Abstract
Threonine needs based on Ross manuals might be 14% of the National Research Council (NRC) recommendation, thus the effect of threonine on mucin2 gene expression, histological characteristics and performance were evaluated in Ross male broilers fed diets containing 0.8% (NRC requirement), 0.87% (average of NRC and Ross requirement), 0.94% (Ross requirement) and 1.01% (more than Ross requirement) total threonine. The dietary treatments consisted of an isonitrogenous corn-soybean meal-based diet with incremental levels of threonine. At the first day of chicken age, 24 pens were equalized to 12 birds per pen, in a completely randomized design and dietary treatments were randomly distributed for the 14-d period. Live performance measurements improved (P<0.05) as dietary threonine increased from 0.8% to 0.87%. The least performance was related to diet containing 1.01% threonine. Histological assays showed that villi height, crypt depth and villi surface increased as dietary threonine increased from 0.8% to 0.87% and decreased in 0.94% and 1.01% threonine. There was no effect of threonine on mucin2 gene expression and goblet cells density. According to these results, threonine needs in starter period in Ross (308) broilers is more than NRC recommendation.
Introduction
Dietary amino acid concentration should closely meet maintenance and tissue growth requirements of commercial broilers and under- and over- formulation of amino acids will decrease performance and increase nitrogen excretion, respectively (CitationKidd et al., 2004). Threonine (Thr) was discovered over 60 years ago and is considered to be the third limiting amino acid for broilers fed corn-soybean meal (CitationWaldroup et al., 2005). The essential amino acid Thr may be of particular interest in terms of intestinal growth and development due to its importance in the structure of mucins (CitationHorn et al., 2009). The nutrient Thr must be considered in dietary formulations for commercial broilers because its excess is costly and its deficiency will decrease the sufficiency of total sulfur amino acid (TSAA) and lysine (Lys) use (CitationKidd, 2000).
The intestinal tract epithelium is covered by a mucus layer composed predominantly of mucin glycoproteins, which are synthesized and secreted by goblet cells distributed along the villi and goblet cells utilize substantial amounts of nutrients for the synthesis of mucins (CitationUni et al., 2003). Mucin affect protecting the gut from acidic chime, digestive enzymes and pathogens, filtering nutrient in the gastrointestinal tract, nutrient digestion and absorption (CitationHorn et al., 2009). Proteins and specific amino acids have been shown to alter mucin secretion and may interact directly with goblet cells or with the enteric nervous system to elicit changes in mucin secretion (CitationMontagne et al., 2000; CitationClaustre et al., 2002; CitationFaure et al., 2005). Studies in piglets showed between 80% and 90% to dietary Thr is used by the intestine (CitationSchaart et al., 2005). The portaldrained viscera (PDV) has a high obligatory visceral requirement for Thr and the high rate of intestinal Thr utilization is due mainly to incorporation into mucosal proteins (CitationSchaart et al., 2005). Threonine is an integral constituent of intestinal mucin proteins (CitationVan Klinken et al., 1995; CitationLien et al., 1997).
Intestinal mucin synthesis is sensitive to dietary Thr supply, which suggests that the gut’s requirement for Thr may comprise a significant proportion of the whole body requirement (CitationNichols and Bertolo, 2008). De novo synthesis of mucosal and mucin proteins is sensitive to luminal Thr concentration, which demonstrates the importance of dietary amino acid supply to gut protein metabolism (CitationNichols and Bertolo, 2008). The structure of Mucin gene (MUC2) is composed by 11% Thr (CitationGum, 1992). The hydroxyl group of Thr and serine is necessary for ester linkages on the mucin amino acid backbone to carbohydrate groups that make up the majority (50% to 80%) of the molecular weight of mucin (CitationMontagne et al., 2004). There is a lack of information about the effects of Thr levels more than CitationNRC (1994) on performance and mucin dynamics in poultry, especially for starter period. However, Ross requirement (0.94%) is 14% more than CitationNRC (1994). Thus, this experiment was conducted to determine whether providing dietary Thr higher than CitationNRC (1994) can effect on performance, type of gut mucins, goblet cell density, and mucin2 (MUC2) mRNA abundance in growing broiler chicks.
