Abstract
The objective of this study was to evaluate the effects of a slow-release coated urea product (CU, 1% as dry matter of diet) on ruminal disappearance and fermentation, as well as on growth performance of beef steers. Soybean meal in control diet was replaced by CU and steam-rolled corn. For the growth performance trial, 20 beef steers (330±20 kg) were used. For the ruminal trial, four ruminally cannulated steers (230±20 kg) were used.
Dry matter intake, daily gain, feed efficiency and carcass dressing were not affected (P>0.05) by CU. Ruminal ammonia N was higher (P<0.05) with CU than control diet. Potential disappearance and total ruminal disappearance of dry matter were increased, while the potential disappearance rate of neutral detergent fibre was reduced with CU. Slow coated urea at 1% of total diet did not affect growth performance and carcass dressing of beef steers.
Introduction
The most common non-protein nitrogen (NPN) source used in ruminant feeding is urea, due to its low cost; thus, when prices of protein feeds escalate (i.e., soybean meal), it is economical to use urea as a nitrogen supplement in ruminant diets. Using the protein equivalent of 281%, incorporation of one unit of urea in a diet can replace five units of soybean meal. However, the final decision is not just a matter of a mathematical substitution. The amount of NPN that can be used is limited due to the rapid hydrolysis of this N source, which causes accumulation and escape of ammonia from the rumen (CitationSatter and Roffler, 1975).
Slow-release NPN compounds fed to domestic ruminants (CitationForero et al., 1980; CitationOwens et al., 1980; CitationLöest et al., 2001) have shown variable responses, probably because the release of N is too fast to optimize microbial protein production (CitationGalo et al., 2003). Although some slow-release urea products have effectively mitigated rapid ammonia release in the rumen (CitationHuntington et al., 2006) and enhanced feed efficiency in dairy cows, when soybean meal was replaced (CitationGolombeski et al., 2006). Coated urea products are comprised of closely sized central particles of concentrated rumen-degradable nitrogen compounds and a non-rumen-degradable semi-permeable membrane coating, which covers the central particles and allows their diffusion through the semi-permeable membranes into the rumen fluid.
It was hypothesized that a slow-release coated urea product (CU) would not affect ruminal variables and growth performance in beef cattle. To test this hypothesis, the objectives of this study were to evaluate the effects of feeding CU (1% as dry matter of diet) on ruminal fermentation and disappearance, as well as on growth performance and dressing carcass in beef cattle.
Materials and methods
This experiment was conducted under the supervision and with the approval of the Academic Committee of Animal Science of the Colegio de Postgraduados according to regulations established by the Animal Protection Law enacted by the Estado of México.
For the growth performance trial, 20 cross-breed (¾ Brown Swiss x ¼ Brahman 75:25) steers (330±50 kg body weight) were de-wormed (Ivermectine, Iverject ADE, Avilab, Jalisco, México), vaccinated (Clostridium chavoei, septicum, sordelli and perfringes C and D and Pasterella multocida type A and D, Mannheimia haemolytic A-1, Bobact8, Intervet, México), implanted (Trembolona acetate plus 17 β-estradiol, Sinovex plus, Fort Dodge, Overland Park, Kansas, USA), housed in individual metabolic pens in dry lot, and finally randomly assigned to the following treatments: 1) Control (standard diet with soybean meal as protein source); 2) a slow-release coated urea product (CU, Optigen 1200, Alltech Inc. Nicholasville, KY USA).
A standard diet commonly used in Mexico feedlots was used as control treatment. It was nutritionally evaluated using NRC (2000) software. The CU diet was re-formulated to maintain a similar percentage of N among control and polymer-coated urea diets. This was largely accomplished by replacing soybean meal with steam rolled corn ().
Table 1 Ingredients and chemical composition of diets.
Dry matter (DM), crude protein (CP) and acid detergent fibre (ADF) were analyzed according to the CitationAOAC (1997). Net energy for gain (NEg) was calculated with NRC model (2000). Neutral detergent fibre (NDF) was analyzed according to CitationVan Soest et al. (1991). Nitrogen fractions were determined using the procedures described by CitationKrishnamoorty et al. (1982). Nitrogen fractions (A, B1, B2, B3 and C) were determined using 5 g of diet (DM basis) mixed with 100 mL carbonate-phosphate buffer (BCP), plus 5 mL t-butyl alcohol 10% and 5 mL BCP. This mixture was maintained at 20±2°C, washed for 10 min and filtered (Whatman No. 54); the residual was washed with 50 mL BCP and 250 mL distilled water. Nitrogen content of the residual samples was determined according to micro-Kjeldahl method; from the soluble nitrogen extracted with BCP, an aliquot was taken and true soluble protein was precipitated with trichloroacetic acid (TCA 10% final concentration). The precipitated protein was calculated according to CitationLowry (1951) using seroalbumin as a standard. A sample was taken from diets and neutral and acid detergents were added (CitationVan Soest et al., 1991); then nitrogen content was measured by micro-Kjeldahl method. The following formulas (CitationLicitra et al., 1996) were used to quantify N fractions: A= soluble N with BCP — soluble true protein (Lowry method); B1= true protein — insoluble N with BCP (true soluble protein); B2= insoluble N with BCP-insoluble protein in neutral detergent (NDIP); B3= NDIP — insoluble protein in acid detergent (ADIP); C= ADIP.
