Abstract
Thirty adult goats were classified at parturition into two body condition score (BCS) groups: BCI (n=16) with a score of 2.7 and BCII (n=14) with a score of 2.0. On the fiftieth day postpartum, oestrus was synchronized by CIDR for 5 days. Upon CIDR removal (Day 0), they received 1 mL of PGF2α IM and mated for 72 hours. Kids were kept with does and weaned at 40 days of age. Blood samples were taken at 0, 1, 4, 8 and 21 days after CIDR removal for progesterone assay. The BCI group showed a greater weight loss compared to the BCII group, and BCS before synchronization was 1.9±0.08 and 1.6±0.07 for the BCI and BCII groups, respectively (P<0.05). The weaning weight of BCI kids was greater when compared to BCII (P<0.001). After CIDR removal, all females were marked and mated. Pregnancy rate was higher in BCI goats (87% vs 36%; P<0.05), as well as prolificacy (1.65 vs 1.25; P<0.05) and twinning rate (0.62 vs 0.25; P<0.05). Progesterone concentration was higher in pregnant does in BCI. A positive relationship was found between progesterone level at CIDR removal and BCS at parturition (0.57; P<0.01), also between progesterone level at 21 days after CIDR removal and BCS at parturition (0.47; P<0.05), or BCS before synchronization (0.51; P<0.05). We conclude that oestrus response to postpartum CIDR synchronization appeared to be slightly dependent on BCS. However, goats with low BCS at oestrus synchronization exhibited a reduction in pregnancy rate.
Introduction
In Northeast Brazil, as in other tropical areas, the main impediment to the utilization of assisted reproduction techniques such as oestrus synchronization in goat herds, for most of the year, is the maintenance of the animal’s body condition (CitationFreitas et al., 2004a). In goats as in other ruminants, the level of energy reserves assumes an even more essential role in postpartum. In this phase, a lower body condition at parturition increases the probability of developing metabolic diseases and reproductive dysfunctions, as well as prolonging the anoestrus period (CitationFreitas et al., 2004b), compromising the response to oestrus synchronization as well as the level of fertility. A positive relationship between decrease in energy reserves and ovarian quiescence was demonstrated for goats by CitationTanaka et al. (2003) and CitationRondina et al. (2005). CitationTanaka et al. (2003), supported by previous observations in cows (CitationImakawa et al., 1986) and goats (CitationTanaka et al., 2002), suggested that a 20% loss of body mass is the cut-off point for anovulation, a value close to that reported by CitationRondina (2010) in goats in Northeast Brazil.
In goats, unfortunately, there is still relatively little information about the effects of body reserves on oestrus synchronization protocols, especially during the postpartum phase. There have been reports that low body condition affects responsiveness to oestrus synchronization in different hormonal protocols (CitationKusina et al., 2001; CitationTanaka et al., 2004; CitationPaula et al., 2005). In contrast, CitationKusina et al. (2000), in comparing synchronization protocols in indigenous African goats maintained on pasture and with low body score, found no differences in reproductive response. Therefore, the objective of this work was to determine the response to oestrus synchronization in the postpartum period, in goats with different body conditions.
Materials and methods
Animals and experimental design
The experiment was conducted on the experimental farm Campo da Semente - Guaiuba - Ceara, which belongs to the Universidade Estadual do Ceara (UECE), located at 4° 2′ S 38° 30′ W, with a constant photoperiod regimen, mean annual temperature of 26 to 28°C and mean annual precipitation of 904.5 mm, during the rainy season (January — June).
We used 30 adult goats of the Anglo-Nubian breed, which were pluriparous and pregnant. The animals were from the same farm and checked over the whole course of gestation for health and reproductive aspects. At parturition, the goats were classified into two groups (P<0.05) based on body condition score (BCS) (CitationMorand-Fehr and Hervieu, 1999): BCI (n=16), with BCS of 2.7±0.27 (mean ± SD), and BCII (n=14), with BCS of 2.0±0.25.
All animals were maintained in similar feeding and management conditions. Goats were kept in the same area when grazed on mixed pasture during the day, and were penned overnight. Herein, animals received a supplement based on sorghum silage and concentrate. Every two weeks, goats were weighed and BCS was determined. The kids remained together with the does and weaned on a regime of creep feeding at 40 days of age. The kidding period was 30 days long.
