Abstract
As its Greek name suggests, neuroglia is the glue of the nervous system, simply referred to as non-neuronal cells, with supporting functions for neurons in the central nervous system (CNS) of mammalian and lower vertebrates. Recent advances in stem cell biology have redefined new functions. In fish, neuroglial cells have been described as supporting cells, and as such require further investigation. Approximately 283 cell lines have been obtained from fish worldwide, yet none from the brain of Sparus aurata, neither in cell lines nor as primary culture. We describe a novel reproducible in vitro neuroglial marine model for establishing primary neuroglial cell cultures by dissociating the whole brain of seabream juveniles. Proliferating neural stem cells produced alongside three generating lineages (neuronal precursor cells, astroglial precursor cells and oligodendroglia precursor cells) developed neurons, astrocytes and oligodendrocytes, respectively. The radial glia, finely described by morphological studies and immunochemical antigen expression, showed a particular spatial distribution, giving rise simultaneously both to astrocytes and neuronal precursors within a highly proliferative assemblage. Radial glial cells were assessed by glial fibrillary acidic protein (GFAP) and vimentin reactivity, astrocytes by GFAP, neurons by the neuron-specific markers for ubiquitin carboxy-terminal hydrolase 1 (UCHL1) and intermediate filament associated protein (NF). Myelinating oligodendrocytes were immunostained with anti-myelin basic protein (MBP) and anti-O4. Findings suggest that seabream neuroglial cells at 3-4 weeks of culture proliferation acquire neuroglial differentiation and oligodendrocyte maturation with myelination, suggesting that mixed neuroglial cultures accelerate maturation of oligodendrocytes and regenerate CNS injury in fish.
Introduction
Over the last decade, it has been understood that neuroglia may play a starring role in the development of the nervous system of mammals, as well as taking part in more sophisticated neural processes, such as synaptic plasticity, functional maintenance of neurons, regulation of central nervous system (CNS) regeneration, and synaptogenesis (Kimelberg, Citation2004, Citation2010; Hinsch and Zupanc, Citation2006; Jeserich et al., Citation2008; Nishiyama et al., Citation2009; Zupanc et al., Citation2012). Cell lines and primary cell cultures represent an essential tool for investigation of molecular genetics, genotoxicity and environmental toxicology, as well as in immunology, physiology, virology, and tumorigenesis (Monod et al., Citation1998; Bols et al., Citation2001; Villena, Citation2003; Schirmer, Citation2006). The complete replacement of experimental animals has been specifically recommended, and new cell-based models have been improved in recent years due to several reasons, including the high costs involved in carrying out in vivo experiments, as well as the sacrifice of a huge number of animals (ICCVAM, Citation2002; Marques et al., Citation2011). Teleost fish cell lines have been developed from a wide range of tissues and organs, allowing the study and monitoring of diseases, especially those of viral origin, known to be organor tissue-specific (Lakra et al., Citation2011). In particular, some authors refer to the need to establish cell cultures from brain due to the spread of neural viral diseases, paralleled in the last few years with the intensification of aquaculture systems (Chi et al., Citation2005; Parameswaran et al., Citation2006; Cutrín et al., Citation2007). In fact, the aquatic environment may be contaminated by chemicals that could directly or indirectly affect the neuronal functions of living organisms (Walum et al., Citation1990). Therefore, the development of brain cell cultures from fish could provide a reliable model that is rapid and cost effective for neurotoxicity screening or to be used in test batteries for risk assessment (Fröjdö et al., Citation2002). Also, in fish, in vitro brain culture is nowadays used in the morphological characterisation and examination of the CNS functional processes that are usually hidden by the cranial structure (Tomizawa et al., Citation2001). In a recent review on the development of fish cell lines, Lakra et al. (Citation2011) reported that approximately 283 cell lines have been established from finfish worldwide. Among these, only 4 were developed from brain tissue: 3 from marine (Lai et al., Citation2001; Wen et al., Citation2008; Ku et al., Citation2009) and one from brackish water (Servili et al., Citation2009) fish. So far, there have been no reports on cell lines or primary cultures developed from the brain of seabream. With regard to the primary cell cultures obtained from fish CNS, out of two were developed from freshwater fish species, such as rainbow trout (Salmo gairdneri) (Tocher and Sargent, Citation1990; Tocher and Wilson, Citation1990; Fröjdö et al., Citation2002) and goldfish (Carassius auratus) (Houalla and Levine, Citation2003), and one from a marine species, such as turbot (Scophthalmus maximus) (Tocher, Citation1993). The culture methods mostly applied in the past were based on tissue slices (Annis et al., Citation1990; Stoppini et al., Citation1991), dispersed cells and embryos (Pitt and Carney, Citation1999), and perfused whole brain (de Curtis et al., Citation1998; Federico and MacVicar, Citation1996), which are very complex techniques (Tomizawa et al., Citation2001). Tocher and Wilson (Citation1990) described for the first time an alternative method for isolation and culturing of astrocytes from rainbow trout. They prepared a dissociated cell suspension from young fish brains by mechanical sieving through nylon gauzes, and cultured the cells in polylysinetreated plastic flasks at 22°C. Recently, Servili et al. (Citation2009) established a long-term culture of neural stem cells from adult seabass through mechanical dispersion of the brain by pipetting, and enzyme digestion using trypsin. These authors characterised a population of neural stem cell progenitors by immunocytochemistry, using various neural antibodies (Servili et al., Citation2009). In all vertebrates, neuroglia is composed of three main cell types: ependymocytes, astrocytes and oligodendrocytes, even though its identification and classification, particularly in non-mammalian species, has long been a subject of discussion (Cuoghi and Mola, Citation2009). Only in the last century, the establishment of new glial animal models and specific mRNA probes have allowed us a better understanding of macroglia morphology, function and immunocytochemical characteristics. Compared to mammals, several differences have been reported in teleost glia cells regarding the evolutive radiation, and distribution in the different parts of the CNS (Cuoghi and Mola, Citation2009). Interestingly, in adult fish, the ability to generate new neurons after injury to several neural zones has been demonstrated, and this at a rate of proliferation much higher than that found in the brain of adult mammals (Hinsch and Zupanc, Citation2006). In addition, the major cells represented in fish neuroglia include radial glia and tanycytes (Arochena et al., Citation2004; Lazzari and Franceschini, Citation2004). Tanycytes are special ependymal cells, bipolar, with deeply elongated and extending processes; they are thought to be genealogical descendants of radial glial cells but do not develop astrocyte (Wen et al., Citation2008).
