des Mollisiodées III. Rev Mycol 25:135–214.
- Le GalM. 1961. Contribution à étude des Mollisioïdées IV. Rev Mycol 26:263–331.
- Le GalM. 1966. Contribution à l’étude des Mollisiodées V. Rev Mycol 31:3–44.
- Le GalMMangenotF. 1956. Contribution à étude des Mollisioïdées I. Note préliminaire: les formes conidiennes. Rev Mycol 21:3–13.
- LiAFaheyTJPawlowskaTEFiskMCBurtisJ. 2015. Fine-root decomposition, nutrient mobilization and fungal communities in a pine forest ecosystem. Soil Biol Biochem 83:76–83, doi:10.1016/j.soilbio.2015.01.019
- LinL-CLeeM-JChenJ-L. 2011. Decomposition of organic matter by the ericoid mycorrhizal endophytes of Formosan rhododendron (Rhododendron formosanum Hemsl.). Mycorrhiza 21:331–339, doi:10.1007/s00572-010-0342-2
- MelinENannfeldtJA. 1934. Researches into the blueing of ground wood-pulp. Skogsvårdsföreningens Tidskrift 32:397–616.
- MenkisAAllmerJVasiliauskasRLygisVStenlidJFinlayR. 2004. Ecology and molecular characterization of dark septate fungi from roots, living stems, coarse and fine woody debris. Mycol Res 108:965–973, doi:10.1017/S0953756204000668
- MenkisAVasiliauskasRTaylorAFStenlidJFinlayR. 2005. Fungal communities in mycorrhizal roots of conifer seedlings in forest nurseries under different cultivation systems, assessed by morphotyping, direct sequencing and mycelial isolation. Mycorrhiza 16:33–41, doi:10.1007/s00572-005-0011-z
- MillerJD. 2011. Foliar endophytes of spruce species found in the Acadian Forest: basis and potential for improving the tolerance of the forest to spruce budworm. In: PirttilaAMFrankAC, eds. Endophytes of forest trees. Netherlands: Springer. p 237–249.
- MillerJDCheridHSumarahMWAdamsGW. 2009. Horizontal transmission of the Picea glauca foliar endophyte Phialocephala scopiformis CBS 120377. Fungal Ecol 2:98–101, doi:10.1016/j.funeco.2009.01.002
- MorrellJJZabelRA. 1985. Wood strength and weight losses caused by soft-rot fungi isolated from treated southern pine utility poles. Wood Fiber Sci 17:132–143.
- MünzenbergerBBubnerBWölleckeJSieberTNBauerRFladungMHüttlRF. 2009. The ectomycorrhizal morphotype Pinirhiza sclerotia is formed by Acephala macrosclerotiorum sp. nov., a close relative of Phialocephala fortinii. Mycorrhiza 19:481–492.
- NagyLGPetkovitsTKovácsGMVoigtKVágvölgyiCPappT. 2011. Where is the unseen fungal diversity hidden? A study of Mortierella reveals a large contribution of reference collections to the identification of fungal environmental sequences. New Phytol 191:789–794.
- NewshamKK. 2011. A meta analysis of plant responses to dark-septate root endophytes. New Phytol 190:783–793, doi:10.1111/j.1469-8137.2010.03611.x
- NilssonT. 1973. Studies on wood degradation and cellulolytic activity of microfungi. Stud Suec 104:40.
- NylanderJ. 2004. MrModeltest 2.2. Uppsala: Evolutionary Biology Centre, Uppsala University: Program distributed by the author.
- OtgonsurenBLeeM-J. 2012. Pinus sylvestris can form Ectomycorrhiza with Phialocephala fortinii. Taiwan J For Sci 27:265–81.
- PetersonRLWaggCPautlerM. 2008. Associations between microfungal endophytes and roots: do structural features indicate function? Botany 86:445–456.