Steers had free access to feed and water. Feed was offered daily at 7:00 h. Body weight (BW) was recorded every experimental period (i.e. 21 d during 84 d), and then average daily gain (ADG) was calculated. Dry matter intake (DMI) and ADG were used to calculate feed efficiency (FE). At the end of the growth performance trial (i.e., 48 d), all steers were slaughtered in an authorized slaughterhouse (Los Reyes, Estado de México). Hot carcass weights were recorded and carcass dressing percentage calculated.
Data (i.e. carcass) were analyzed as a completely randomized design with the MIXED procedure of CitationSAS (1999) using steer as a random in the model; continuous data collected over time (i.e. BW, DMI) were analyzed as repeated measurements according to CitationLittell et al. (1998) using the MIXED procedure. Initial body weight was tested as a covariate in the model, but it was not significant (P>0.05). Because the interaction treatment x time was not significant, only overall values were showed. Significant differences were accepted at P≤ 0.05.
For the ruminal trial, four Holstein steers (230±20 kg body weight) fitted with ruminal cannulas, housed in metabolic pens in a naturally ventilated barn were completely randomized to both diets described previously (control or CU). Experimental periods were 14-d with 12-d for adaptation and 2-d for sample collection and ruminal incubations.
For the in sacco disappearance trial, bags (10 × 20 cm; pore 52±10 µm) with 5 g DM of diet (ground 2 mm, Thomas Willey) were placed in the rumen at 8:00 h and removed at 3, 6, 9, 12, and 48 h. The CU treatment diet was ground without the CU, which was added afterwards in order to avoid damaging the polymer-coated urea. To determine soluble fraction of diets at 0 h, bags were washed with water (39°C, 20 min). After ruminal incubation, residuals of DM and NDF were determined.
Ruminal fluid samples were collected at 3, 6, 9, 12 and 24 h after the morning feeding. Ruminal pH was recorded immediately (ORION SA 210) and samples were acidified with 3 M metaphosphoric acid (1:10 dilution), then cooled (4°C) for 30 min and centrifuged (25,000 xg; 4°C; 20 min). Supernatant fluid samples were kept and frozen for the further analysis. Volatile fatty acid (VFA) concentration was determined (CitationErwin et al., 1961) with a gas chromatograph (Hewlett Packard 6890). Lactate (CitationMadrid et al., 1999) and ammonia N (NH3N; CitationMcCullough, 1967) of ruminal fluids were determinate with a UV-VIS spectrophotometer (Varian, Cary-1-E).
In sacco DM and NDF disappearance kinetics were estimated using the model described by CitationØrskov and McDonald (1979) as follows: P= a + b(1 − e−kt)] where P= denotes the proportion of the material disappearing (lost through the bag) at time t; a= soluble fraction at 0 h; b= is the insoluble but potentially degradable fraction; and k= rate of degradation (/h). The DM remaining at each incubation time was fitted to the nonlinear regression model using the procedure “NLIN” of CitationSAS (1999). The experimental design was a cross over design as follows: Yijk= μ+ sequencei + steerij + periodk + treatmenth + eijk where Yijk= the measurement during the kth period of the jth steer in the ith group (i= 1,2; j= 1, 2, 3, 4; k = 1,2); μ= the overall mean effect; sequencei = the effect of the ith sequence group (i= 1,2); steerij= the effect of the jth steer on the ith sequence (j= 1, 2, 3, 4), steerij N(0, σ2steer); periodk = the effect of the kth period (k= 1,2); trth= the effect of the hth treatment (h= 1,2; being a function of i and k); eijk= the random error, eijk N(0, σ2e). Parameters of the model are the mean (μ), the effect of the sequence group (seqi), the variance amongst animals (experimental units)(σ2steer), the effect of periods, the effect of the treatment (treatmenth), and the random residual variation (σ2e). Period was considered as a fixed component in the model. Ruminal variables collected over time were analyzed as repeated measurements by the MIXED procedure of CitationSAS (1999) according to CitationLittell et al. (1998). Ruminal disappearance kinetic was analyzed with MIXED option using the cross-over model described previously. Because the interaction treatment x time was not significant, only overall values were showed. Significant differences were accepted when P≤0.05.