Tomizawa and colleagues (Citation2001) have cultured ex vivo the whole brain from adult zebrafish, without perfusion but simply by placing it on a sterile porous membrane. They demonstrated the preservation of Purkinje neurons also in fish by the use of monoclonal antibobody (M1), staining large neurons with huge dendrites in the molecular layer. Furthermore, the zebrafish has recently been identified as an additional valuable model organism for the study of myelination, and potential remyelination therapies, as many of the molecular and cellular mechanisms underlying gliogenesis and myelination were found conserved in this fish (Monk and Talbot, Citation2009; Larson et al., Citation2010). Other studies on zebrafish mutants have led to the identification of genes involved in glial development and myelin sheath formation (Pogoda et al., Citation2006; Woods et al., Citation2006; Voas et al., Citation2007; Lyons et al., Citation2009; Monk and Talbot, Citation2009; Larson et al., Citation2010). Although mechanisms involved in spacing of oligodendrocytes, before and during myelinating processes, are not known, remodelling and migration pathways seem to be influenced by the contact with neighbouring oligodendrocyte progenitor cells (OPCs) (Kirby et al., Citation2006). Nonetheless, by taking into account the beststudied animal models currently available, rat and zebrafish, an in vitro myelination model derived from CNS remains to be established. From the point of view of in vitro models, new challenges have been made to overcome this. Recently, a novel modified neuron-oligodendrocyte co-culture model was described in rat to investigate the mechanisms of CNS-related myelin deficits (Pang et al., Citation2012), whereas in zebrafish, an ex vivo system was applied by using time-lapse confocal microscopy (Kirby et al., Citation2006), zebrafish OPCs in this study were found to extend continuously and retract numerous filopodium-like processes as they migrate to settle into their final positions in proximity to axons.
Lastly, additional data have been gathered from in vivo or ex vivo neurosusceptibility to xenobiotic studies in marine species, but never with in vitro systems. For instance, several authors have investigated the modulation of Aflatoxin B1 (AFB1) metabolism in salmonid fish, such as rainbow trout, coho salmon, as well as in zebrafish and channel catfish almost in vivo (Santacroce et al., Citation2008). Only recently, AFB1 adverse effects were studied on primary monolayer cultures of Sparus aurata hepatocytes, but metabolic and neurotoxic effects in gilthead seabream brain remain to be accomplished, due to the need for a model that closely replicates in vivo conditions of seabream CNS (Centoducati et al., Citation2009b; Santacroce et al., Citation2011a, Citation2011b).
Therefore, this present study aims to isolate and cultivate cells from the brain of young specimens of gilthead seabream using an innovative method, which consists in the characterisation and recognition of the different types of neuroglial cells by phase contrast microscopy and immunocytochemical analysis. This work aims to: i) describe the morphology, functions and types of neuroglial cell in the seabream primary culture; ii) examine the sequence of differentiation of glial cell types during long-term culture; and iii) compare the fish glial model with those already reported in mammalian CNS.
Materials and methods
Animals
Gilthead seabream (Sparus aurata) juveniles of 35±5 g mean body weight (n=50) were purchased from high-quality stock bred at a commercial fish farm (Panittica Pugliese, Brindisi, Italy) and transported to the laboratory of Aquaculture at the Department of Public Health and Animal Science (Faculty of Veterinary Medicine, University of Bari, Italy) in two oxygenated transport tanks (60 L/each) that were filled with oversaturated natural seawater (O2 at 10 mg L–1, salinity at 37.5%, temperature at 18±1°C) at a density of 14.6 kg/m3. Upon arrival at the laboratory, after no more than 90 min, fish were anaesthetised by immersion in a tank with seawater plus 3-aminobenzoic acid ethylester methanesulfonate (MS222, 0.02%; Sigma-Aldrich) for all handling and fine dissection procedures. They were then taken out individually, wiped with 70% ethanol and rapidly sacrificed by spinal cord transection with a scalpel blade for decapitation. For each sampling, only healthy individuals were selected after anatomopathological examination. Whole brain tissue samples (weighing approx. 0.1-0.2 g/brain) were removed aseptically, pooled and collected in a sterile pre-cooled Hank’s Balanced Salt Solution (HBSS, Sigma-Aldrich) (without Ca2+ and Mg2+) supplemented with 10 mM HEPES, 0.5 mM EDTA, 25 mM NaHCO3, 200 U mL–1 penicillin, 200 μg mL–1 streptomycin, 200 μg mL–1 amphotericin B and 100 μg mL–1 gentamicin (Washing Solution, WS) (Centoducati et al., Citation2009a; Santacroce et al., Citation2011c). Unless specified, all chemicals were from Sigma-Aldrich-Aldrich Ltd., Milan, Italy.
Isolation and preparation of neuroglial cell cultures
Primary neuroglial cultures were rapidly isolated from freshly enucleated brains. In brief, the cerebral hemispheres of juvenile seabream were removed with sterile surgical blade and scissors, and carefully cleaned of meninges and blood vessels, in a laminar vertical flow microbiology safety hood. After three washings with three volumes of HBSS (WS), brains were crushed by pressing a glass pestle into a stainless-steel sieve with 380 μm mesh for mechanical disruption. Cell suspension was filtered and collected with Digestion Medium (DM) lacking enzymes (7 mM CaCl2, 200 U mL–1 penicillin, 200 μg mL–1 streptomycin, 200 μg mL–1 amphotericin B/100 μg mL– 1 gentamicin, 10 mM HEPES, 25 mM NaHCO3 in Leibovitz’s L-15). The homogenate was then extracted by adding a cocktail of four enzymes including 0.05% Collagenase Type IV, 0.03% Hyaluronidase Type IV-S, 0.3% Dispase Type II, 0.03% DNase Type I for 20 min dissociations at 20°C to the homogenate in DM. The enzyme digestion was blocked adding Leibovitz’s L-15 medium supplemented with 10% foetal bovine serum (FBS; BioWhittaker, Lonza Walkersville Inc., Italy). The digestion mixture was filtered through 104 μm and 60 μm stainless-steel filters, supernatant was discarded, and the pellet was resuspended in cold 1X phosphatebuffered saline (PBS) 5% foetal calf serum (FCS) and centrifuged twice at 150 g for 10 min at 4°C. The white pellet was recovered, washed and dissociated by adding 0.45 μg mL-1 DNase I in cold PBS to dissociate cell clumps by Pasteur pipette, then centrifuged at 70 g for 5 min at 4°C. The final collected pellets were resuspended in 10% FBS/L-15 medium and cell number was counted. Viability of cells was estimated by trypan blue exclusion. Brain cell suspension yielded 5.2×107 viable cells g–1 per tissue weight with a viability of 92.8±0.06%.
Neuroglial maintenance
The suspension of purified neuroglia was adjusted to a density of 1x106 cells mL–1 in basal culture medium (BM) consisting of Leibovitz’s L-15 with 2 mM L-glutamine, 10% FBS, 100 U mL–1 penicilin/100 μg mL–1 streptomycin/100 μg mL–1 amphotericine/50 μg mL–1 gentamycin, 1 mM Na Pyruvate, 5 mM D-Glu, 10 mM HEPES, 12 mM NaHCO3, supplemented with 0.05% ITS plus (insulin/transferrin/sodium selenite plus oleic acid/linoleic acid/BSA), 0.01 mM MEM non-essential amino acid (MEM-NEAA; BioWhittaker), 0.01 mM MEMvitamin mix (BioWhittaker) and 0.1 mM ascorbic acid. The BM osmolality was adjusted to seabream serum osmolarity (369 mOsm kg–1) by adding 20 mM NaCl as reported by other authors (Pagés et al., Citation1995; Lopez-Castejon et al., Citation2008).
