Abstract
Kit Radicaux Libres (KRL) test is a biological application, successfully applied on humans, helpful for the study of the total antiradical activity. In the present work, the first objective was to test on a subset of pig blood samples in order to determine the maximum time of storage able to provide reliable results. Blood samples were collected from 46 piglets and determinations were carried out on the blood samples after three h from collection (T1) and thereafter at 24 (T2) and 48 (T3) h. Successively blood samples from 313 piglets (171 castrated males and 142 females) were collected and analysed in order to determine reference intervals. Results are expressed as half-haemolysis time (HT50 in min), that is a reference point for blood susceptibility to free radical attack.
Our findings showed that for samples analysed at T1 and T2 there were no significant changes but significantly increased values (P<0.05) were obtained when samples were analysed after 48 h from collection, underlining biological and analytical interference due to the hemolysis of the samples. The reference values found in the subjects, expressed as ET AL50 were 46.6-68.7 min (males) and 52.5-86.8 min (females) in RBC, 59.8-93.6 min (males) and 70.5-113.0 (females) in whole blood.
In conclusion, a prolonged time (till +48 h) caused haemolysis, therefore the use of freshly collected blood is strictly recommended. The reference values obtained are considered to represent valid reference ranges for healthy pigs starting after weaning to 175 days of age under modern husbandry conditions.
Introduction
Generation of free radicals is an integral feature of normal cellular function. In contrast, excessive generation and/or inadequate removal of free radicals results in destructive and irreversible damage to the cell (Lopaczyski and Zeisel, Citation2001). A stressful condition leads to the excessive production of the radicals, which results in oxidative stress, an imbalance in the oxidant/antioxidant system (Khadija et al., Citation2009). The action of free radicals, if uncontrolled, seems to be cause of pathologies in man and animal such as atherosclerosis, arthritis, heart attack, cardiovascular disease, ageing progression and destruction of endothelial cells of blood vessels. In farm animals, oxidative stress is involved in a number of pathological conditions, including those associated with animal production, reproduction and welfare (Lykkesfeldt and Svendsen, Citation2007). Hallwell and Gutteridge (Citation1999) described several lines of defense against reactive oxygen species in animals represented especially by enzymes.
Cellular antioxidant defences consist of a complex interacting network and more than forty molecules involved in the oxido-reduction metabolism are now used as markers of oxidative stress (Montuschi et al., Citation2004; Shishehbor and Hazen, Citation2004; Tsimikas, Citation2008). However, exploration of these molecules is generally targeted and gives only a partial view of the oxidoreduction metabolism. The study of the whole cellular antioxidant defenses can yield a clear understanding of the cell redox status; moreover, the early detection of oxidative imbalance by simple and reliable methods is important to prevent such consequences.
The Kit Radical Libres (KRL) test is a biological test that evaluates the antioxidant status of an organism by testing the antioxidant defense systems of both plasma and red blood cells (RBC). KRL test evaluates the total antioxidant activity of blood by measuring the time required to haemolyse 50% of the RBC exposed to a controlled free radical attack. KRL test (Prost, Citation1989, Citation1992) is a biological method helpful for the study of the overall antioxidant defense used especially in human studies in which it showed the effectiveness of natural or pharmaceutical treatments or to discover acute processes such as trauma and ischemia or inflammatory disease; moreover it allowed to discriminate welfare conditions depending on medium or high stress, or tobacco smoking (Lesgards et al., Citation2002). In in vitro studies, KRL has been used to assess the antioxidant power of various synthetic and natural substances (Blache et al., Citation1991; Rossi et al., Citation2009). Some studies on the antioxidant defences in animals, using the KRL test, have been published, particularly on rats (Taleb-Senouci et al., Citation2009) and birds (Bertrand et al,. Citation2006). Since the method is tested in vitro and successfully applied on humans, livestock data as well as reference values are lacking. Moreover, the analysis on KRL test as reported from the manufacturer (Kirial International/Laboratories Spiral, Couternon, France) requires the use of fresh blood; anyway the maximum permitted storage periods (number of hours) of blood samples is not specified.
Blood samples collected from animals in remote locations (farms) often wait for laboratory processing. Consequently, results of hematological determinations of improperly handled blood samples, such as time, can yield misleading results (Cohle et al., Citation1981; Livesey and Dolamore, Citation2010). Profitable swine production requires a rapid increase in body weight and lean tissue, raising the demand on the metabolic system of these animals, which can lead to increased oxidative stress. Brambilla et al. (Citation2002) have considered the response to oxidative stress an effective parameter for assessment of welfare in pigs. Since the assessment of the antiradical blood capacity status in normal pigs is important for monitoring the health and welfare, in this paper at first the maximum time between blood sampling and measurement not affecting resulting data was established (trial 1); subsequently the whole blood and red blood cell (RBC) KRL reference values in pigs have been calculated (trial 2).
Materials and methods
Animals
For trial 1, a total of 46 piglets (Dalland) of the same age (52 days) were selected from a single farm. Blood samples were collected, stored at 4°C, forwarded to laboratory and analysed for the overall antiradical capacity in whole blood and in RBC within 3 h of collection of blood (time 1,T1), and then repeated after 24 (time 2, T2) and 48 h (time 3, T3).