Fifty days after parturition, oestrus was induced in the goats using a controlled intravaginal drug release (CIDR®) device impregnated with 0.33 g progesterone (Eazi-Breed CIDR®, InterAg, Hamilton, New Zealand), which was left in the cranial portion of the vagina for 5 days. Upon removal of the device (time zero), the goats received 1 mL of prostaglandin Pgf2α (Lutalyse®, Upjohn, Kalamazoo, USA), and 24 h after removal of the device, they were exposed to two Anglo-Nubian bucks of proven fertility, equipped with marker pigtails, which remained with the females for 72 consecutive hours. After the does were mated, they were separated from the bucks. All procedures used in this study were approved by Ethics Committee in Animal Experimentation of Ceará State University (No. 09656828/09, CEUA — UECE).
Blood sample and hormone assay
At CIDR® removal (day 0) and at 1, 4, 8 and 21 days after CIDR removal, before the animals were released to pasture, blood samples were collected in heparinized tubes by venipuncture. Plasma progesterone was measured by microparticle enzyme immunoassay (MEIA) (Abbott Diagnostics AxSYM® SYSTEM) using a commercial kit (Axsym Progesterone, Abbott Japan Co., Ltda,Tokyo, Japan). The sensitivity of the assay was 0.2 ng/mL. The intra- and interassay coefficients of variation were 7.9% and 3.3%, respectively.
Pregnancy diagnosis
Pregnancy diagnosis was performed transrectally at 45 days after mating, using a Pie Medical Scanner (Falco 100, Pie Medical equipment B.V., Maastricht, Netherlands) attached to a 6.0/8.0 MHz linear array transducer. Pregnancy failure (0–21 days) was classified in the negative goats at pregnancy diagnosis by ultrasound, when the progesterone level at 21 days after CIDR removal was below 2 ng/mL, whereas mortality (22–45 days) was noted when the progesterone level at 21 days after CIDR removal was above 2 ng/mL.
Statistical analysis
All data were analyzed using the SAS program software (SAS, Inc., Cary, NC, USA). For live weight and plasma progesterone concentration, the effect tested was the group (BCI and BCII). For BCS, the effects tested were group, interval of assessment considered (time) and interaction. In both models, data were analyzed using PROC GLM. For the number of marked goats and reproductive response variables, effect of group was analyzed by the PROC NPAR1WAY. Differences between means were determined by Student’s t test. Comparison between numbers was performed using the chi-squared test. Correlations were assessed using the Spearmen’s test. Values were expressed as the mean ± SEM.
Results
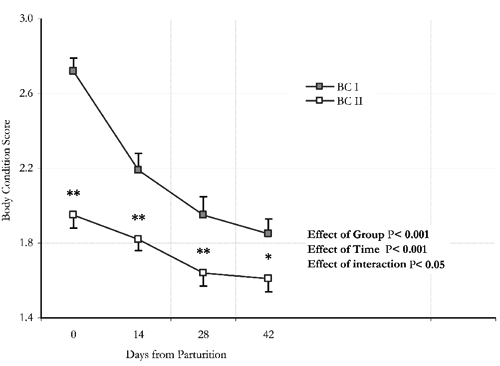
Based on the body condition score at parturition (2.7±0.07 vs 2.0±0.07; P<0.01), the two groups BCI and BCII, showed a difference of more than 8 kg live weight (P<0.001) (). For 42 days postpartum, a marked decline in live weight was observed in all animals, which was more intense in the BCI group with a decrease of more than 13% of initial weight versus 10% in the BCII group (P<0.05) (). The substantial decrease in body mass accounted for body condition scores of 1.9±0.08 and 1.6±0.07 at 42 days postpartum in the BCI and BCII groups (), respectively (P<0.05). Litter size and twinning rate () were similar in the two groups (P>0.05) with pooled means of 1.63 and 0.56, respectively. During the experiment, one of the goats in BCI was found dead in the pasture. Live birth weight of the kids was statistically similar in the two groups (P>0.05) (), while at parturition in BCII, three animals lost their kids and were therefore removed from the study. At weaning (), the kids in group BCI had a live weight, daily weight gain and an increase in weight in relation to initial live weight that were statistically higher when compared to BCII (P<0.001).
