Abstract
Captive-induced behavioural deviations may involve many aspects of fish behaviour such as swimming activity and enhancement of individual aggressiveness. We studied seabass (Dicentrarchus labrax) behaviour as a function of manual and automatic feeding distribution modes. Under manual mode, the food is distributed over an extended area for a longer period, and its precise location is not always predictable, while with pneumatic automatic feeders, fish receive the same amount of resource, which is concentrated in the same surface area over a shorter period. We compared seabass behaviour under automatic and manual conditions collecting video image recordings before, during, and after feeding distribution, in the morning and in afternoon, on two different days, and analysing data within independent sessions of measurements. Feeding modes significantly affected swimming behaviour: automatically-fed fish were characterised by vertical movements through the water column (towards the surface and bottom) and by horizontal swimming. Manually-fed fish were instead characterised by sharp direction changes during their swimming, mostly towards the surface. Feeding distribution induced changes in collision frequency and elicited aggressive behaviour. In particular, agonistic behaviour (i.e. a fish attacks another fish) was almost exclusively recorded during the feeding under automatic distribution, whereas it was constantly expressed during all the distribution phases under manual mode.
Introduction
Captivity in cultivated fish might involve simple alterations of swimming activities (CitationHammer, 1997; CitationBégout Anras et al., 2004), feeding capacities (CitationAndrew et al., 2004a), or enhancement of individual aggressiveness as evidenced in rainbow trout, in salmon and in both European seabass (Dicentrarchus labrax) and seabream (CitationMagnuson, 1962; CitationRuzzante, 1994; CitationAdams and Huntingford, 1996; CitationKadri et al., 1997; CitationAdams et al., 1998; CitationAndrew et al., 2002; CitationEllis et al., 2002; CitationAndrew et al., 2004b; CitationKristiansen and Ferno, 2006; CitationNoble et al., 2007a, Citation2007b). Some studies on captive fish have tested that foraging activities can be influenced by the food distribution mode used. For example, a large study carried out in Greece and Spain with seabream and seabass showed that on-demand feeding regime, compared with the pneumatic feeding, was particularly efficient in reducing competition (CitationAndrew et al., 2002), aggression (CitationNoble et al., 2007b) and improving, in turn, the growth (CitationAzzaydi et al., 1999).
However, although the major efficiency of on-demand feeding regime has been largely demonstrated also in Atlantic species (e.g. salmon; CitationNoble et al., 2007a, Citation2007b and many references therein), most Mediterranean fish farmers still use mainly two different feeding modes, the manual one in smaller circular open and the centralised automatic mode in larger cages (e.g. Farm-Ocean Inc., Bergen, Norway). Under manual feeding regime, food is distributed by hand over an extended area (over 50% of the whole available surface of the cage) for a longer period (20–30 minutes) until satiation (i.e. until no fish feeding was observed at surface), and its precise location is not always predictable. In contrast, with pneumatic automatic feeding systems, fish always receive the same amount of food concentrated in the same surface area (not larger than 2–6 m of diameter) for shorter periods (about 45 times of 2 minutes each), both in early morning and late evening. Satiation of fish, in this case, is assumed on the basis of studies that provide results on the food to be delivered allowing the most efficient fish growth to the market size (CitationPaspatis et al., 1999). Although altered behaviour in response to automatic feeding has been already demonstrated (CitationAndrew et al., 2002), behaviour under manual feeding regime has never been studied in Mediterranean species like seabass.
This investigation would be of particular importance when assessing the choice of distribution mode to be adopted by fish farmers to optimize their activities efficiency. Accordingly, the main aim of this study was to test whether two different modes of food distribution had a differential effect on its behavioural responses in the form of i) water column position and swimming, ii) the number of casual collisions, and lastly iii) the number of attacks as an expression of aggressiveness.