For trial 2, a total of 313 Dalland healthy pigs (171 castrated males and 142 females) average liveweight 22.28 kg, range 7.1-117 kg were selected from different farms. Analyses were performed the same day of collection.
Blood collection
Blood samples were collected from healthy fasting animals by the same trained person in the January-July period. Blood was drawn from the jugular vein into 2-mL K3 EDTA vacuum tubes (Vacutainer; Becton-Dickinson, Le Pont de Claix, France), and forwarded to the laboratory within 3 h from bleeding. In the trial 2, with the aim to assess the possible influence of haematocrit (Ht) on antioxidant total antiradical capacity, the correlation and a robust linear regression were calculated, considering a subset of analysed samples (n=110). Haematocrit values were determined by centrifugation of a capillary sample and read with a microhematocrit reader.
Procedures involving animals were carried out in accordance with European Communities Council Directive (86/609/EEC, 1986) and approved by the Italian Ministry of Health (Law N. 116/92).
KRL test analysis
KRL test (Spiral Laboratories) is currently used in human contest to assess the capability of erythrocytes to resist a standardized production of free radicals generated from the thermal decomposition of a 27 mmol/L solution of 2,2′-azobis (2-amidinopropane) hydrochloride (AAPH) at 37°C (Lesgards et al., Citation2005; Stocker et al., Citation2003; Bauguil et al., Citation2009). The thermal decomposition of AAPH proceeds at a constant speed rate of 1.36 10-6 [AAPH] mol/l/sec, ensuring a constant free-radical flow during all the hemolysis process. The extracellular and intracellular antioxidant defenses contribute to maintain red blood cell membrane integrity and function until cell lysis. Whole blood and RBC samples diluted to 1/25 and 1/50 respectively were submitted in isotonic saline solution to organic free radicals produced by AAPH solution (Spiral, Dijon, France).
The cell lysis was recorded using a 96-well microplate reader by measuring the optical density decays at 620 nm. Results were expressed as the time required to reach 50% of maximal haemolysis [half-haemolysis time (HT50) in minutes], which refers to the whole blood resistance to free-radical attack, and as the lag-time of lysis, i.e., the latence time before the onset of lysis. A blood control with HT50 known was used as internal control. Performance of KRL instrument provided by the company, indicates a CV of the repeatability less than 2.5% and of reproducibility less than 4% (Laboratoires Spiral).
Statistical analysis
Data related to storage time were evaluated by performing a repeated measurements analysis using SPSS software (SPSS Inc., Chicago, IL, USA).
Raw data related to trial 2 were processed for descriptive statistics and assessed for Normality by the D’Agostino-Pearson test; summary data were calculated as mean ± standard deviation and median value. The relation between the antioxidant total antiradical activity and the haematocrit was tested by a Huber robust linear regression model. In order to evaluate possible differences between genders in the antioxidant total antiradical capacity, the t- test or the Kruskal-Wallis nonparametric test was applied to the group (male/female) data. The choice between methods was based on the Normality of data. Reference values for the total antiradical capacity were calculated, applying the method based on the Normal distribution (2.5th-97.5th percentile), or the nonparametric percentile method (CLSI C28 A-3). Analyses were performed using the MedCalc ver. 11.4.4.0 for Windows platform, except Huber’s regression (NCSS 2007 for Windows).
Results and discussion
-Trial 1: Results on blood and RBC at T1, T2 and T3 are reported in and , respectively. At T1 and T2 there were no significant changes but significantly increased values were obtained in blood (P<0.004) and RBC (0.028) when samples were analysed after 48 h from collection showing analytic hemolysis interference occurrence.
-Trial 2: The linear regression model did not evidence a relation (P>0.05) between haematocrit and HT50 in either blood or RBC; therefore, reference values were calculated on a larger sample of subjects, as reported in the Materials and Methods section.
In the univariate descriptive statistics related to blood and RBC HT50 in males and females are reported. The t- test/Kruskal-Wallis test showed highly significant differences between genders, suggesting the separate calculation of reference values. The two distinct reference intervals and their limits for HT50, the lag-time are reported in . In intensive husbandry, pigs are often exposed to stress factors such as mixing with unfamiliar animals, inconsistent handling procedures and exposure to new environments that can lead to increased generation of free radicals and other ROS in the body. Oxidative stress can be measured by directly detecting free radical production, or by indirectly detecting antioxidant defences or molecules marker of oxidative damage. In the present study we measured the antioxidant capacity using a biological test that allows the assessment of the overall individual resistance against free radical aggression. An analytical method that provides rapid and reliable results in animals is crucial. KRL test may represent a valid approach. Since the method was successful used on humans, some preliminary tests discussed in this paper (blood storage time and reference values) necessarily had to be addressed for proper development of the test in animals. In general, hematology results are often influenced by the time between blood sampling and measurement, as well as storage conditions (e.g., temperature and time) during sample delivery between laboratories may further affect the resulting data. Haematological changes may occur in the results as a consequence of delayed analysis and may complicate interpretation of the data.