Materials and methods
Experimental design
One-day-old chicks were feather sexed, and male birds were selected. Chicks were then transported to a research facility and placed in pens at this age. The pens were 0.9×1.2 m and contained a tube feeder and nipple water line. Birds were reared at 32°C and
29°C from days 1 to 7 and 8 to 14, respectively. Birds had access to continuous lighting and unrestricted access to feed and water. Management and husbandry practices were in accordance with current standards.
At day 1, 24 pens were equalized to 12 birds per pen, in a completely randomized design and dietary treatments were randomly distributed for the 14-day period. Diets were formulated to provide a minimum of 100% of CitationNRC (1994) amino acid recommendations for 1 to 14 days of age. The dietary treatments consisted of isonitrogenous corn-soybean meal-based diets with 4 levels of total Thr (0.8%, NRC requirement; 0.87%, average of NRC and Ross requirement; 0.94%, Ross requirement; and 1.01%, more than Ross requirement). Treatments were achieved by the addition of crystalline L-Thr (98.5 % Thr, Degussa Co., Essen, Germany) at the expense of filler in the test diet containing 0.8% Thr (). Supplementation of L-Glutamic acid served to make the diets isonitrogenous.
Table 1 Composition of basal diet fed to male broilers of 1 to 14 day of age.
Corn and soybean meal were analyzed for CP (CitationAOAC, 1990) and amino acids (all samples were analyzed by Degussa Co., in Essen, Germany) prior to formulation. The experimental protocols were reviewed and approved by the Animal Care Committee of the Ferdowsi University of Mashhad, Iran.
Tissue sampling
At day 14, one chicken from each replicate was killed and intestinal segments removed. Samples (approximately 4 cm) were taken from the midpoint between the point of entry of the bile duct and Meckel’s diverticulum (jejunum). These segments were cut in half and one part was freezed in −80°C and the other part was flushed with 0.9% (wt/vol) NaCl and then was fixed in fresh 4% formaldehyde buffer. Jejunum was of particular interest because it is a major site of nutrient absorption in poultry (CitationHorn et al., 2009).
Analysis of histological samples
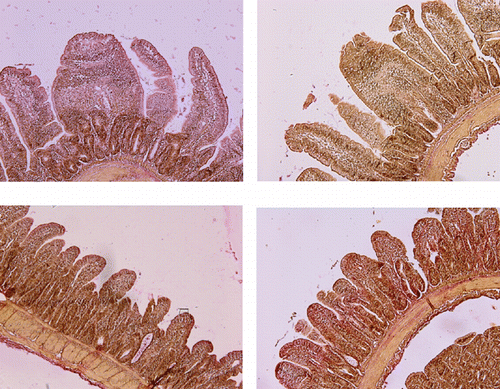
After fixation, soaked samples were rinsed several times in absolute alcohol, and then embedded in paraffin. Serial 6-µm longitudinal sections were cut on Leica Rotary Microtome (RM 2145, Leica Microsystems, Wetzlar, Germany) and placed on glass slides. Then, slides routinely stained with Gill’s hematoxylin and eosin (H&E). For the histochemical evaluation of gut mucins, other representative sections were stained with 1% Alcian blue (AB) for the demonstration of all acidic mucins (sialomucins and sulfomucins). Periodic acid Schiff was used for detection of neutral mucins (CitationLaw et al., 2007).
Neutral and acid mucin staining
Neutral mucin was measured by staining 6 µm sections with periodic acid-schiff (PAS) (CitationUni et al, 2003). Briefly, procedure steps consist of i) deparaffinize and hydrate to eliminate the contribution of sialic acid residues before PAS staining; ii) oxidize in 0.5% periodic acid solution for 15 min; iii) rinse in distilled water; iv) immerse in Schiff reagent for 30 min (sections become light pink color during this step); v) wash in warm water for 10 min (immediately sections turn dark pink color); vi) dehydrate with ethanol and mount in glass slide. The number of PAS positive (PAS+) along the villi were counted by light microscopy.