Materials and methods
Study area, rationale and sample collection
The study was carried out in October-November 2004 in the Gulf of Castellammare, at the northern coast of Sicily (latitude 38° 02′ 31″ N; longitude 12° 55′ 28″ E) when the temperature of seawater was about 21°C. Gulf waters are known as highly oligotrophic (CHL-a concentrations lower than 1.0 µg L−1 ), with a low degree of turbidity (nephelometric standard unit <5 and Secchi disk depth up to 25 m throughout the year) and low current velocity (∼12 cm s−1 ; CitationSarà et al., 2006). Experiments were carried out in large cages (Farmocean, Norway; volume=4,500 m3 ) and in circular cages (volume=1,000 m3 ), which were positioned in the Eastern part of the Gulf (latitude 38° 04′ 53″ N; longitude 13° 02′ 04″ E) and moored to the bottom at a depth of about 32 m, about 0.7 nm off the coastline. The cages were filled with seabass (Dicentrarchus labrax) of the same size (∼280–300 g) and at the same density (∼20 kg m3 ; about 66 fishes per m3 ). In both cage types, the supplied food was produced by BioMar (France) and Hendrix (Italy).
The food was supplied automatically in the large cages and by hand in the circular cages. In both cases, the total daily amount of food was divided in two meals. The first meal was early in the morning (8:00 a. m.; hereafter the a.m. period), while the second one was provided in the afternoon (3:00 p.m.; hereafter the p.m. period). In the case of submersible cages, about 400–500 kg of food per meal was supplied through an automatic pneumatic feeding system (hereafter automatic mode, 800–1000 kg per day). The time length of each meal distribution was about 2 hours both in the a.m. and p.m. periods. During each meal, the food was supplied in 3 kg bouts every 2–3 minutes until the end of the programmed food amount. In manual distribution (hereafter manual mode), the total amount of food (about 200 kg per day) was provided by hand by an operator in about 30 minutes for each meal. The food available to each fish per delivery was on average 4–6% of wet weight (CitationMazzola and Rallo, 1981), which is the amount considered to satiate Mediter-ranean cultivated fish and to allow their fastest growth in the shortest period of time.
The experiments consisted in the collection of video images by SONY Hi-8 video cameras encased in waterproof boxes (NIMAR Inc., Italy) fixed inside the submersible and circular cages at a depth of about 1.5 m beneath the surface. Collection of video images lasted 30 minutes before the meal (hereafter the before phase), 30 minutes during the meal (hereafter the during phase), and 30 minutes after the meal (hereafter the after phase). The experiment started one hour after the placement of cameras inside the cages to allow the fish to acclimate to the presence of the cameras and to avoid any interference due to the human presence. In automatic mode, once recording of the before phase was carried out, the meal started to be provided for at least 3 hours. In this phase (during the meal), video images were recorded for a period of 30 minutes chosen in the middle of the meal. The after phase started once the meal finished, and lasted for another 30 minutes. In the case of manual mode, operators reached the cages, positioned cameras, then moved out of sight in a boat anchored upstream at about 30 meters from the cage, waited for 1 h to allow the fish to acclimate to the presence of the cameras and to avoid any interference due to the human presence, and then the session started (before phase). At the end of the before phase, operators moved close to the cages by pulling a rope, and then they started supplying food for about 30–40 minutes; once finished with the meal, the operators moved away from the cages. The experiments were repeated on two different days, with an interval of at least 7 days. Video images were analysed in the lab and each 30 minutes of video recording was divided into two sessions of 15 minutes each. Within each session, we choose 75 two-second frames randomly extracted by using the digit tables (CitationZar, 1999). The two-second frames represented the best compromise to observe a fish moving throughout the image field on the TV screen. During each frame, we followed the behaviour of at least 60 fishes (i.e. repeating the vision of frame 60 times), and the occurrence of each category was counted in all 60 fish and then transformed into a frequency. shows the list of behavioural categories recorded as single events. Positioning at the surface or in the middle of the water column were considered as the expression of general position in the cage, whereas swimming behaviour was composed by horizontal swimming, swimming towards the surface, swimming towards the bottom, and direction changing.
Table 1 Description of swimming behaviours of seabass.