Table 1 In vivo performance of goats and kids from parturition to before oestrus synchronization and reproductive response after oestrus synchronization in goats of the BCI and BCII groups. Values are given in means ± SEM.
presents the results of oestrus synchronization. All females (100%) were marked by the buck, and in both groups more than 60% showed paint marking in the first 24 h after CIDR removal (P<0.05). At 30 days after mating, ultrasound diagnosis detected a pregnancy rate that was statistically higher (P<0.05) in BCI versus BCII (87% vs 36%) (); these values were also found at parturition and thereby determined the kidding rate. The litter size differed significantly between the groups (P<0.05), where the animals in BCI had a greater rate of multiple births (0.62 vs 0.25; P<0.05) (). Of the seven animals in the BCII group that tested negative for pregnancy, 86% (6/7) () had a pregnancy loss before 21 days after CIDR removal, compared to only 1 of the 2 animals negative on ultrasound in the BCI group. In these goats, progesterone remained, on average, below 1 ng/mL (), with the exception of one animal in the BCII group, which on day 4 showed a level of 1.68 ng/mL, a sign of possible regression of the corpus luteum.
Table 2 Distribution of goats marked according to the time (hours) from the CIDR removal in goats of the BCI and BCII groups.
The plasma progesterone concentration determined on the day of CIDR removal (day 0) (), was higher (P<0.05) in the pregnant does of the BCI group when compared to the pregnant animals in the BCII group and to the two groups of animals that tested negative for pregnancy. Four days after CIDR removal, the plasma progesterone level in the BCI group was above 1 ng/mL and higher than in the other animals (P<0.05). From day 8, plasma progesterone increased gradually, with values over 1 ng/mL, also in the BCII group and in the animals of the group with embryonic mortality 21 days after CIDR removal, indicating the persistence of the gestational corpus luteum, where plasma levels were higher in animals with early pregnancy loss (P<0.05) ().
Correlation analysis () indicated significant and positive coefficients between body condition score at parturition and plasma progesterone concentration on CIDR removal (0.57; P<0.01) or at 21 days after removal (0.47; P<0.05). There was also a positive relation between body condition score at 21 days after CIDR removal and progesterone level in the same interval (0.51; P<0.05), as well as between the two progesterone concentrations (just after device removal and after 21 days) (0.39; P<0.05).
Table 4 Correlation coefficients (n=26) between body condition scores at parturition (BCP), before oestrus synchronization (BCES) and progesterone concentration at CIDR removal (PRCIDR) or 21 days after (PRTW).
Discussion
The marked reduction of body mass recorded during our experiment, in both groups, reflected the effects of negative energy balance characteristics of the postpartum period. An inadequate intake of nutrients in this phase usually induces in goats a decline in body condition score and a prolongation of the interval between births. During the experimental period, it was also observed that animals with a higher BCS had a less acute form of weight loss. CitationLago et al. (2001) reported that dairy cows with a higher BCS at parturition showed a marked reduction in weight and body condition during the lactation period in relation to those with a low BCS. Females with more adipose reserves at parturition tend to mobilize these reserves markedly, thereby showing a direct relation between weight loss and fat mobilization (CitationChillard, 1998). Body condition is one of the principal factors that contribute to a decrease in feeding at parturition in sheep (CitationParr et al., 1993). Dairy cows with an elevated body condition score exhibit a greater loss of appetite, developing a more pronounced negative energy balance, because it causes a greater mobilization of body reserves and larger accumulation of triglycerides in the liver (CitationRukkwamsuk et al., 1999). The intense mobilization of body mass in the BCI group was the likely reason for the higher performance of its kids. Females with larger reserves of body fat in the postpartum period have higher circulating levels of non-esterified fatty acids and utilize larger amounts of these reserves for the synthesis of milk fat in relation to those with smaller deposits of body fat during the lactation phase (CitationWaltner et al., 1993), which can affect the weight of the young at weaning.
Despite the marked decline in body mass, an elevated response to the oestrus synchronization protocol was observed in both groups. In contrast, CitationTanaka et al. (2004) and CitationNation et al. (2000) respectively reported that in undernourished goats and in cows in the postpartum period, with reduced nutritional state, the application of oestrus synchronization via CIDR was not successful. In goats, the extent of energy deficit in the postpartum period is usually related to the prolongation of the anovulatory period and delayed return to ovarian cyclicity (CitationFreitas et al., 2004b; CitationMalau-Aduli et al., 2005). However, CitationMbayahaga et al. (1998) reported that there is no correlation between the manifestation of the first oestrus after parturition and loss of body weight in goats. CitationLlewelyn et al. (1992) studied indigenous African goats and observed that the return of ovarian activity after parturition occurred prior to improvement in body condition and weight. Furthermore, CitationCiccioli et al. (2005) found in beef heifers that the response to oestrus synchronization in the first oestrus cycle in the postpartum period is not influenced by body condition at parturition.