Acid mucin measured by staining 5 µm sections with AB, pH 2.5 (CitationLev and Spicer, 1964). Briefly, method steps consist of i) dissolving dye in 3% acetic acid to provide a solution with PH 2.5; ii) bringing sections to distilled water; iii) staining in the AB solution pH 2.5 for 15 min; iv) wash well in running tap water for 5 min; v) rinse in distilled water; vi) counterstain with neutral red stain for 1 min; vii) rapidly dehydrate in absolute alcohol, clear and mount. The number of AB positive cells (AB+) along the villi was counted with light microscopy by using EPIX XCAP software (Image pro Express, Mediacybernetic, Atlanta, GA, USA).
Histomorphometric analysis was performed on H&E-stained tissue sections. The parameters measured were as follows: villus height (measured from the tip of the villus to the villus-crypt junction), crypt depth (measured from the crypt-villus junction to the base of the crypt), and villus surface area [(π×mh×h)+ (π×mh/2)2], where mh is the width at the midvillus height and h is villus height (CitationLaw et al., 2007). Villi length and width were measured from 5 villi per bird and only complete, vertically oriented villi were measured. Goblet cell counts were taken from the same 5 villi per bird and the average value was used. Density of goblet cells was calculated as the number of goblet cells per unit of surface area (mm2).
RNA extract, reverse transcription and real-time PCR
Relative real-time PCR was performed to assess MUC2 gene expression in jejunum of broiler chicken. Total RNA was extracted from jejunum samples using the RNXTM (-Plus) (RN7713C, Cinnagen Inc., Tehran, Iran) according to manufacturer’s instructions. The quantity and integrity of isolated RNA were determined by using UV absorbances (260/280). To remove residual DNA, was used RNase-free DNase I (EN0521, Fermentas, Opelstrasse 9, Germany), ribonuclease inhibitor (E00311, Fermentas, Germany) and buffer with MgCl2. DNase I was inactivated by EDTA and was incubated at 65°C for 10 min. Reverse transcription was performed by using a RevertAid™ First Strand cDNA Synthesis Kit (K1622, Fermentas) according to the manufacturer’s instructions and RT reaction was used of Random hexamer. We provided a control (one RNA sample without cDNA) for DNA contamination in PCR. All RNA samples were reversed transcribed simultaneously for minimize variations. We applied real time PCR for measurement mucin 2 gene mRNA abundance. The primers for MUC2 (CitationHorn et al., 2009) (Intestinal mucin 2; Gallus gallus, XM_421035) were as follows: forward: 5′- TCA CCC TGC ATG GAT ACT TGC TCA-3′, reverse: 5′- TGT CCA TCT GCC TGA ATC ACA GGT-3′ and with primers from the Gallus gallus 18S ribosomal RNA gene (CitationSmirnov et al., 2006) (GI7262899; forward: 5′-CGATGCTCTTAACTGAGT-GT-3′, reverse: 5′CAGCTTTGCAACCATACTC- 3′). Real time PCR was executed in triplicate. Initial step contained 10 min at 95°C, and followed by 40 cycles of a two-phase PCR (denaturation at 95°C for 30s; annealing and extension at 62°C for 1 min). To confirm PCR product from misannealed primer was runned a dissociation curve following the real time PCR. The thermal profile for dissociation curve is, 95°C for 15 s, 60°C for 1 min, 95°C for 15 s and 60°C for 15 s. Reaction mixtures for real-time PCR included 2 µL cDNA as template, 10 Power SYBRR Green PCR Master Mix (ABI, USA), 0.2 mM of each forward and reverse primers and 7.4µL double distilled water. We used of ΔΔCt model. In this model, Ct value or threshold cycle is the first cycle in PCR reaction in which the fluorescence signal increases significantly. ΔCt was calculated by subtracting the Ct amount of mucin 2 gene from Ct of 18sRNA for each sample, Then ΔΔCt was calculated from subtract ΔCt each treatments of ΔCt control. We selected the diet containing 0.8% Thr as control and then 2−ΔΔcT was calculated. 2−ΔΔCT is a good method to analyze the relative changes in gene expression (CitationBustin et al., 2009).
Statistical analysis
Data were analyzed using GLM procedures of SAS software (CitationSAS, 2006) in a completely randomized design. Differences between means were tested using CitationDuncan’s test (1995). Differences were considered significant at P<0.05.