Statistical analyses and elaborations
Behavioural data were analysed in order to test the null hypothesis, i.e. that there was no difference in fish position in the water column, or swimming behaviours under different feeding modes (automatic vs manual distribution) using a three-way PERMANOVA (Permutational Multivariate Analysis of Variance) design (CitationAnderson, 2001) on square root-transformed percent matrixes, using the Euclidean distance and 9999 permutations. Thus, FEEDING (FEED: automatic vs manual distribution, 2 levels), PERIOD (PER: feeding in the morning [a.m.] vs feeding in the afternoon [p.m.], 2 levels), and PHASE (PH: before, during, and after distribution of feed, 3 levels) were treated as fixed factors in the experimental design. Two different and independent days of measurement (Day, 2 levels) were treated as random factors and nested in the interaction FEED × PER × PH, while four different sessions of measurement were treated as random and orthogonal factors (Session, 4 levels). The homogeneity of multivariate dispersions was tested by the PERMDISP test (CitationAnderson, 2006). A similar experimental design was used to test the same hypothesis of two other variables (collisions and agonistic behaviours). In this case, the homogeneity of variance was tested with the Cochran’s C test and Student-Newman-Keuls was used as a post-hoc-comparison test (CitationUnderwood, 1997). Statistical analyses were performed by means of the software PRIMER 6 plus PERMANOVA (CitationClarke and Warwick, 1994; CitationAnderson, 2006; personally licensed to Chiara Romano; National Research Council, Italy) and GMAV 5.0 (University of Sidney, AU; personally licensed to Gianluca Sarà).
Results
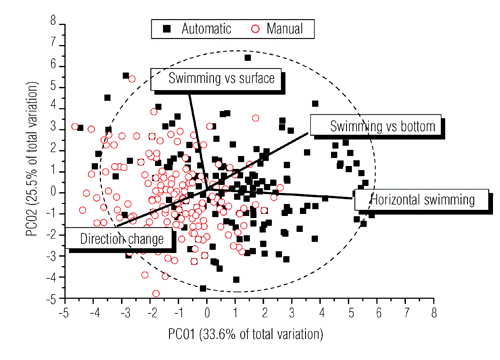
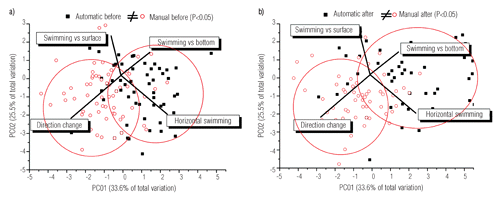
Measures of behavioural variables recorded under different treatments are reported in . The feeding regime did not affect the position of the fish in the water column (). Instead, the position of fish groups changed significantly between periods of the day (a.m.>p.m.; P<0.05): fish mostly stayed in the middle of the water column in the morning, whereas they typically stayed close to surface in the afternoon. Further, they moved from the middle column during the food distribution phase (P<0.05) and returned to this position in the before and after phases. As a general picture, swimming behaviour was significantly affected by feeding distribution modes (; ) as automatically-fed fish were significantly characterised by vertical movements through the water column (towards the surface and bottom) and by horizontal swimming. Manually-fed fish were instead significantly characterised by sharp direction changes during their swimming, mostly towards the surface (P<0.05). These differences among groups were maintained both in the a.m. and p.m. phases of the day (PERMANOVA pairwise test P<0.05) and throughout both the before and after phases ( a and b; PERMANOVA pairwise test P<0.05). Under automatic distribution, the overall behavioural responses during each feeding phase were different, as before the food arrived, fish were mostly swimming at the surface horizontally, during food distribution they showed quick vertical swimming, and finally, in the period after they swam horizontally some meters beneath the surface (P<0.05). Under manual distribution, the overall response during the food distribution was characterized by constant direction changes that resulted in a significant difference (P<0.05) compared with the overall response expressed before and after feeding, which was characterized by both swimming beneath the surface and minor direction changes (P>0.05).
Table 2 Behavioural frequencies (mean ±SE) during food distribution.
Table 3 PERMANOVA carried out on water column position and swimming variables to test the null hypothesis of no difference between automatic and manual modes throughout different phases of the study (BEFORE, DURING, and AFTER).
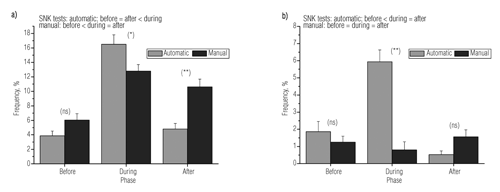
The feeding distribution elicited aggressive behaviours and induced changes in collision frequency among fish (). Under automatic distribution, collisions were significantly higher during feeding than before and after phases ( a). Under manual distribution, collisions were less frequent during the before phase, whereas they were elicited throughout the during phase, and still maintained higher during the after phase (see SNK test inside a). Agonistic behaviour (i.e. a fish attacks another fish) was basically elicited in the during phase under automatic distribution, and almost absent in the before and after phases (ANOVA, P<0.05; b). Under manual distribution, no difference in agonistic behaviour was observed () among feeding phases.