Results
The DM, CP, NDF, ADF, ash and NEg were similar for experimental diets (). There were apparently differences in CP fractions; the CU diet had a higher A protein fraction than control.
The DMI was increased and ADG and FE were decreased by time. There were no time x treatment interactions for FE, hot carcass weight and carcass dressing percentage. The CU treatment did not affect final BW, DMI, FE, or carcass weight as compared to the control diet ().
Table 2 Overall body and carcass weight, total gain, intake, and carcass dressing of beef Cattle fed diets with soybean meal or coated urea.
The soluble fraction and disappearance rate were similar for both diets. The potentially disappearing fraction and total disappearance of DM was lower for the CU diet (). The potentially disappearing fraction for NDF was also lower for CU than control. There were no differences for NDF disappearance rate.
Table 3 Ruminal disappearance of DM and NDF of diets with soybean meal or coated Urea.
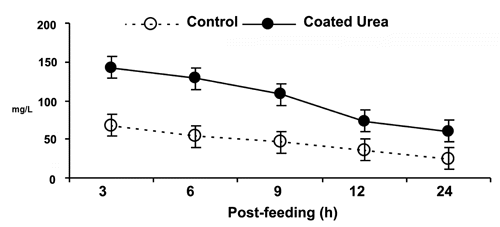
As post-feeding time increased, ruminal pH values and lactate concentrations decreased. Volatile fatty acid proportions were not affected by time. Ruminal pH, lactate, volatile fatty acid concentration and molar proportion of acetate, propionate and butyrate were not different with experimental diets (). The CU diet induced higher ammonia N concentrations than control diet ().
Table 4 Overall ruminal pH, lactate and volatile fatty acids (VFA), of steers fed diets with Soybean meal or coated urea.
Discussion
Growth performance was not affected by CU, such as was previously found by CitationTedeschi et al. (2002) and CitationWahrmund and Hersom (2007) using the same CU for beef steers. Other slow-release NPN products also did not affect growth performance in lambs (CitationVirk et al., 1989) and cattle (CitationCampbell et al., 1963; CitationLöest et al., 2001). The negative effects of CU on in sacco disappearance were contrary to the findings from CitationHarrison et al. (2007), who found that NPN contained in a CU product increased apparent DM digestion in rumen-simulating fermenters. Maybe the high ammonia N concentrations in the rumen with CU could affect the in sacco degradation. These findings suggest that N from the CU diet could be degraded faster than N from control diet with soybean meal, but probably slower than common urea. Indeed, the chemical analysis of diets confirmed that protein fraction A was higher for CU than control diet. This idea was in part confirmed by CitationGarcia-Gonzalez et al. (2007) and CitationHarrison et al. (2007), who found that ruminal N ammonia concentrations with CU were lower than those from common urea. CitationPeyton and Conrad (1982) found that digestible DM intake in cows fed on soybean meal was higher than those fed on urea. CitationLana et al. (1997) showed that steers fed on soybean meal had higher DM intake, ADG and feed efficiency than those fed urea. This result is consistent with the effect of amino nitrogen on bacterial growth rate (CitationVan Kessel and Russell, 1996).
The CU did not affect ruminal fermentation patterns (i.e, pH and AGV) as compared to the control diet, which agree with previous studies using the same CU product (CitationGarcia-Gonzalez et al., 2007; CitationHarrison et al., 2007).
Conclusions
The analysis of the results suggests that CU can replace soybean meal in diets for beef steers without any negative effect on growth performance. Besides, the high ruminal ammonia concentration and the reduction of in sacco degradation could be associated with the increase in the soluble N in the CU diet.
References
- AOAC 1997 Official Methods of Analysis 16th ed. Association of Official Analytical Chemists Washington, DC, USA
- Campbell T.C. Loosli J.K. Warner R.G. Tasaki I. 1963 Utilization of biuret by ruminants J. Anim. Sci 22 139–145
- Erwin E.S. Marco G.J. Emery E. 1961 Volatile fatty acid analysis of blood and rumen fluid by gas chromatography J. Dairy Sci 44 1768–1771
- Forero O. Owens F.N. Lusby K.S. 1980 Evaluation of slow-release urea for winter supplementation of lactating range cows J. Anim. Sci 50 532–538
- Galo E. Emanuele S.M. Sniffen C.J. White J.H. Knapp J.R. 2003 Effect of a polymer-coated urea product on nitrogen metabolism in lactating Holstein dairy cattle J. Dairy Sci 86 2154–2162
- Garcia-Gonzalez R. Tricarico J.M. Harrison G.A. Meyer M.D. McLeod K.R. Harmon D.L. 2007 Optigen® is a sustained release source of non-protein nitrogen in the rumen J. Anim. Sci 85 Suppl. 1 98–98 (abstr.)