Results
Performance measurements
The corn-soybean meal basal diet was formulated to contain 21.16% CP. Analyzed diet showed 21.15% CP. Growth performance measurements as affected by dietary Thr presented in . Broilers fed 1.01% Thr had a poorer (P<0.05) feed to gain ratio response vs. broilers fed 0.8, 0.87 and 0.94% Thr. There was not any significant difference between feed intake. The lowest and the highest feed intake and body weight gain observed in birds fed 1.01% and 0.87% Thr, respectively. Body weight of chickens decreased (P<0.05) in diets containing 1.01% Thr when compared with those of 0.8, 0.87 and 0.94% Thr.
Table 2 Live performance measurements of male broiler fed incremental levels of dietary Thr from 1 to 14 days of age.
Histomorphometry
Data for intestinal villus height, crypt depth and villus surface area are presented in . Dietary treatments affected villus height, crypt depth and villus surface area in jejunum of broiler chicks fed levels of Thr for 14 days. Villus height increased (P<0.05) to 634.09 µm in 0.87% containing diets in comparison to the 0.94 and 1.01 % Thr treatments. Crypt depth was the lowest (P<0.05) in birds fed 0.94 and 1.01% Thr.
Table 3 Effect of dietary threonine on histomorphological parameters in 14 day of age.
Villus surface area was approximately equal as birds fed 0.94 and 1.01% Thr. Birds fed 0.80% and 0.87 % Thr had the highest (P<0.05) villus surface area than the other two groups ().
Histochemistry
The histochemical methods employed in this study stained the surface mucus of the intestinal mucosa, mucus granules of goblet cells. The histochemical staining results were interpreted as follows: with PAS, neutral mucins were staining red and AB 2.5, stained all acidic mucins (sialomucins and sulfomucins) blue. There was a tendency for an increase in goblet cells density as dietary Thr increased from 0.8% to 0.87%, however it was not significant and then decreased in 0.94 and 1.01%Thr (). Most of the goblet cells numbers that contained neutral mucins were equal with numbers of acidic mucin-containing goblet cells in all treatments. Goblet cells in all treatments randomly distributed along the villus-crypt axis.
Table 4 Effect of dietary threonine on goblet cells density in broiler chicks in 14 days of age.
Real-time PCR
shows the average CT results for treatments and how these CTs are manipulated to determine ΔCt, ΔΔCt and the relative amount of MUC2 mRNA. There was not any significant effect of the different levels of Thr on MUC2 gene mRNA abundance in the jejunum for broiler chickens. MUC2 gene mRNA abundance in chicks fed 1.01% Thr decreased in comparison to the others, however it was not significant.
Table 5 Relative quantification using the comparative CT method.
Discussion
The essential amino acid Thr is typically the third limiting amino acid behind TSAA and Lys in commercial broiler diets composed of corn or sorghum, soybean meal and meat meal (CitationKidd and Kerr, 1996; CitationKidd, 2000). Thr is not only an essential amino acid for growth in young chicks, but also its preferential utilization by the gut for mucus synthesis makes it disproportionately essential for maintenance requirements. Up to 90% of dietary Thr is extracted by the portal-drained viscera (versus only about a third for other essential amino acids) (CitationStoll et al., 1998; CitationVan Goudoever et al., 2000; CitationVan Der Schoor et al., 2002; CitationSchaart et al., 2005). Thr’s requirement for maintenance functions in the gut would be particularly sensitive to Thr supply. The protective mucus layer in the gut predominantly consists of mucins, glycoproteins that are particularly rich in Thr. More ever, mucins are continuously synthesized and very resistant to small intestinal proteolysis and hence recycling; therefore, mucin synthesis is largely an irreversible loss of Thr (CitationVan Der Schoor et al., 2002). As a result, a substantial and constant supply of Thr is necessary to maintain gut function and structure (CitationLaw et al., 2007). It is important to note that CitationRoss (2007) has estimated Thr requirements 0.14% more than CitationNRC (1994). To our knowledge, previous studies have been published examining the effects of the less Thr levels on the different characteristics. As a result, in the current experiments, dietary Thr levels increased to 0.07, 0.14, and 0.21% more than CitationNRC (1994) recommendation. In the study presented herein and in others evaluating Thr needs of starting (CitationHorn et al., 2009), growing (CitationKidd et al., 2004) and finishing (CitationKidd et al., 1999) male broiles, diets containing surfeit Thr allowed for better growth however performance decreased in 1.01% Thr.