- PicklesBJGorzelakMAGreenDSEggerKNMassicotteHB. 2015. Host and habitat filtering in seedling rootassociated fungal communities: taxonomic and functional diversity are altered in ‘novel’soils. Mycorrhiza 25:517–531.
- RambautA. 2014. FigTree 1.4.2: tree drawing tool. Available at http://tree.bio.ed.ac.uk/software/Figtree
- ReiningerVGrünigCRSieberTN. 2012. Host species and strain combination determine growth reduction of spruce and birch seedlings colonized by rootassociated dark-septate endophytes. Environ Microbiol 14: 1064–1076, doi:10.1111/j.1462-2920.2011.02686.x
- RonquistFHuelsenbeckJP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574, doi:10.1093/bioinformatics/btg180
- SeifertKAMorgan-JonesGGamsWKendrickB. 2011. The genera of Hyphomycetes. Utrecht, the Netherlands: CBS-KNAW Fungal Biodiversity Centre. 997 p.
- ShenoyBDJeewonRWangHAmandeepKHoWHBhatDJCrousPWHydeKD. 2010. Sequence data reveals phylogenetic affinities of fungal anamorphs Bahusutrabeeja, Diplococcium, Natarajania, Paliphora, Polyschema, Rattania and Spadicoides. Fungal Divers 44:161–169, doi:10.1007/s13225-010-0059-8
- SokolskiSPicheYChauvetÉBérubéJA. 2006. A fungal endophyte of black spruce (Picea mariana) needles is also an aquatic hyphomycete. Mol Ecol 15:1955–1962, doi:10.1111/j.1365-294X.2006.02909.x
- StillerJWHallBD. 1997. The origin of red algae: implications for plastid evolution. Proc Natl Acad Sci U S A 94:4520–4525, doi:10.1073/pnas.94.9.4520
- StoykeGCurrahRS. 1993. Resynthesis in pure culture of a common subalpine fungus-root association using Phialocephala fortinii and Menziesia ferruginea (Ericaceae). Arctic Alpine Res 25:189–193, doi:10.2307/1551812
- SuY-YQiY-LCaiL. 2012. Induction of sporulation in plant pathogenic fungi. Mycology 3:195–200.
- SumarahMWAdamsGWBerghoutJSlackGJWilsonAMMillerJD. 2008. Spread and persistence of a rugulosin-producing endophyte in Picea glauca seedlings. Mycol Res 112:731–736, doi:10.1016/j.mycres.2008.01.007
- SumarahMWMillerJDAdamsGW. 2005. Measurement of a rugu-losinproducing endophyte in white spruce seedlings. Mycologia 97:770–776, doi:10.3852/mycologia.97.4.770
- SumarahMWPunianiESørensenDBlackwellBAMillerJD. 2010. Secondary metabolites from anti-insect extracts of endophytic fungi isolated from Picea rubens. Phytochem 71:760–765.
- SuttonB. 1976. Anavirga dendromorpha and its Phialocephala phialidic state. Trans Br Mycol Soc 67:269–274.
- TellenbachCGrünigCRSieberTN. 2011. Negative effects on survival and performance of Norway spruce seedlings colonized by dark-septate root endophytes are primarily isolate-dependent. Environ Microbiol 13:2508–2517, doi:10.1016/S0007-1536(76)80133-7
- TellenbachCSieberTN. 2012. Do colonization by dark-septate endophytes and elevated temperature affect pathogenicity of oomycetes? FEMS Microbiol Ecol 82:157–168.
- TellenbachCSumarahMWGrünigCRMillerJD. 2012. Inhibition of Phytophthora species by secondary metabolites produced by the dark septate endophyte Phialocephala europaea. Fungal Ecol 6:12–18, doi:10.1016/j.funeco.2012.10.003
- TerhonenEKeriöSSunHAsiegbuFO. 2014. Endophytic fungi of Norway spruce roots in boreal pristine mire, drained peatland and mineral soil and their inhibitory effect on Heterobasidion parviporum invitro. Fungal Ecol 9:17–26, doi:10.1016/j.funeco.2014.01.003
- VohníkMAlbrechtováJVosátkaM. 2005. The inoculation with Oidiodendron maius and Phialocephala fortinii alters phosphorus and nitrogen uptake, foliar C: N ratio and root biomass distribution in Rhododendron cv. Azurro. Symbiosis 40:87–96.