In general, the CIDR-PGF2α hormone protocol is a highly efficient treatment in synchronizing oestrus in goats (CitationOliveira et al., 2001) and in postpartum cows (CitationLucy et al., 2001; CitationFlores et al., 2006), demonstrating better efficiency in simulating corpus luteum function. These reasons also account for the successful use of the CIDR protocol in the induction of oestrus in prepuberty goats (CitationMaffili et al., 2006) and acyclic heifers (CitationLucy et al., 2001). The administration of exogenous progesterone during the anoestrus period sensitizes the hypothalamus-hypophysis-ovarian axis directly or indirectly, besides unblocking the release of gonadotrophins and activating the behavioral centers, thereby stimulating the expression of oestrus in a large proportion of animals (CitationRhodes et al., 2003).
Based on hormone analysis, plasma progesterone concentration at CIDR removal positively correlated with body condition score at parturition. At the time of removal of the device, the pregnant animals of the BCI group showed progesterone levels similar to those reported by CitationTanaka et al. (2004) in Shiba goats and higher levels in relation to the group with lower body condition at parturition. This last difference, however, is not explainable only based on exogenous hormone supply, because plasma progesterone concentrations at the time of removal of the device is also a product of the quality of the corpus luteum during hormonal treatment.
The plasma progesterone concentration during oestrus synchronization with CIDR in goats has been investigated relatively little, but the relation between body reserves and progesteronemia can be explained by the dependence of this hormone on the insulin-IGF-I system. In ruminants, the modifications in systemic concentrations of IGF-I after parturition are directly related to the plasma insulin level, which functions as an important initiator of the recovery of ovarian activity (CitationWebb et al., 2004). In addition, IGF-I stimulates the production of luteal progesterone in vitro, since receptors for this growth factor have been detected in bovine corpus luteum (CitationWoad et al., 2009).
The information collected in the present study indicated that the evaluation of body condition at paring is one the most reliable indicators of reproductive response in the postpartum period. Thus, a larger number of pregnant animals were recorded in the BCI group, although this showed greater loss of body mass. According to CitationCiccioli et al. (2003), animals with moderate BCS normally display greater pregnancy rates in relation to animals with low BCS. In addition, the latter show a more prolonged negative energy balance, which leads to a greater mobilization of body reserves for maintenance activities and lactation, to the detriment of ovarian activity. In goats, embryonic mortality occurs mostly during the first 30 days of gestation (CitationMartinez et al., 1998), like in the present study. CitationDixon et al. (2007) observed that the majority of pre-natal deaths in sheep also occur between the 2nd and 30th day of gestation. The principal causes of early pregnancy loss are due to failures in ovulation or in the process of fertilization, and in this phase progesterone concentrations exert an essential role in the success of ovulation as in maternal recognition during the initial steps of embryonic development (CitationInskeep, 2004). Thus, in this study, plasma progesterone concentrations at the moment of CIDR removal and after 21 days correlated with body condition at parturition. CitationAshworth et al. (1989) reported that in sheep the progesterone level during metaoestrus and dioestrus substantially affects later pregnancy rate. These observations are in accordance with findings in cows by CitationKerbler et al. (1997), in which luteal secretion of progesterone is essential for the production of an oocyte of good quality and for embryonic survival. In relation to the notion that exogenous progesterone administration by means of CIDR can lead to fertility, the results in the literature are not conclusive. CitationOliveira et al. (2001) compared various protocols of induction and synchronization of oestrus in Saanen breed goats and found a lower pregnancy rate, contrary to that reported by CitationMotlomelo et al. (2002) in Boer goats, indigenous to South Africa, and in postpartum beef cows (CitationLucy et al., 2001; CitationFlores et al., 2006).
Conclusions
We can conclude that the reproductive response in postpartum goats demonstrated a complex interaction with body reserves. While the success of oestrus synchronization with CIDR showed little dependence on body condition score, circulating progesterone concentrations measured in this period, as well the pregnancy rate, were shown to be related to body reserves.
Acknowledgements:
D. Rondina is senior investigator of CNPq/Brazil.
The authors also extend their thanks to the staff of Ceará State University, Campo da Semente Farm Experimental Station for the care, handling and management of the experimental animals.