Discussion
In the investigated Sicilian farm, seabass showed significant changes of behaviour under two different investigated feeding distribution modes. Specifically, from the analysis of the behavioural responses, it was evident that fish responded differently to manual and automatic feeding modes during periods of food distribution and the periods just preceding and/or following it. Further differences were also recorded between the responses occurring with daylight (a.m.) and the ones occurred in conditions of subdued light (p.m.) (CitationBlyth et al., 1999). The position in the water column was not affected by feeding distribution as fish occupied the layers in the cages in the same way under automatic and manual distribution. However, they responded to different day phases, as they occupied deeper layers in the morning, then moved towards superficial layers in the evening. This observation is in accordance with evidence in the literature indicating that seabass prefer to occupy surface layers in experimental conditions (CitationSchurmann et al., 1998). Since most fish farmer activities occur in the morning, the preference for deeper water column layers might be simply explained as an avoidance response to noisy manoeuvres (i.e. boat engines approaching cages, farmers walking and shouting on gangways around cages; CitationRichardson et al. 1995; CitationSarà et al. 2007).
Swimming behaviour was basically affected by different food distribution modes. Automatically fed fish were mainly characterised by circular horizontal swimming around a restricted central area of the cages (i.e. exactlywhere the food usually fell from the pneumatic feeders) and by sudden and continuous vertical micromovements (of tens of centimetres), from the surface to deeper layers and vice-versa (CitationHammer, 1997). These movements strictly occurred below the surface where the sinking pellets fell. Conversely, manually-fed fish were characterised by continuous direction changes (i.e. turning behaviour; CitationNoble et al. 2007a, Citation2007b; CitationSarà et al., 2007) across the larger area of feed supply in the cage, indicating a response to the spreading of food by farmers. This type of behaviour was maintained over time in both the before and after phases, in the a.m. and p.m. periods. Behaviour of manually-fed fish appeared to be responsive not to limited resource availability (i.e. the proviso in production farms is that food should be provided until satiation of each fish), but to the high unpredictability of the whereabouts of the food supplied. Random searching of food which position is not predictable would, in fact, induce fish to remain close to the surface, continuously swimming with significant levels of turning behaviour. This would cause an increasing of energy expenditure which, in ultimis, may have effects on growth performances reducing economic incoming for farmers (CitationAndrew et al., 2002; CitationNoble et al., 2007a, Citation2007b). Automatic mode behaviour could have induced a foraging strategy which characteristics were imposed by an extreme competition triggered by high density of individuals restricted within a small, as predictable, food patch (CitationPaspatis et al., 1999; CitationAndrew et al., 2002). The food amount delivered in automatic mode is chosen to assure fish satiation (CitationPaspatis et al., 1999) and, therefore, to allow fish farmers to benefit of a profitable aquaculture activity. The same amount delivered over and over, however, did not reduce agonistic behaviours, which function has been explained to be a hard-wire response to cope, in constrained conditions, with despotic individuals with better competitive abilities (CitationMessier et al., 1990; CitationHawkins et al., 2005; CitationPurchase and Hutchings, 2008). An automatic schedule would elicit the conditioned behaviour that rewards fish remaining in the feeder supply area and moving continuously around it (CitationAndrew et al., 2002; CitationHuntingford and Adams 2005). This result, along with the differences recorded in the automatically-fed fish, substantiates that rearing conditions may induce aberrant behaviours particularly when captivity regimes provide settings disrupting natural biological rhythms (CitationBégout Anras and Lagardere, 2004). The automatic food distribution mode also enhanced collisions and aggressive behaviour. Automatically-fed fish significantly reduced their inter-individual distances during food distribution, but they recovered their usual behaviour (i.e. a lower number of collisions similar to the before phase) within a few minutes after the end of feeding. In contrast, the frequency of collisions under manual mode was lower than automatically-fed fish, but these fish maintained a higher frequency of collisions for a longer time after the end of feed distribution, recovering the former behaviours slower than fish fed in automatic mode. The increase in collisions under rearing conditions has been documented in many farmed species (e.g. rainbow trout; see CitationEllis et al., 2002) as it seems a regular effect of over-crowding and is favoured by the limited availability of space when the feed source is pulsing (i.e. automatic distribution). In contrast, when the food is spread over a larger area (i.e. manual distribution), collisions were reduced. However, it is not clear why the recovery time was slower under manual than automatic distribution and this deserves further research.