Goblet cells secrete mucins (CitationHorn et al., 2009) and mucins are polymeric glycoproteins representing an important component of the mucus layer that covers the epithelium of the gastrointestinal tract (CitationMontagne et al., 2004).The mucin is an important component of the mucus and contributes to the lubrication of the gut epithelium, the protection of the intestinal lumen from an acidic environment and bacterial protease, colonization resistance, and the repair of the epithelium (CitationMontagne et al., 2004). Fractional protein synthesis rate of small intestinal mucosal protein and mucins were reduced by an imbalanced intake of dietary Thr (both a Thr deficiency and an excess) (CitationWang et al., 2007). There was a tendency for an increase in goblet cell density as dietary Thr increased from 0.80 to 0.87% but it was not significant. However, by 1.01% Thr, goblet cell density decreased. Thrdeficient diet had dramatically lower numbers of goblet cells in neonatal piglets (CitationLaw et al., 2007). Goblet cell density in broilers numerically decreased when dietary treatments contained approximately 40, 70 and 100% of the Thr requirement based on CitationNRC (1994) (CitationHorn et al., 2009). In this study, no significant difference between neutral and acidic mucin-containing goblet cells was seen in all treatments and the number of goblet cells was almost equal. This finding is similar to observation of CitationWang et al (2007). They reported, there was an acidic mucins before birth in jejunum and between birth and weaning neonatal piglets, the ratio of neutral mucins to acidic mucins increase when they fed with threonine adequate diet. Acidic mucins (oligosaccharide chains terminated with sialic acid or sulfate groups) protect against microbial penetration and translocation because these mucins are more resistant to bacterial mucolytic activities (CitationDeplancke and Gaskins, 2001). The physiological relevance of distinct mucin subtypes is not well understood. It has been suggested that acidic mucins protect against bacterial translocation as sulfated mucins appear to be less degradable by bacterial glycosidases and host protease (CitationFontaine et al.,1996; CitationRobertson and Wright, 1997). Mucin-producing cells were observed in the small intestine from 3 days before hatch, and at this time contained only acidic mucin. After hatch and until day 7 posthatch, the proximal, middle, and distal segments of the small intestine contained similar proportions of goblet cells producing acidic and neutral mucins (CitationUni et al., 2003). These observations suggested that, adequate Thr intake supported the production of mixtures of neutral and acidic mucins in the small intestine. Amino acids maintain intestinal viability and mass, in addition to providing energy for normal intestinal function. As gastrointestinal tissues have relatively high protein turnover rate, high protein diets provide nutrients for basal metabolism and causes a developed small intestine. On the other hand, Thr is of vital nutritional importance, because it is the single most used essential amino acid by the metabolism of portal-drained visera (CitationSchaart et al., 2005). Villus surface area was numerically decreased from 0.628 mm2 to 0.42 mm2 as the Thr levels increased from 0.87% to 1.01% Thr. There was a tendency for a decrease in crypt depth when dietary Thr increased from 0.80% to 1.01%. These results were in consistent with the report by CitationLaw et al. (2007) who found that villus height in piglet receiving Thr deficient diet decreased compare to those of receiving the Thr adequate diet.
More than 20 human mucin genes have been catalogued by the National Library of Medicine. Mucins are usually subdivided into two groups, the secreted mucins (gel-forming and non-gel-forming) and the membraneanchored mucins. The second group consists of the two large mucins MUC3 and MUC4, containing EGF-like motifs, and the small mucin MUC1, MUC6, MUC2, MUC5AC and MUC5B are the secreted gel-forming mucins (Desseny et al., 2000). MUC2 in gallus gallus is on choromosom 5 and encods a gel-forming protein and is expressed in the small intestine and other mucus membrane-containing organs. Intestinal loss of MUC2 affects the protective capacities of the mucus layer and leads to colonic inflammation in mice (CitationSluis et al., 2009). The inflammation in these mice is characterized by a thickening of the mucosa, loss of epithelial integrity, and an increase in infiltrating cells (CitationSluis et al., 2009). Each subunit of a mucin protein backbone contains a central domain that is rich in serin, Thr, proline, alanine, and glycine, and two extending peptides (N and C terminal) that contain cystein. The splanchnic tissues of preterm infants use more than three quarters of the total dietary Thr intake, irrespective of the amount of enteral Thr delivered (CitationSluis et al., 2009).