References
- Agricultural and Food Research Council 1998 The nutrition of goats Technical Committee on Responses to Nutrients, Report No. 10 CAB Int. Wallingford, UK
- AshworthC.J. SalesD.I. WilmutI. 1989 Evidence of an association between the survival of embryos and the periovulatory plasma progesterone concentration in the ewe J. Reprod. Fertil 87 23 32
- ChillardY. BocquierF. DoreauM. 1998 Digestive and metabolic adaptations of ruminants to undernutrition, and consequences on reproduction Reprod. Nutr. Dev 38 131 152
- CiccioliN.H. Charles-EdwardsS.L. FloydC. WettemannR.P. PurvisH.T. LusbyK.S. HornG.W. LalmanD.L. 2005 Incidence of puberty in beef heifers fed high- or low- starch diets for different periods before breeding J. Anim. Sci 83 2653 2662
- CiccioliN.H. WettemannR.P. SpicerL.J. LentsC.A. WhiteF.J. KeislerD.H. 2003 Influence of body condition at calving and postpartum nutrition on endocrine function and reproductive performance of primiparous beef cows J. Anim. Sci 81 3107 3120
- DixonA.B. KnightsM. WinklerJ.L. MarshD.J. PateJ.L. WilsonM.E. DaileyR.A. SeidelG. InskeepE.K. 2007 Patterns of late embryonic and fetal mortality and association with several factors in sheep J. Anim. Sci 85 1274 1284
- FloresR. LooperM.L. KreiderD.L. PostN.M. RosenkransC.F.Jr. 2006 Estrous behavior and initiation of estrous cycles in postpartum Brahman-influenced cows after treatment with progesterone and prostaglandin F2{alpha} J. Anim. Sci 84 1916 1925
- FreitasV.J.F. RondinaD. Lopes JúniorE.S. TeixeiraD.I.A. PaulaN.R.O. 2004a Hormonal treatments for the synchronization of estrus in dairy goats raised in the tropics Reprod. Fert. Develop 16 415 420
- FreitasV.J.F. RondinaD. NogueiraD.M. SimplicioA.A. 2004b Post-partum anoestrus in Anglo-Nubian and Saanen goats raised in semi-arid of North-eastern of Brazil Livest. Prod. Sci 90 219 226
- ImakawaK. DayM.L. Garcia-WinderM. ZaleskyD.D. KittokR.J. SchanbacherB.D. KinderJ.E. 1986 Endocrine changes during restoration of estrous cycles following induction of anestrus by restricted nutrient intake in beef heifers J. Anim. Sci 63 565 571
- InskeepE.K. 2004 Preovulatory, postovulatory, and postmaternal recognition effects of concentrations of progesterone on embryonic survival in the cow J. Anim. Sci 82 24 39
- KerblerT.L. BuhrM.M. JordanL.T. LeslieK.E. WaltonJ.S. 1997 Relationship between maternal plasma progesterone concentration and interferon-tau synthesis by the conceptus in cattle Theriogenology 47 703 714
- KusinaN.T. ChinuwoT. HamudikuwandaH. NdlovuL.R. MuzanenhamoS. 2001 Effect of different dietary energy level intakes on efficiency of estrus synchronization fertility in Mashona goat does Small Ruminant Res 39 283 288
- KusinaN.T. TarwireiH. HamudikuwandaH. AgumbaG. MukwenaJ. 2000 A comparison of the effects of progesterone sponges and ear implants, PGF2a, and their combination on efficacy of estrus synchronization and fertility of Mashona goat does Theriogenology 53 1567 1580
- LagoE.P. PiresA.V. SusinI. FariaV.P. LagoL.A. 2001 Efeito da condição corporal ao parto sobre alguns parâmetros do metabolismo energético, produção de leite e incidência de doenças no pós-parto de vacas leiteiras Rev. Bras. Zootecn 30 1544 1549
- LlewelynC.A. OgaaJ.S. ObwoloM.J. 1992 Plasma progesterone concentrations during pregnancy and onset of ovarian activity postpartum in indigenous goats in Zimbabwe Trop. Anim. Health Prod 24 242 250
- LucyM.C. BillingsH.J. ButlerW.R. EhnisL.R. FieldsM.J. KeslerD.J. KinderJ.E. MattosR.C. ShortR.E. ThatcherW.W. WettemannR.P. YelichJ.V. HafsHD. 2001 Efficacy of an intravaginal progesterone insert and an injection of PGF2alpha for synchronizing estrus and shortening the interval to pregnancy in postpartum beef cows, peripubertal beef heifers, and dairy heifers J Anim Sci 79 982 995