Cells were cultured in complete Leibovitz’s L-15 (BM) and seeded in 0.01% collagen type Icoated plates and flasks at a density of 3×104 cells/cm2, respectively, into 25 cm2 Falcon Primaria™ culture flasks for morphological characterisation of primary cell types by phase contrast, and on 12-well plates Falcon BD Biosciences (BD Biosciences, Sismed Srl, Italy) for immunophenotypisation by immunofluorescence. Firstly, cells were allowed to attach for 12 h, then fresh BM was added, replaced every 48 h thereafter. Cells were cultured in a refrigerate incubator in humidified atmosphere at 97% air/3% CO2 at 18°C (CO2-170 Innova, New Brunswick Scientific, PBI, Milano, Italy). Cultures were used after approximately 3-4 weeks when the cells had become confluent monolayers. To obtain a glial cell-enriched culture, after the first week SaGliPs were cultured in enriched differentiation medium (EDM) consisting of L-15 BM supplemented with 0.01 μg mL–1 epidermal growth factor (EGF), 0.2 μg mL–1 hydrocortisone, 10 mM glucose, 10 μg mL–1 insulin and 4 mM glutammine. For assessment of cytostructural features, phase contrast microscopy images were taken daily on adherent cultured SaGliPs with a CCD camera on a Motic AE31 inverted epi-fluorescence microscope.
Antibodies
For indirect immunolabelling, the following polyclonal (pAb) or monoclonal (mAb) primary antibodies were used at the dilutions indicated: mAb mouse IgG anti-glial fibrillary acidic protein (GFAP), (G3893, clone No. G-A-5, Sigma-Aldrich, 1:500); pAb rabbit anti-UCHL1 (HPA005993, Sigma-Aldrich, 1:1000); mAb mouse IgM Anti-Vimentin, (V5255, clone VIM-13.2, Sigma-Aldrich, 1:200); pAb rabbit IgG anti-neurofilament 200 (NF), (N4142, Sigma-Aldrich, 1:200); pAb rabbit Anti-CD44 (HPA005785, Sigma-Aldrich, 1:1000); pAb rabbit Anti-MBP (M3821, Sigma-Aldrich, 1:200); mAb mouse IgM Anti-O4 (O7139, Sigma-Aldrich, 1:500).
The secondary antibodies used were the followings: goat anti-mouse IgG TRITC conjugate (T7028, Sigma); goat anti-rabbit IgG1 fluorescein isothiocyanate (FITC)-conjugate (F6005, Sigma); goat anti-mouse IgG FITC conjugate (F0257, Sigma); goat anti-rabbit IgG-FITC (F9887) at a 1:50 dilution for 45 min at RT. All primary and secondary antibodies were diluted in PBS/3% BSA.
For direct immunolabelling, the following monoclonal (mAb) antibody was used at the dilutions indicated: mAb mouse anti-proliferating cell nuclear antigen (PCNA) FITC-conjugate (F0167, clone PC10, Sigma-Aldrich, 1:100).
Microscopy analysis
Bright field and fluorescence microscopy analysis was performed by a Motic AE31 epifluorescent inverted microscope, equipped with DAPI/TRITC/FITC fluorescence filter cube set (DAPI/Hoechst set: Exciter D350/50x/Emitter D460/50m, FITC/RSGFP/Fluo 3/Dio Acridine set: Ex. D480/30x/Em. D535/40m, TRITC/DiI/Cy3 set: Ex. D540/25x/Em. D605/55m). Digital image capture was performed by Moticam 3000C Cooled CCD digital colour camera (3.3 Megapixel, 1/2” CCD), capture system in origin Live Cam 1.0 (32-32) and Motic Images Advanced (v. 3.2) acquisition software (Motic, Seneco, Milano, Italy). Image analysis and assemblage were performed with Motic Images Advanced (v. 3.2) software (Motic, Seneco) and by using the Adobe Photoshop 8.0 (Adobe, Inc.) software for reunion from different filters.
Indirect immunofluorescence
Cells were rinsed three times with PBS, fixed with 4% paraformaldehyde/PBS for 15 min at RT, washed twice with PBS and permeabilised with 0.2% Triton X-100/PBS for 10 min at RT. After three washes with PBS each of 5 min, cells were blocked in 3% BSA/PBS for 30 min, then incubated in primary antibodies diluted in blocking buffer for 60 min at RT. For indirect labelling, after three PBS washes, cells were incubated with the appropriate secondary antibody for 60 min at RT. After another three PBS washes, nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI; 1.5 μg ml–1) for 10 min at RT. To avoid photobleaching of fluorescent dyes, stained cells were embedded with anti-fade mounting medium PBS/glycerine/1,4-diazabicyclo[2.2.2]octane (90% Glycerol/100 mg mL–1 DABCO/PBS), adding the anti-fade directly onto the wells before microscope visualisation. As a negative control, cells were stained with the same procedure without the primary antibody labelling.