The condition of differential access to food implicitly involved a higher level of aggressiveness under automatic distribution. Thus, while agonistic responses were higher in automatic than manual mode, they were restricted to the during phase only. In the after phase, fish attacks waned within a few minutes, suggesting that aggressiveness was related only to the access to food. Thus, if other behavioural traits like continuous circular swimming in automatically-fed fish and direction change in manually-fed fish appeared to be maintained over time throughout all phases of this study, agonistic traits were limited to situations when fish competed for resources under automatic feed mode.
Conclusions
In conclusion, present results suggest that captive seabass responded i) differently to manual and automatic feeding modes during periods of food distribution and the periods just preceding and/or following it and ii) the response was different between day phases (a.m. vs p.m.). Also, the aggressiveness seems to be limited only when it results in an increased chance to access the resource which is consistent with the literature suggesting that domestication can sometimes influence aggressiveness (CitationHuntingford and Adams, 2005), and that the direction of its effect depends on feeding regimes (CitationRuzzante, 1994). Different conditional processes modes used to feed our seabass induced different behavioural changes (e.g. increased aggression and conditioned swimming activity), which were maintained over time potentially impairing the welfare of cultivated fish.
Acknowledgments:
the authors would like to thank students of Experimental Ecology Lab (University of Palermo, Italy) for their kind help in the field, Mr. F. Cascio and other owners of fish farming “Ittica Trappeto” (Balestrate, Italy) for their logistic assistance, and Dr. C. Romano (CNR-Italy) for her kind help in statistical analyses with Primer software.
References
- AdamsC.E. HuntingfordF.A. 1996 What is a successful fish? Determinants of competitive success in Arctic char (Salvelinus alpinus) in different social contexts Can. J. Fish. Aquat. Sci 53 2446–2450
- AdamsC.E. HuntingfordF.A. TurnbullJ.F. BeattieC. 1998 Alternative competitive strategies and the cost of food acquisition in juvenile Atlantic salmon (Salmo salar) Aquacultur 167 17–26
- AndersonM.J. 2001 Permutation tests for univariate or multivariate analysis of variance and regression Can. J. Fish. Aquat. Sci 58 626–639
- AndersonM.J. 2006 Distance-based test for homogeneity of multivariate dispersions Biometric 62 245–253
- AndrewJ.E. HolmJ. HuntingfordF.A. 2004a The effect of pellet texture on the feeding behaviour of gilthead sea bream (Sparus aurata L) Aquacultur 232 471–479
- AndrewJ.E. HolmJ. KadriS. HuntingfordF.A. 2004b The effect of competition on the feeding efficiency and feed handling behaviour in gilthead sea bream (Sparus aurata L) held in tanks Aquacultur 232 317–331
- AndrewJ.E. NobleC. KadriS. JewellH. HuntingfordF.A. 2002 The effect of demand feeding on swimming speed and feeding responses in Atlantic salmon Salmo salar L gilthead seabream Sparus aurata L and European seabass Dicentrarchus labrax L in sea cages Aquac. Res 33 501–507
- AzzaydiM. MartinezF.J. ZamoraS. Sanchez-VasquezF.J. MadridJ.A. 1999 Effect of meal size modulation on growth performance and feeding rhythms in Euro-pean sea bass (Dicentrarchus labrax L.) Aquacultur 170 253–266
- Bégout AnrasM.L. LagardereJ.P. 2004 Domestication et comportement chez les poissons téléostéens INRA Prod. Anim 17 211–215
- Bégout AnrasM.L. LagardereJ.P. CovèsD. 2004 Swimming activity of seabass: comparing patterns obtained in natural environment and in re-circulating tanks under high density J. Fish Biol 65 314–315