In this experiment, there was no effect of diet on MUC2 mRNA abundance. CitationSmirnov et al. (2006) reported increase in mucin mRNA expression from day 17 of incubation to 3 days of posthatch. There was no change of diets containing 3.3 and 8.2 g of Thr/kg for 7 days on MUC2 mRNA abundance in chicks and there was no relationship between crude mucin excretion and MUC2 mRNA abundance in chicks (CitationHorn et al., 2009). Thr deficiency restricts the majority of mucin synthesis at the translational level. There are some researches about the effect of deficiency of Thr on intestinal mucin dynamics but the results about an excess intake of dietary Thr is limited. However, the Ross requirement is more than NRC requirement. The mechanism by which a Thr deficiency or excess can change mucin synthesis is not clear.
It has been shown that increasing the consumption of Thr elevates plasma, liver and muscle levels of Thr (CitationCastagné et al., 1993). If Thr plasma level was raised, the transport of neutral amino acids is inhibited and then its effect on Thr entry into brain can modify the brain concentration of serotonine and related compounds (CitationCastagné et al., 1993). A reduction in the uptake of neutral amino acids (including branched-chain amino acids) limit local synthesis of both global and specific proteins and imbalance of Thr intake may affect the secretion of local and systemic hormones that regulate intestinal protein metabolism (CitationWang et al., 2007).
Conclusions
The aim of this study was to investigate the effect of threonine levels on mucin2 gene expression, intestinal histology and performance of broiler chickens. Body weight, villus height and villus surface was affected (P<0.05) in chicks fed with the various levels of threonine in this experiment. Mucin2 cDNA in jejunum tissue in broiler chicken was characterized. Mucin2 gene expression was not affected in chicks, whereas there was a tendency for a decrease in broilers fed diet containing 1.01% threonine. Additional studies need to investigate the role of threonine on mucin 2 gene expression anf intestinal histology in broiler chickens.
References
- AOAC 1990 Official methods of analysis 15th ed. AOAC Washington, DC, USA
- BustinS.A BenesV GarsonJ. A HellemansJ HuggettJ KubistaM MuellerR NolanT PfafflM.W ShipleyG.L VandesompeleJ WittwerC.T 2009 The MIQE guideline: minimum information for publication of quantitative realtime PCR experiments Clin. Chem 55 611 622
- CastagnéV MoennozD FinotP MaireJ 1993 Effects of diet-induced hyperthreoninemia Life Sci 53 1803 1810
- ClaustreJ ToumiF TrompetteA JourdanG GuignardH ChayvialleJ. A PlaisancieP 2002 Effects of peptides derived from dietary proteins on mucus secretion in rat jejunum Am. J. Physiol 283 521 528
- DeplanckeB GaskinsH. R 2001 Microbial modulation of innate defence: goblet cells and the intestinal mucus layer Am. J. Clin. Nutr 73 1131S 1141S
- DesseynJ AubertJ PorchetN LainA 2000 Evolution of the large secreted gelforming mucins Mol. Biol. Evol 17 1175 1184
- DuncanD.B 1955 Multiple range and multiple F tests Biometrics 11 1 42
- FaureM MoennozD MontigonF MettrauxC BreuilleD BallevreD 2005 Dietary threonine restriction specifically reduces intestinal mucin synthesis in rats J. Nutr 135 486 491
- FontaineN MeslinJ. C LoryS AndrieuxC 1996 Intestinal mucin distribution in the germ-free rat and in the heteroxenic rat harbouring a human bacterial flora: Effect of inulin in the diet Brit. J. Nutr 75 881 892
- GumJ.RJr 1992 Mucin genes and the proteins they encode: structure, diversity, and regulation Am. J. Resp. Cell Mol 7 557 564