Results
Morphological characterisation of neuroglial cell types by phase contrast microscopy
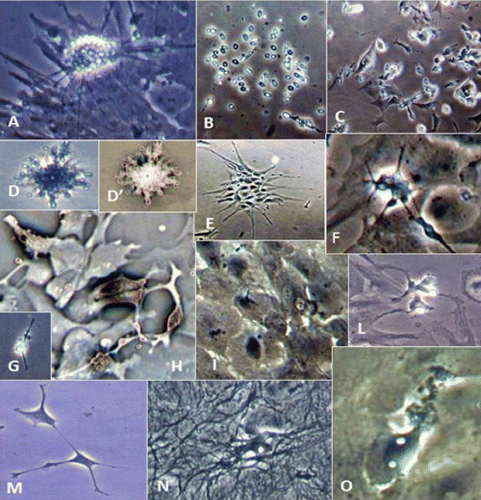
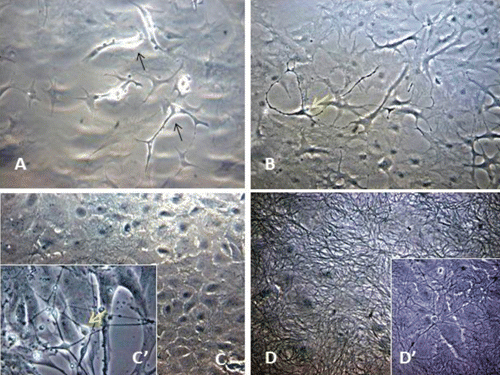
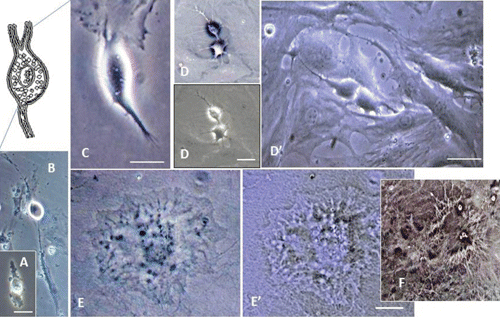
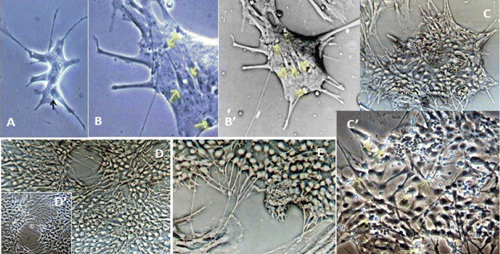
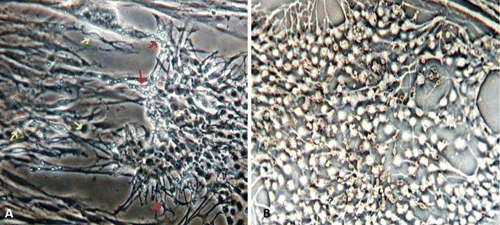
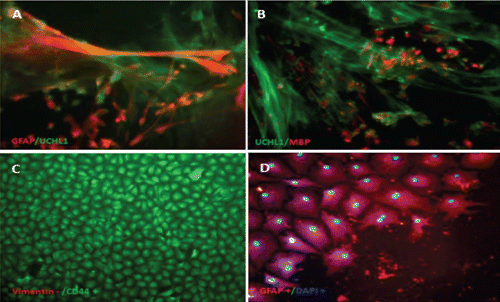
Microscopy images indicating development and differentiation of Sparus aurata neuroglial cells (SaGliPs) in primary culture are shown in . Soon after 24 h of plating, SaGliPs cells showed a typical rounded neuro-staminal morphology (). Neurons, astrocytes and oligodendrocytes in the SaGliPs cultures were morphologically identified by inverted phase contrast microscopy, and analysed using acquisition software (Motic, Seneco, Milan, Italy) (). Overall, during the first 2 weeks of culture, the microscopic identification of cell morphology confirmed that the population in culture clearly enclosed the following cell types: i) the radial glia progenitors (RGPs) () visualised in clusters with high proliferative capacity, polygonal and irregular in shape when adherent; ii) the staminal neural cells (SNc) with the characteristic ovoid shape at Day 1, and bipolar elongation from Day 2 ( and ) to Day 6 ( and ); iii) neuron precursor cells (NPC), which generate neurons but not glia (); iv) pro-oligodendrocyte precursors (POPC), as bipolar cells with a globoid cell body in the first 2 days (); v) oligodendrocyte precursors (OPCs) as a branched polygonal cells in 1-week old cultures (); vi) astrocyte precursors (). Representative images of oligodendrocyte lineage maturation are shown in . Immature oligodendrocytes exhibited a spindle-shaped bipolar morphology similar to those of mammalian species (). The round cell body of mature oligodendrocytes appeared enriched of cytoplasmic granules, and a dense filamentous meshwork in the peripheral areas often enwrapping other neuroglial processes (, and ). Within 1 week after plating, the radial progenitor cells extend their basal process in a radial direction all around the colony, thus gaining the typical form of radial glial cells (, and ). Inside the radial glia, a homogeneous population of cells with a typical neuronal perikaryal appearance started to proliferate directly from the foot of cone-shaped radial cell, thus forming a cluster of highly proliferative cells (, , and ). Soon after being generated, those newly formed cells (that reside in the proliferative colony) leave the proliferative zones migrating horizontally (). During the post-mitotic migration, post-mitotic neurons migrate along the radial glial processes giving a secondary proliferative population, until they reach the border of the colony where the longer and thicker radial processes arborise to fill the empty layer (, and ). The small new cells, formed all around the radial gliall colony, acquired a spherical refractive body and bipolar spindle shape ( and ).
Their characteristic formation and distribution suggested that they had to evolve to a different lineage, and to migrate independently of radial glial fibres. Next, as newborn neurons grew, long and branched neurites could be observed at the beginning of week 2, whereas the convolution and length of neurites increase with time in culture ( and ), reaching their maximum at 2 months of culture ( and ).
The astrocytes displayed a heterogeneous phenotype, including highly branched fibrous astrocytes and polygonal stellate cells ( and and and ); perhaps polygonal astrocytes progress morphologically in stellate shape cells during differentiation. After 2 weeks of culture, SaGliPs in enriched medium started acquiring features of differentiation, such as the typical appearance of mature astrocyte forming monolayer and generating neurons ( and ). Next, after 4 weeks of culture, neurons grown on the monolayer of astrocytes exhibited dense dendrite and neurite branching ( and ). Finally, microglial cells were the smallest of the glial cells found, and showed an elongated and flattened cell body, with few short fine highly branched processes. However, these cells were rarely observed on the astrocyte monolayer ().
Immunofluorescence staining and lineage identification
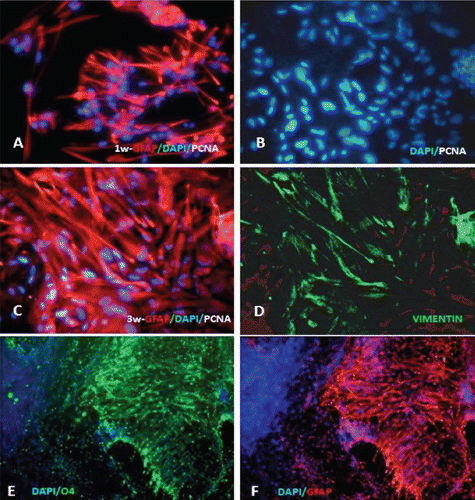
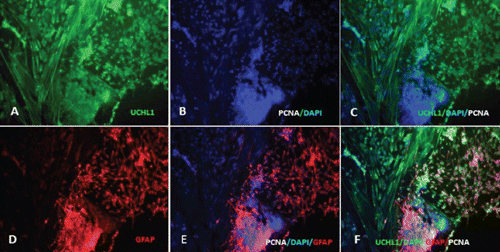
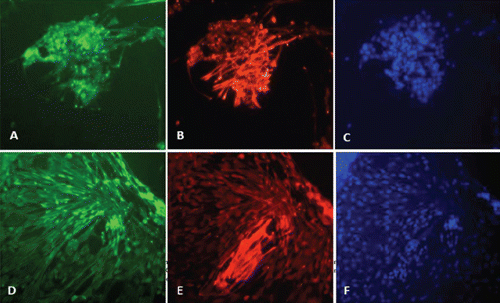
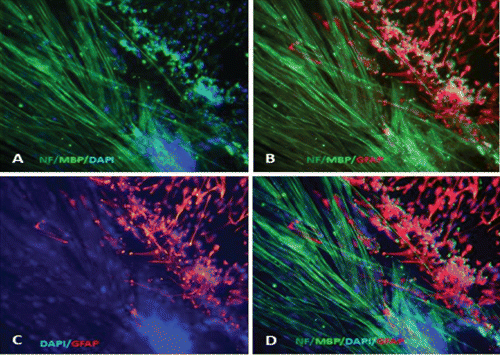
Characterisation of the timing and levels of expression of neuroglial markers was performed by phenotypisating cells in immunofluorescence. Cells within the radial glial colonies were actively proliferating, as revealed by proliferating cell nuclear antigen (PCNA) labelling in indirect immunofluorescence (). PCNA is a cell cycle dependent intranuclear protein that assists the delta-DNA polymerase during DNA replication whereby its expression is considered a marker of DNA synthesis, being detectable in G1/S phase (Boldogh et al., Citation2001). In cultures of SaGliPs radial glia, a consistent number of replicating nuclei were detectable in the central region in relation to mature peripheral areas (, , , ). Cells within the central region showed a strong fluorescence signal for DAPI and PCNA (). Within the first week of culture, the early replicating cells grown inside the radial colonies began to differentiate into mature neurons along the boundary of the island, and further completed maturation from 2 weeks up to 2 months in the long-term culture ( and ). To differentiate between the two radial glial populations (clonally-related glial cells and neurons), the GFAP labelling was combined with immunofluorescence for ubiquitin carboxyterminal hydrolase 1 (UCHL1), a highly specific marker to neurons. Considering the specific localisation of UCHL1 in the brain, UCHL1 antibodies are specifically used to identify neurons and their processes, as this is the most abundant protein in the brain and is not expressed in non-neuronal cells (Lewis et al., Citation2010). The areas GFAP positive in the clusters were UCHL1 negative, whereas the newly formed clusters were UCHL1 positive and PCNA/DAPI positive, thus suggesting