- BlythP.J. KadriS. ValdimarssonS.K. MitchellD.F. PurserG.J. 1999 Diurnal and seasonal variations in feeding patterns of Atlantic salmon Salmo salar L in sea cages Aquac. Res 30 539–544
- ClarkeK.R. WarwickR.M. 1994 Change in marine communities: an approach to statistical analysis and interpretation Natu-ral Environment Research Council publ Swindon, Wiltshire, UK
- EllisT. NorthB. ScottA.P. BromageN.R. PorterM. GaddD. 2002 The relationships between stocking density and welfare in farmed rainbow trout J. Fish Biol 61 493–531
- HammerC. 1997 The spontaneous swimming activity of juvenile whiting (Merlangius merlangus L) and cod (Gadus morhua L) under tank conditions with regard to feeding levels Arch. Fish. Mar. Res 45 1–16
- HawkinsL.A. ArmstrongJ.D. MagurranA.E. 2005 Aggregation in juvenile pike (Esox lucius): interactions between habitat and density in early winter Funct. Ecol 19 794–799
- HuntingfordF.A. AdamsC.E. 2005 Behavioural syndromes in farmed fish: implications for production welfare Be-haviour 142 1207–1221
- KadriS. MetcalfeN.B. HuntingfordF.A. ThorpeJ.E. 1997 Daily feeding rhythms in Atlantic salmon I: Feeding and aggression in parr under ambient environmental conditions J. Fish Biol 50 267–273
- KristiansenT.S. FernoA. 2006 Individual behaviour and growth of halibut (Hippoglossus hippoglossus L) fed sinking and floating feed: Evidence of different coping styles Appl. Anim. Behav. Sci 104 236–250
- MagnusonJ.J. 1962 An analysis of aggressive behaviour growth and competition for feed and space in Medaka (Oryzias latipes -Pisces Cyprinodonitidae) Can. J. Zool 40 313–363
- MazzolaA. RalloB. 1981 Furher experiences in the intensive culture of seabream (Sparus aurata L.) J. World Maricult. Soc 12 137–142
- MessierF. VirglJ.A. MarinelliL. 1990 Density-dependent habitat selection in musk-rats: a test of the ideal free distribution model Oecologi 84 380–385
- NobleC. KadriS. MitchellD.F. HuntingfordF.A. 2007a Influence of feeding regime on intraspecific competition, fin damage and growth in 1+ Atlantic salmon parr (Salmo salar L.) held in freshwater production cages Aquac. Res 38 1137–1143
- NobleC. KadriS. MitchellD.F. HuntingfordF.A. 2007b The effect of feed regime on the growth and behaviour of 1+ Atlantic salmon post-smolts (Salmo salar L.) in semi-commercial sea cages Aquac. Res 38 1686–1691
- PaspatisM. BatariasC. TiangosP. KentouriM. 1999 Feeding and growth responses of sea bass (Dicentrarchus labrax) reared by four feeding methods Aquacultur 175 293–305
- PurchaseC.F. HutchingsJ.A. 2008 A temporally stable spatial pattern in the spawner density of a freshwater fish: evidence for an ideal despotic distribution Can. J. Fish. Aquat. Sci 65 382–388
- RichardsonW.J. GreeneC.R.Jr MalmeC.I. ThomsonD.H. 1995 Marine Mammals and Noise San Dieg Academic Press San Diego, CA, USA
- RuzzanteD.E. 1994 Domestication effects on aggressive and schooling behavior in fish Aquacultur 120 1–24
- SaràG. DeanJ.M. D'AmatoD. BuscainoG. OliveriA. GenoveseS. FerroS. BuffaG. Lo MartireM. MazzolaS. 2007 Effect of shipping traffic on behaviour of bluefin tuna Thunnus thynnus in the Mediter-ranean Sea Mar. Ecol. Prog. Ser 331 243–253
- SaràG. ScilipotiD. MilazzoM. ModicaA. 2006 Use of stable isotopes to investigate the dispersion of fish farming waste as a function of hydrodynamics Mar. Ecol.Prog. Ser 313 261–270
- SchurmannH. ClaireauxG. ChartoisH. 1998 Changes in vertical distribution of sea bass (Dicentrarchus labrax L.) during a hypoxic episode Hydrobiologi 371/372 207–213
- UnderwoodA.J. 1997 Experiments in ecology. Their logical and interpretation using analysis of variance Cambridg Cambridge University Press Cambridge, UK
- ZarJ.H. 1999 Biostatistical analysis 4th ed. Prentice-Hall Upper Saddle River, NJ, USA