- Golombeski G.L. Kalscheur K.F. Hippen A.R. Schingoethe D.J. 2006 Slow-release urea and highly fermentable sugars in diets fed to lactating dairy cows1 J. Dairy Sci 89 4395–4403
- Harrison G.A. Tricarico J.M. Meyer M.D. Dawson K.A. 2007 Effects of Optigen® on fermentation, digestion, and N partitioning in rumen-simulating fermenters J. Anim. Sci 85 Suppl. 1 98–98 (abstr.)
- Huntington G.B. Harmon D.L. Kristensen N.B. Hanson K.C. Spears J.W. 2006 Effects of a slow-release urea source on absorption of ammonia and endogenous production of urea by cattle Anim. Feed Sci. Tech 130 225–241
- Krishnamoorty U. Muscato T.V. Snifeen C.J. Van Soest P.J. 1982 Nitrogen fractions in selected feedstuffs J. Dairy Sci 65 217–225
- Lana R.P. Fox D.G. Russell J.B. Perry T.C. 1997 Influence of monensin on Holstein steers fed high-concentrate diets containing soybean meal or urea J. Anim. Sci 75 2571–2579
- Licitra G. Hernandez T.M. Van Soest P.J. 1996 Standardizations of procedures for nitrogen fractionation of ruminant feeds Anim. Feed Sci. Tech 57 347–348
- Littell R.C. Henry P.R. Ammerman C.B. 1998 Statistical analysis of repeated measures data using SAS procedures J. Anim. Sci 76 1216–1231
- Löest C.A. Titgemeyer E.C. Drouillard J.S. Lambert B.D. Trater A.M. 2001 Urea and biuret as nonprotein nitrogen sources in cooked molasses blocks for steers fed prairie hay Anim. Feed. Sci. Tech 94 115–126
- Lowry O.H. Rosenbrough N.J. Farr A.L. Randall R.J. 1951 Protein measurement with the folin phenol reagent J. Biol. Chem 193 265–275
- Madrid J. Martínez T.A. Hernández F. Megías M.D. 1999 A comparative study on the determination of lactic acid in silage juice by colorimetric, high-performance liquid chromatography and enzymatic methods J. Sci. Food Agr 79 1722–1726
- McCullough H. 1967 The determination of ammonia in whole blood by a direct colorimetric method Clin. Chim. Acta 17 297–304
- Ørskov E.R. McDonald I. 1979 The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage J. Agr. Sci 92 499–503
- Owens F.N. Lusby K.S. Mizwicki K. Forero O. 1980 Slow ammonia release from urea: rumen and metabolism studies J. Anim. Sci 50 527–531
- Peyton S.C. Conrad H.R. 1982 Corncobs as energy with urea nitrogen in dairy rations J. Dairy Sci 65 1465–1471
- SAS 1999 SAS User’s Guide: Statistics version 8 Institute Inc Cary, NC, USA
- Satter L.D. Roffler R.E. 1975 Nitrogen requirement and utilization in dairy cattle J. Dairy Sci 58 1219–1237
- Tedeschi L.O. Baker M.J. Ketchen D. J. Fox D. G. 2002 Performance of growing and finishing cattle supplemented with a slow-release urea product and urea Can. J. Anim. Sci 82 567–573
- Van Kessel J.S. Russell J.B. 1996 The effect of amino nitrogen on the energetics of ruminal bacteria and its impact on energy spilling J. Dairy Sci 79 1237–1243
- Van Soest P.J. Robertson J.B. Lewis B.A. 1991 Methods for dietary fibre, neutral detergent fibre, and non-starch polysaccharides in relation to animal nutrition J. Dairy Sci 74 3583–3597
- Virk A.S. Steingass H. Menke K.H. 1989 Studies on in vitro degradation and in vivo digestion of a slow ammonia releasing urea product Arch. Anim. Nutr 39 167–176
- Wahrmund J.L. Hersom M.J. 2007 Evaluation of dried distillers grains or soybean hulls with and without Optigen II® to background beef calves J. Anim. Sci 85 Suppl. 1 410–410 (abstr.)