- VrålstadTMyhreESchumacherT. 2002. Molecular diversity and phylogenetic affinities of symbiotic rootassociated ascomycetes of the Helotiales in burnt and metal-polluted habitats. New Phytol 155:131–148.
- WangCJKSuttonB. 1984. Two new synnematous genera: Chalarodendron and Paradidymobotryum. Mycologia 76: 569–572, doi:10.2307/3793345
- WangCJKZabelRA. 1990. Identification manual for fungi from utility poles in the eastern United States. Rockville, Maryland: American Type Culture Collection. 356 p.
- WangZBinderMSchochCLJohnstonPRSpataforaJWHibbettDS. 2006a. Evolution of helotialean fungi (Leotiomycetes, Pezizomycotina): a nuclear rDNA phylogeny. Mol Phylogenet Evol 41:295–312, doi:10.1016/j.ympev.2006.05.031
- WangZJohnstonPRTakamatsuSSpataforaJWHibbettDS. 2006b. Toward a phylogenetic classification of the Leotiomycetes based on rDNA data. Mycologia 98: 1065–1075, doi:10.3852/mycologia.98.6.1065
- WebsterJDescalsE. 1975. The phialidic state of Casaresia sphagnorum. Trans Br Mycol Soc 64:529–531, doi:10.1016/S0007-1536(75)80160-4
- WhiteTJBrunsTLeeSTaylorJ. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: InnisMAGelflandDHSninskyJJWhiteTJ, eds. PCR protocols: a guide to methods and applications. San Diego, California: Academic Press. p 315–322.
- WilcoxHEWangCJK. 1987. Mycorrhizal and pathological associations of dematiaceous fungi in roots of 7 mo old tree seedlings. Can J For Res 17:884–899, doi:10.1139/x87-140
- WilsonBJAddyHDTsunedaAHambletonSCurrahRS. 2004. Phialocephala sphaeroides sp. nov., a new species among the dark-septate endophytes from a boreal wetland in Canada. Can J Bot 82:607–617, doi:10.1139/b04-030
- WongPTWDongCMartinPMSharpPJ. 2015. Fairway patch—a serious emerging disease of couch (syn. bermudagrass) [Cynodon dactylon] and kikuyu (Pennisetum clandestinum) turf in Australia caused by Phialocephala bamuru P.T.W. Wong & C. Dong sp. nov. Australas Plant Pathol 44:545–555 doi: 10.1007/s13313-015-0369-0
- WuLGuoS. 2008. Interaction between an isolate of dark-septate fungi and its host plant Saussurea involucrata. Mycorrhiza 18:79–85, doi:10.1007/s00572-007-0159-9
- ZaffaranoPLDuòAGrünigCR. 2010. Characterization of the mating type (MAT) locus in the Phialocephala fortinii s.l.-Acephala applanata species complex. Fungal Genet Biol 47:761–772, doi:10.1016/j.fgb.2010.06.001
- ZaffaranoPLQuelozVDuòAGrünigCR. 2011. Sex in the PAC: a hidden affair in dark-septate endophytes? BMC Evol Biol 11:282, doi:10.1093/molbev/msi097
- ZijlstraJDvan’t HofPBaarJVerkleyGJSummerbellRCParadiIBraakhekkeWGBerendseF. 2005. Diversity of symbiotic root endophytes of the Helotiales in ericaceous plants and the grass, Deschampsia flexuosa. Stud Mycol 53:147–162, doi:10.3114/sim.53.1.147