- MaffiliV.V. TorresC.A.A. BruschiJ.H. FonsecaJ.F. VianaJ.H.M. 2006 Estrous induction in Toggenburg goats using intravaginal devices Arq. Bras. Med. Vet. Zoo 58 367 372
- Malau-AduliB.S. EduvieL.O. LakpiniC.A.M. Malau-AduliA.E.O. 2005 Influence of crop residue ration supplementation on the attainment of puberty and postpartum reproductive activities of Red Sokoto goats J. Anim. Physiol. An. N 89 11 19
- MartinezM.F. BoschP. BoschR.A. 1998 Determination of early pregnancy and embryonic growth in goats by transrectal ultrasound scanning Theriogenology 49 1555 1565
- MbayahagaJ. MandikiS.N.M. BisterJ.L. 1998 Body weight oestrus and ovarian activity in local Burudian ewes and goats after parturition in the dry season Anim. Reprod. Sci 51 289 300
- Morand-FehrP. HervieuJ. 1999 Apprécier l'éat corporel des chèvres: Intérêt et méthod Reussir La Chevre 231 22 34
- MotlomeloK.C. GreylingJ.P.C. SchwalbachL.M.J. 2002 Synchronization of oestrus in goats: the use of different progestagen treatments Small Ruminant Res 45 45 49
- NationD.P. BurkeC.R. PartonG. StevensonR. MacmillanK.L. 2000 Hormonal and ovarian responses to a 5-day progesterone treatment in anoestrous dairy cows in the third week post-partum Anim. Reprod. Sci 63 13 25
- National Research Council 2007 Nutrient requirements of small ruminants National Academy Press Washington, DC, USA
- OliveiraM.A.L. GuidoS.I. LimaP.F. 2001 Comparison of different protocols used to induced and synchronize estrus cycle of Saanen goats Small Ruminant Res 40 149 153
- ParrR.A. DavisI.F. MilesM.A. SquiresT.J. 1993 Feed intake affects metabolic clearance rate of progesterone in sheep Res. Vet. Sci 55 306 310
- PaulaN.R.O. GaleatiG. TeixeiraD.I.A. Lopes JúniorE.S. FreitasV.J.F. RondinaD. 2005 Responsiveness to progestagen-eCG-cloprostenol treatment in goat food restricted for long period and refed Reprod. Domest. Anim 40 108 110
- RhodesF.M. McDougallS. BurkeC.R. VerkerkG.A. MacmillanK.L. 2003 Invited Review: Treatment of Cows with an Extended Postpartum Anestrous Interval J. Dairy Sci 86 1876 1894
- RondinaD. FreitasV.J.F. SpinaciM. GaleatiG. 2005 Effect of nutrition on plasma progesterone levels, metabolic parameters and small follicles development in unstimulated goats reared under constant photoperiod regimen Reprod. Domest. Anim 40 548 552
- RondinaD. GaleatiG. 2010 Nutritional effects on reproduction in small ruminants and its importance for the success of reproductive biotechnologies Acta Scientiae Veterinariae 38 337 345
- RukkwamsukT. KruipT.A.M MeijerG.A.L. WensingT. 1999 Hepatic fatty acid composition in periparturient dairy cows with fatty liver induced by intake of a high energy diet in the dry period J. Dairy Sci 82 280 287
- TanakaT. AkaboshiN. InoueY. KamomaeH. KanedaY. 2002 Fasting-induced suppression of pulsatile luteinizing hormone secretion is related to body energy status in ovariectomized goats Anim. Reprod. Sci 72 185 196
- TanakaT. FujiwaraK.I. KimS. KamomaeH. KanedaY. 2004 Ovarian and hormonal responses to a progesterone-releasing controlled internal drug releasing treatment in dietary-restricted goats Anim. Reprod. Sci 84 135 146
- TanakaT. YamaguchiT. KamomaeH. KanedaY. 2003 Nutritionally induced body weight loss and ovarian quiescence in Shiba goats J. Reprod. Develop 49 113 119
- WaltnerS.S. McnamaraJ.P. HillersJ.K. 1993 Relationships of body condition score to production variables in high producing Holstein dairy cattle J. Dairy Sci 76 3410 3419
- WebbR. GarnsworthyP.C. GongJ.G. ArmstrongD.G. 2004 Control of follicular growth: Local interactions and nutritional influences J. Anim. Sci 82 63 74
- WoadK.J. PearsonS.M. HarrisS.E. GersakK. ShellingA.N. 2009 Investigating the association between inhibin alpha gene promoter polymorphisms and premature ovarian failure Fertil. Steril 91 62 66