that the active proliferating cells were derived from radial glia (). Results show that in cultures of SaGliPs, radial glial progenitors can generate both neurons and astrocytes in vitro, as revealed by the positive immunostaining of UCHL1 for neurons and GFAP immunoreactivity for astrocytes, and radial glial cells (); these findings are in agreement with those of Pollard and Conti (Citation2007). At late stages of differentiation, proliferating nuclei persist, as shown by the staining of the vital DNA dye (DAPI) (), whereas the outer cells sustained a progressive differentiation, which occurred following a gradient from the periphery to the centre (). Within 1 month, SaGliPs formed a confluent monolayer of tightly packed polygonal-stellate shaped cells which stained negatively for vimentin but were immunoreactive for the CD44 marker of astrocyte lineage, and strongly expressed the astrocyte marker GFAP ( and ). In contrast, cells of radial glia were characterised by the large colony, elongated spindle spread projections, and showed intense positive immunostaining for both vimentin and GFAP ( and ). Threeweek old neuroglial cultures were positively immunostained for neurofilament (NF), and myelin basic protein (MBP) as a marker for oligodendrocyte functionally active cells and visualised on axons ( and , and ). To visualise axon fibres, neurons were immunostained for NF and cells nuclei were DAPI counterstained for labelling. Oligodendrocyte immunoreactive for MBP produced myelin sheath, as visualised in fragments associated with the axons (, and ). The co-localisation of MBP and GFAP near axons positive for NF demonstrated that immature oligodendrocyte differentiated into myelinating oligodendrocytes, and these colocalise with astrocyte while enwrapping axons (, and ). In our cultures, oligodendrocyte cell bodies showed firstly a bipolar shape (), almost morphologically similar to those in mammals (Nishiyama et al., Citation2009), whereas later in culture oligodendrocyte cell bodies appeared rounded and flattened with abundant branching processes ( and and , and ).
Discussion
An ideal neuroglial model should support the development of all potential staminal lineages, allowing well-balanced growth, maturation and communication of cultured nervous cells without preferentially expanding only one of them (Pang et al., Citation2012). From this point of view, mixed neuroglial cultures represent a suitable environment to ensure maturation of myelinating oligodendrocytes, as well as proliferation and differentiation of supporting astrocytes and neurons, thus showing the potential to increase CNS regeneration.
However, an in vitro neuroglial marine model derived from the CNS of gilthead seabream has never been established, neither in cell lines nor as primary culture. In order to achieve this, a mild tissue extraction and a well-designed culture medium remain fundamental. Indeed, during morphogenesis and development of nervous system, growth factors represent crucial molecules in the neuron-glia communication, exerting mitogenic and trophic effects (Bramanti et al., Citation2007). Aloisi et al. (Citation1987) had previously shown that the astroglial-conditioned medium (ACM), collected from epidermal growth factor (EGF) and insulin-like growth factor-I (IGF-I)-treated astrocytes, is important for the development and maturation of cultured nervous cells, as it can modulate neuron-glia interactions. In addition, EGF and insulin (INS) were found to be powerful mitogenic or trophic factors for both neurons and astrocytes (Casper et al., Citation1994; Morrison et al., Citation1988). EGF was found to stimulate proliferation and differentiation of cultured rat primary astrocytes (Spina-Purrello et al., Citation2002; Bramanti et al., Citation2007), whereas INS plus EGF induce astroglial cell differentiation according to the stage of culture (Zelania et al., Citation2000). Furthermore, the high concentration of insulin in enriched medium has been shown to promote oligodendrocyte survival and proliferation via activation of IGF-1 receptor (D’Ercole et al., Citation1996; Ebner et al., Citation2000).
By modifying culture medium (BM) in enriched differentiation medium (EDM), we also experimented both neurotrophic and neurite-promoting activities (i.e. axonal sprouting) that are important for long-term culturing of neurons (Beckh et al., Citation1987). According to Pang and colleagues (Citation2012), the combination of two environmental factors such as the addition of a highly enriched medium and the presence of growth factors secreted by neurons and glia may enhance the initial survival and differentiation of cells. It has also been suggested that these newly produced growth factors may support myelination of oligodendrocytes that also provide trophic signals to nearby neurons (Du and Dreyfus, Citation2002). Another positive feedback of the secreted factors is that they can sustain the myelination process by affecting oligodendrocyte differentiation (Xiao et al., Citation2009). Our results strongly uphold the usefulness of EDM medium, (selectively enriched with INS plus EGF, high glucose and glutamine) in supporting neuronal growth, glial and oligodendrocyte differentiation. In our cultures, EDM produced high levels of neurite sprouting with widespread evidence of myelination along nervous fibres. During 2 months of long-term culture, EDM enhanced proliferative activity in radial glia producing astrocyte, resulting also in neuronal proliferation without any apparent signal of degeneration. Recently, Wen et al. (Citation2008) isolated and characterised a neural progenitor cell line from adult tilapia (Oreochromis sp.) brain, reporting that the cells co-expressed GFAP and vimentin, and showed the appearance of radial glial cells. In these primary cultures, macrophages and neurons as well as dendritic melanocytes and multipolar cells appeared frequently in small numbers. A confluent monolayer usually consisted of slender fibroblast-like cells that grew in parallel, but islands of epithelioid cells were also occasionally observed.