- HornN.L DonkinS.S ApplegateT.J AdeolaO 2009 Intestinal mucin dynamics: Response of broiler chicks and white Pekin ducklings to dietary threonine Poultry Sci 88 1906 1914
- KiddM.T 2000 Nutritional consideration concerning threonine in broilers World Poultry Sci. J 56 139 151
- KiddM.T KerrB.J 1996 L-threonine for poultry: A review J. Appl. Poultry Res 5 358 367
- KiddM.T LernerS.P AllardJ.P RaoS.K HalleyJ.T 1999 Threonine needs of finishing broilers: Growth, carcass, and economic responses J. Appl. Poultry Res 8 160 169
- KiddM.T McDanielC.D BrantonS.L MillerE.R BorenB.B FancherB.I 2004 Increasing amino acid density improves live performance and carcass yields of commercial broilers J. Appl. Poultry Res 13 593 604
- LawG.K BertoloR.F Adjiri-AwereA PencharzP.B BallR.O 2007 Adequate oral threonine is critical for mucin production and gut function in neonatal piglets Am. J. Physiol.-Gastr. L 292 G1293 G1301
- LevR SpicerS.S 1964 Specific staining of sulphate groups with Alcian blue at low pH J. Histochem. Cytochem 12 309 309
- LienK.A SauerW. C FentonM 1997 Mucin output in ileal digesta of pigs fed a protein-free diet Z. Ernahrungswiss 36 182 190
- MontagneL PielC LallesJ.P 2004 Effect of diet on mucin kienetics and composition: Nutrition and health implications Nutr. Rev 62 105 114
- MontagneL ToullecR LallesJ.P 2000 Calf intestinal mucin: Isolation, partial characterization, and measurement in ileal digesta with an enzyme-linked immunosorbent assay J. Dairy Sci 83 507 517
- National Research Council 1994 Nutrient Requirements of poultry 9th rev. ed. National Academy Press Washington, DC, USA
- NicholsN.L BertoloR.F 2008 Luminal threonine concentration acutely affects intestinal mucosal protein and mucin synthesis in piglets J. Nutr 138 1298 1303
- RobertsonA.M WrightD.P 1997 Bacterial glycosulphatases and sulphomucin degradation Can. J. Gastroenterol 11 361 366
- Ross Broiler Nutrition Specification 2007 Available from: http://www.aviagen.com/.
- SAS 2006 STAT User's Guide. Version 9.1 SAS Inst. Inc. Cary, NC, USA
- SchaartM.W SchierbeekH Van Der SchoorS.R StollB BurrinD.G ReedsP Van GoudoeverJ.B 2005 Threonine utilization is high in the intestine of piglets J. Nutr 135 765 770
- SluisM SchaartM KoningB SchierbeekH VelcichY RenesI GoudoeverJ 2009 Threonine metabolism in the intestinal of mice: loss of mucin2 induces the threonine catabolic pathway J. Pediatr. Gastr. Nutr 49 99 107
- SmirnovA TakoE FerketP.R UniZ 2006 Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in Ovo feeding of carbohydrates Poultry Sci 85 669 673
- StollB HenryJ ReedsP.J YuH JahoorF BurrinD 1998 Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets J. Nutr 128 606 614
- UniZ SmirnovA SklanD 2003 Pre- and posthatch development of goblet cells in the broiler small intestine: Effect of delayed access to feed Poultry Sci 82 320 333
- Van DerSchoorS.R ReedsP.J StollB HenryJ.F RosenbergerJ.R BurrinD.G Van GoudoeverJ.B 2002 The high metabolic cost of a functional gut Gastroenterology 123 1931 1940
- Van GoudoeverJ.B StollB HenryJ.F BurrinD.G ReedsP.J 2000 Adaptive regulation of intestinal lysine metabolism P. Natl. Acad. Sci. USA 97 1620 11625
- Van KlinkenB.J DekkerJ BullerH.A EinerhandA.W 1995 Mucin gene structure and expression: protection vs. adhesion Am. J. Physiol.-Gastr. L 269 G613 G627
- WaldroupP.W JiangQ FrittsC.A 2005 Effects of glycine and threonine supplementation on performance of broiler chicks fed diets low in croud protein Int. J. Poultry Sci 4 250 257
- WangX QiaoS YinY YueL WangZ 2007 A deficiency or excess of dietary threonine reduces protein synthesis in jejunum and skeletal muscle of young people J. Nutr 137 1442 1446