In fish CNS, as previously reported by Wen and colleagues (Citation2008), in contrast to mammals, radial glial cells are the most abundant type of neuroglial cells. As in mammalian CNS, radial glial cells are clearly defined by their long radial processes that contain vimentin and GFAP. The mammalian neocortex contains embryonic precursor cells capable of generating both neurons and glia. Therefore, the concept that proliferative radial glia generate neurons was unquestionably supported by Noctor et al. who found that radial glia remained mitotically active throughout neurogenesis, developing astrocytes after neuronal migration was completed (Noctor et al., Citation2001). Our results agree well with those previously reported, as we also found that neurons, in order to reach their proper destination, were guided by radially directed processes of their founder cells, suggesting that the radial glia promotes a robust control in fish brain development by regulating neuronal migration. On the other hand, the formation of cell clustering, which occurs during the development of mammalian neocortex, reveals the existence of amassed dividing cells which are clonally related (Cai et al., Citation1997; Noctor et al., Citation2001), and closely resembles what we found in clones of mitotic radial glia and post-mitotic neurons (Pollard and Conti, Citation2007). In our results, the central zone of the colony of radial glia showed an intense granular production of neurons, and this was positively marked with the anti-UCHL1 antibody. This is a highly specific neuron marker, reported to be a member of the ubiquitin carboxy-terminal hydrolase (UCH) family of deubiquitinating enzymes, and it is thought to play a role in protecting neurons from lipid peroxidation (Nagamine et al., Citation2010). During CNS injury and a variety of brain diseases, a persistent release of UCHL1 due to neuronal death was found in CSF, suggesting the potential use of this protein as a biomarker of brain lesions, both in humans and animals (Lewis et al., Citation2010).
The morphology of neuroglial cell bodies from gilthead seabream was clearly defined in brain slices by Nogueira et al. (Citation2010) during a study of the occurrence of neurotoxic signs triggered by domoic acid neurotoxicity. Domoic acid, a harmful algal phycotoxin, is able to induce neuro-excitatory and neurotoxic effects by binding to the brain glutamate receptor. Investigation of this kind of neurotoxicity is feasible through the use of such a novel marine neuroglial model. All the previously cited morphological findings are in accordance with our results, as we developed enriched neuroglial networks in 3-4 week old cultures that resemble the neuropil, which is a dense intricate feltwork of interwoven fine glial processes. In the aforementioned studies, as in our 2-month-old long-term cultures, fibrils cross-interacted with astrocytes and oligodendrocytes, synaptic terminals, axons, and dendrites, and were interspersed among the bodies of the nervous and glial cells, including the connections between glial cells filopodia and axonal branching.
Additional data gathered from neurotoxicity assays in gilthead seabream have revealed that the species neurosusceptibility was investigated exclusively through in vivo systems. For instance, toxicant neurosusceptibility to xenobiotics in marine species has been studied only by performing in vivo exposures or by using brain slices ex vivo, but never using in vitro systems. Again, until now, few marine cell lines have been available for studying the viral neurosusceptibility or host’s vulnerability to virusinduced neurological damage in Mediterranean species. Considering that several lentiviral groups give rise to important dysfunctions in fish CNS, resulting in inflammation and neuronal injury, new in vitro research models for studying the relationship between neuropathology and host response would also be preferred and well received. New lentiviral animal models also allow specific evaluation of viral evolution with host pathogenic responses, and thus represent invaluable tools to widen our understanding of lentivirus neuropathogenesis (Patrick et al., Citation2002).
Conclusions
In conclusion, we present a reliable and reproducible protocol for assembling an in vitro model of marine bony fish CNS. This study shows that the radial glia isolated from the brain of juvenile seabream can be considered a multipotent stem cell complex with a high replicative potential, where the enhanced self-renewal cell ability allowed the generation of neurons, astrocytes and oligodendrocytes. Furthermore, we provide first evidence of involvement of myelin forming olygodendrocytes in the crosstalk either with axonal neurofilaments or with astroglial processes, thus establishing a stable neurofilament network tool for a possible modulation of stimulatory neurotransmitters. The described animal model closely resembles in vivo seabream CNS conditions, and may, therefore, contribute to future studies of pharmaco-toxicology, physiology, neuropathology and neuroinfenction in marine fields. Future challenges using this new model might be to study the discrepancy of myelin formation involved in neurodegenerative diseases, deficiency in neuron-glia interactions or to evaluate the effect of marine neurotoxicants at a cellular and molecular level.
Acknowledgments
The authors would like to thank the Panittica Pugliese S.p.A. for providing the experimental animals. The excellent critical reading of Dr. Rosa Anna Cavallo and Dr. Maria Immacolata Acquaviva, (Institute for Coastal Marine Environment, National Research Council, Taranto, Italy) is gratefully acknowledged. The authors would also like to thank Dr. Selene Pastore, Giovanna Calzaretti and Francesco D’Onghia for their helpful technical support.
References
- AloisiF. AgrestiC. LeviG. 1987. Glial conditioned media inhibit the proliferation of cultured rat cerebellar astrocytes. Neurochem. Res. 12:189-195.
- AnnisC.M. EdmondJ. RobertsonR.T. 1990. A chemically-defined medium for organotypic slice cultures. J. Neurosci. Methods 32:63-70.
- ArochenaM. AnadónR. Díaz-RegueiraS.M. 2004. Development of vimentin and glial fibrillary acidic protein immunoreactivities in the brain of graymullet (Chelon labrosus), an advanced teleost. J. Comp. Neurol. 469:413-436.
- BeckhS. MullerH.W. SeifertW. 1987. Neurotrophic and neurite-promoting activity in astroglial conditioned medium. In: AlthaeusH.H. SeifertW. ( eds.) Glial-neuronal communication in development and regeneration. Springer, Heidelberg, Germany, pp 67-111.
- BoldoghI. MilliganD. LeeM.S. BassettH. LloydR.S. McCulloughA.K. 2001. hMYH cell cycle-dependent expression, subcellular localization and association with replication foci: evidence suggesting replication-coupled repair of adenine: 8-oxoguanine mispairs. Nucleic Acids Res. 29:2802-2809.
- BolsN.C. BrubacherJ.L. GanassinR.C. LeeL.E.J. 2001. Ecotoxicology and innate immunity in fish. Dev. Comp. Immunol. 25:853-873.
- BramantiV. CampisiA. TomassoniD. CostaA. FisichellaA. MazzoneV. DenaroL. AvitabileM. AmentaF. AvolaR. 2007. Astroglial-Conditioned Media and Growth Factors Modulate Proliferation and Differentiation of Astrocytes in Primary Culture. Neurochem. Res. 32:49-56.
- CaiL. HayesN.L. NowakowskiR.S. 1997. Synchrony of clonal cell proliferation and contiguity of clonally related cells: production of mosaicism in the ventricular zone of developing mouse neocortex. J. Neurosci. 17:2088-2100.
- CasperD. RobozG.J. BlumM. 1994. Epidermal growth factor and basic fibroblast growth factor have independent actions on mesencephalic dopamine neurons in culture. J. Neurochem. 62:2166-2177.
- CentoducatiG. SantacroceM.P. ConversanoM.C. CrescenzoG. 2009a. Biotechnological process for isolation of hepatocytes from marine organisms. Bulletin 2009/39, European Patent Office Publ., Paris, France.
- CentoducatiG. SantacroceM.P. LestingiA. CasalinoE. CrescenzoG. 2009b. Characterization of the cellular damage induced by Aflatoxin B1 in sea bream (Sparus aurata Linnaeus, 1758) hepatocytes. Ital. J. Anim. Sci. 8(Suppl.2):848-850.
- ChiS.C. WuY.C. ChengT.M. 2005. Persistent infection of betanodavirus in a novel cell line derived from the brain tissue of barramundi Lates calcarifer. Dis. Aquat. Org. 65:91-98.
- CuoghiB. MolaL. 2009. Macroglial cells of the teleost central nervous system: a survey of the main types. Cell Tissue Res. 338:319-332.
- CutrínJ.M. DopazoC.P. ThiéryR. LeaoP. OlveiraJ.G. BarjaJ.L. BandínI. 2007. Emergence of pathogenic betanodaviruses belonging to the SJNNV genogroup in farmed fish species from the Iberian Peninsula. J. Fish. Dis. 30:225-232.
- D’ErcoleA.J. YeP. CalikogluA.S. Gutierrez-OspinaG. 1996. The role of the insulin-like growth factors in the central nervous system. Mol. Neurobiol. 13:227-255.
- de CurtisM. BiellaG. BuccellatiC. FolcoG. 1998. Simultaneous investigation of the neuronal and vascular compartments in the guinea pig brain isolated in vitro. Brain Res. Protoc. 3:221-228.
- DuY. DreyfusC.F. 2002. Oligodendrocytes as providers of growth factors. J. Neurosci. Res. 68:647-654.
- EbnerS. DunbarM. MckinnonR.D. 2000. Distinct roles for PI3K in proliferation and survival of oligodendrocyte progenitor cells. J. Neurosci. Res. 62:336-345.
- FedericoP. MacVicarB.A. 1996. Imaging the induction and spread of seizure activity in the isolated brain of the guinea pig: the roles of GABA and glutamate receptors. J. Neurophysiol. 76:3471-3492.
- FröjdöE.M. WesterlundJ. IsomaaB. 2002. Culturing and characterization of astrocytes isolated from juvenile rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Phys. A 133:17-28.
- HinschK. ZupancG.K.H. 2006. Isolation, cultivation, and differentiation of neural stem cells from adult fish brain. J. Neurosci. Meth. 158:75-88.
- HouallaT. LevineR.L. 2003. The isolation and culture of microglia-like cells from the goldfish brain. J. Neurosci. Meth. 131:121-131.
- ICCVAM, 2002. Expert panel evaluation of the validation status of in vitro test methods for detecting endocrine disruptors. Available from: http://iccvam.niehs.nih.gov
- JeserichG. KlempahnK. PfeifferM. 2008. Features and functions of oligodendrocytes and myelin proteins of lower vertebrate species. J. Mol. Neurosci. 35:117-126.
- KimelbergH.K. 2004. The problem of astrocyte identity. Neurochem. Int. 45:191-202.
- KimelbergH.K. 2010. Functions of mature mammalian astrocytes: a current view. Neuroscientist 16:79-106.
- KirbyB.B. TakadaN. LatimerA.J. ShinJ. CarneyT.J. KelshR.N. AppelB. 2006. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci. 9:1506-1511.
- KuC.C. TengY.C. WangC.S. LuC.H. 2009. Establishment and characterization of three cell lines derived from the rockfish grouper Epinephelus quoyanus: use for transgenic studies and cytotoxicity testing. Aquaculture 294:147-151.
- LaiY.S. MuraliS. ChiuH.C. JuH.Y. LinY.S. ChenS.C. GuoI.C. FangK. ChangC.Y. 2001. Propagation of yellow grouper nervous necrosis virus (YGNNV) in a new nodavirus susceptible cell line from yellow grouper, Epinephelus awoara (Temminck & Schlegel), brain tissue. J. Fish. Dis. 24:299-309.
- LakraW.S. SwaminathanT.R. JoyK.P. 2011. Development, characterization, conservation and storage of fish cell lines: a review. Fish. Physiol. Biochem. 37:1-20.
- LarsonT.A. GordonT.N. LauH.E. ParichyD.M. 2010. Defective adult oligodendrocyte and Schwann cell development, pigment pattern, and craniofacial morphology in puma mutant zebrafish having an alpha tubulin mutation. Dev. Biol. 346:296-309.
- Lazzari M. Franceschini V. 2004. Glial fibrillary acidic protein and vimentin immunoreactivity of astroglial cells in the central nervous system of the African lungfish, Protopterus annectens (Dipnoi: Lepidosirenidae). J. Morphol. 262: 741-749. doi:10.1002/jmor.10274
- LewisS.B. WolperR. ChiY-Y. MiraliaL. WangY. YangC. ShawG. 2010. Identification and preliminary characterization of ubiquitin C terminal hydrolase 1 (UCHL1) as a biomarker of neuronal loss in aneurysmal subarachnoid hemorrhage. J. Neurosci. Res. 88:1475-1484.
- Lopez-CastejonG. SepulcreM.P. MuleroI. PelegrinP. MeseguerJ. MuleroV. 2008. Molecular and functional characterization of gilthead seabream Sparus aurata caspase-1: the first identification of an inflammatory caspase in fish. Mol. Immunol. 45:49-57.
- LyonsD.A. NaylorS.G. ScholzeA. TalbotW.S. 2009. Kif1b is essential for mRNA localization in oligodendrocytes and development of myelinated axons. Nat. Genet. 41:854-858.
- MarquesA. LourençoH.M. NunesM.L. RoseiroC. SantosC. BarrancoA. RainieriS. LangerholcT. CencicA. 2011. New tools to assess toxicity, bioaccessibility and uptake of chemical contaminants in meat and seafood. Food Res. Int. 44:510-522.
- MonkK.R. TalbotW.S. 2009. Genetic dissection of myelinated axons in zebrafish. Curr. Opin. Neurobiol. 19:486-490.
- MonodG. DevauxA. ValotaireY. CravediJ.P. 1998. Primary cell cultures from fish in ecotoxicology. In: BraunbeckT. HintonD.E. StreitB. ( eds.) Fish ecotoxicology. Birkhauser Ed., Basel, Switzerland, pp 39-60.
- MorrisonR.S. KeatingR.F. MoskalJ.R. 1988. Basic fibroblast growth factor and epidermal growth factor exert differential trophic effects on CNS neurons. J. Neurosci. Res. 21:71-79.
- NagamineS. KabutaT. FurutaA. YamamotoK. TakahashiA. WadaK. 2010. Deficiency of ubiquitin carboxy-terminal hydrolase-L1 (UCH-L1) leads to vulnerability to lipid peroxidation. Neurochem. Int. 57:102-110.
- NishiyamaA. KomitovaM. SuzukiR. ZhuX. 2009. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 10:9-22.
- NoctorS.C. FlintA.C. WeissmanT.A. DammermanR.S. KriegsteinA.R. 2001. Neurons derived from radial glial cells establish radial units in neocortex. Nature 409:714-720.
- NogueiraI. Lobo-da-CunhaA. AlfonsoA. RiveraS. AzevedoJ. MonteiroR. CervantesR. Gago-MartinezA. VasconcelosV. 2010. Toxic effects of domoic acid in the Seabream Sparus aurata. Mar. Drugs 8:2721-2732.
- PagésT. GomezE. SunerO. ViscorG. TortL. 1995. Effects of daily management stress on haematology and blood rheology of the gilthead seabream. J. Fish. Biol. 46:775-786.
- PangY. ZhengB. KimberlyS.L. CaiZ. RhodesP.G. LinR.C. 2012. Neuron-oligodendrocyte myelination co-culture derived from embryonic rat spinal cord and cerebral cortex. Brain Behav. 2:53-67.
- ParameswaranV. ShuklaR. BhondeR. HameedA.S. 2006. Establishment of embryonic cell line from sea bass (Lates calcarifer) for virus isolation. J. Virol. Methods 137:309-316.
- PatrickM.K. JohnstonJ.B. PowerC. 2002. Lentiviral neuropathogenesis: Comparative neuroinvasion, neurotropism, neurovirulence, and host neurosusceptibility. J. Virol. 76:7923-7931.
- PittJ.A. CarneyE.W. 1999. Evaluation of various toxicants in rabbit whole-embryo culture using a new morphologically-based evaluation system. Teratology 59:102-109.
- PogodaH.M. SternheimN. LyonsD.A. DiamondB. HawkinsT.A. WoodsI.G. BhattD.H. Franzini-ArmstrongC. DominguezC. AranaN. JacobsJ. NixR. FetchoJ.R. TalbotW.S. 2006. A genetic screen identifies genes essential for development of myelinated axons in zebrafish. Dev. Biol. 298:118-131.
- PollardS.M. ContiL. 2007. Investigating radial glia in vitro. Prog. Neurobiol. 83:53-67.
- SantacroceM.P. ConversanoM.C. CasalinoE. LaiO. ZizzadoroC. CentoducatiG. CrescenzoG. 2008. Aflatoxins in aquatic species: metabolism, toxicity and perspectives. Rev. Fish Biol. Fisher. 18:99-130.
- SantacroceM.P. NarracciM. AcquavivaI. CavalloR.A. ZacchinoV. CentoducatiG. 2011a. New development in Aflatoxin research: from aquafeed to marine cells. In: Torres-PachecoI. ( ed.) Aflatoxins, vol. 2. InTech, Online Publ., pp 209-234.
- SantacroceM.P. NarracciM. AcquavivaI. ZacchinoV. Lo NoceR. CentoducatiG. CavalloR.A. 2011b. Effects induced in vitro by Aflatoxin B1 on Vibrio fischeri and on primary cultures of Sparus aurata hepatocytes. Chem. Ecol. 27:67-76.
- SantacroceM.P. ZacchinoV. CasalinoE. MerraE. TateoA. De PaloP. CrescenzoG. CentoducatiG. 2011c. Expression of a highly differentiated phenotype and hepatic functionality markers in gilthead sea bream (Sparus aurata L.) long-cultured hepatocytes: first morphological and functional in vitro characterization. Rev. Fish. Biol. Fisher. 21:571-590.
- SchirmerK. 2006. Proposal to improve vertebrate cell cultures to establish them as substitutes for the regulatory testing of chemicals and effluents using fish. Toxicology 224:163-183.
- ServiliA. BufalinoM.R. NishikawaR. de MeloI.S. Muñoz-CuetoJ.A. LeeL.E.J. 2009. Establishment of long term cultures of neural stem cells from adult sea bass, Dicentrarchus labrax. Comp. Biochem. Phys. A 152:245-254.
- Spina PurrelloV. CormaciG. DenaroL. RealeS. CostaA. LalicataC. SabbatiniM. MarchettiB. AvolaR. 2002. Effects of growth factors on nuclear and mitochondrial ADP-ribosylation processes during astroglial cell development and aging in culture. Mech. Age Dev. 123:511-520.
- StoppiniL. BuchsP.A. MullerD. 1991. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Meth. 37:173-182.
- TocherD.R. 1993. Elongation predominates over desaturation in the metabolism of 18:3n-3 and 20:5n-3 in turbot (Scophthalmus maximus) brain astroglial cells in primary culture. Lipids 28: 267-272.
- TocherD.R. SargentJ.R. 1990. Incorporation into phospholipid classes and metabolism via desaturation and elongation of various 14C-labelled (n-3) and (n-6) polyunsaturated fatty acids in trout astrocytes in primary culture. J. Neurochem. 54:2118-2124.
- TocherD.R. WilsonR. 1990. Primary culture of astrocytic glial cells from rainbow trout, Salmo gairdneri L., brain. J. Neurosci. Meth. 33:93-100.
- TomizawaK. KuniedaJ. NakayasuH. 2001. Ex vivo culture of isolated zebrafish whole brain. J. Neurosci. Meth. 107:31-38.
- VillenaA.J. 2003. Applications and needs of fish and shellfish cell culture for disease control in aquaculture. Rev. Fish. Biol. Fisher. 13:111-140.
- VoasM.G. LyonsD.A. NaylorS.G. AranaN. RasbandM.N. TalbotW.S. 2007. AlphaIIspectrin is essential for assembly of the nodes of Ranvier in myelinated axons. Curr. Biol. 17:562-568.
- WalumE. HanssonE. HarveyA.L. 1990. In vitro testing of neurotoxicity. ATLA-Altern. Lab. Anim. 18:153-179.
- WenC.M. LeeC.W. WangC.S. ChengY.H. HuangH.Y. 2008. Development of two cell lines from Epinephelus coioides brain tissue for characterization of betanodavirus and megalocytivirus infectivity and propagation. Aquaculture 278:14-21.
- WoodsI.G. LyonsD.A. VoasM.G. PogodaH.M. TalbotW.S. 2006. Nsf is essential for organization of myelinated axons in zebrafish. Curr. Biol. 16:636-648.
- XiaoJ.H. KilpatrickT.J. MurrayS.S. 2009. The role of neurotrophins in the regulation of myelin development. Neurosignals 17:265-276.
- ZelenaiaO. SchlagB.D. GochenauerG.E. GanelR. SongW. BeesleyJ.S. GrinspanJ.B. RothsteinJ.D. RobinsonM.B. 2000. Epidermal growth factor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NFkB. Mol. Pharmacol. 57:667-678.
- ZupancG.K. SîrbulescuR.F. IlieI. 2012. Radial glia in the cerebellum of adult teleost fish: implications for the guidance of migrating new neurons. Neuroscience 210:416-430.