The increase of HT50 (min) found in the present paper at T3, could depend on hemolysis, evidenced by the red color of the plasma, causing the release of the hemoglobin and other intrinsic components into the surrounding fluid. Analytic hemolysis is masked when the constituents of the plasma are at lower concentrations than the constituents in erythrocytes. The release of erythrocytic constituents can result in increased values. Similarly Koseoglu et al. (Citation2011) reported that hemolysis affects plasma concentration of a whole range of biochemical parameters, whereas the most prominent effect of hemolysis is observed for AST, LD, potassium and total bilirubin. However a waiting time of 24 h till analysis is sufficient to ensure the delivery of the samples and performing blood tests.
The KRL test, used to analyze whole blood, allows the measurement of both the intracellular and extracellular defences, taking into account their synergic effects. For this reason, the mean values for the total antiradical power of whole blood are higher than the values observed in RBC. The analysis performed on RBC refers to the intracellular defense status, and the two measurements are complimentary. The average life span of RBCs is 60-85 days, KRL analysis of RBCs antioxidant defences reflects the free radical aggression of the last two-three months, while KRL analysis of whole blood gives an idea of total antioxidant defences of the organism at the time of sampling. The same conclusions can be drawn for the lag time. It has been reported that the response to oxidative stress can be an effective parameter for assessment of welfare in pigs. In a previous study conducted on pigs reared on two different kinds of floor, solid or slatted, KRL analyses showed RBC values significantly lower in pigs allotted on slatted floor than solid floor underlining a sign of a chronic stress (Rossi et al., Citation2012).
The present study is revealing gender difference in the susceptibility of whole blood or RBC to free radicals suggested two distinct reference values according to Thorn (Citation2000) for other haematological parameters in pig or rabbit specie (Burnett et al., Citation2006). There is a good evidence to show that sex differences in oxidative status exist in different species. In many species, females live longer than males and it is probably associated with free radicals which are in lower amount in the mitochondria of females than males (Sastre et al., Citation2002). The longer lifespan in females may be due to the higher gene expression of antioxidants and the lower oxidative damage of mitochondria (Borras et al., Citation2003). Moreover, there is evidence for the strong antioxidant properties of estrogen (Tudus, Citation2000) but not for progesterone and testosterone (Barp et al,. Citation2002). On the other hand an hormonal difference between male and female is appreciable since intrauterine life (Goxe et al., Citation1993). Erythrocytes of female pigs resulted more refractory (+ 15.4%) to oxidative stress than males. Similar results were reported by Liu et al. (Citation2008) who found in male mice higher levels of malondiadehyde and lower contents of glutathione, hypothesizing that sex-responded differences may be attributed to the influence of sex hormones.
The herein reported limits are slightly lower than those reported in humans (84-101 min in blood and 66-75 min in RBC, determined as a whole group) and overlapping with the results already reported for castrated male piglets weighing 10-30 kg (59.34-93.6 min in blood, 43.94-66.9 min in RBC) (Pastorelli et al., Citation2009) suggesting that this parameter does not related to body weight.
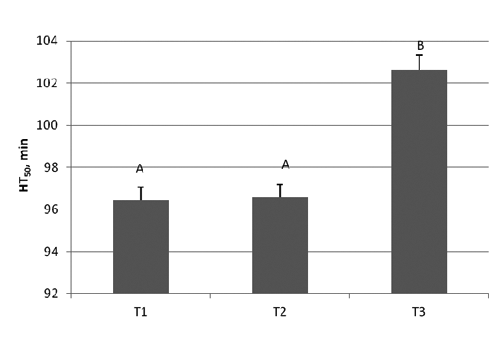
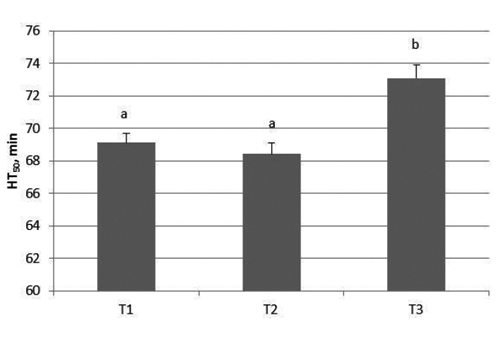
Table 1. Descriptive statistics for HT50 and lag time in whole blood and red blood cell.
Table 2. Reference values for the overall antiradical capacity and lag time in castrated males and females.
Conclusions
Data related to storage time indicated that a prolonged time (till +48 h) of analysis of blood samples caused haemolysis. If the assay is performed within 24 h after sample collection, the specimens are not affected by haemolysis. The reference values found provide guidelines for interpreting observations on total antiradical activity as well as for monitoring the health status of similar aged pigs using KRL test.
The early detection of oxidative imbalance by simple and reliable method as the one herein reported is important to prevent such consequences. Further investigations are needed to evaluate possible differences in different genetic strains and to compare the normal values with animals with different pathologies.
Acknowledgments
This research was supported by the Lombardia Region (ProZoo Project 2009) “Genomics application to fertility problems, disease resistance and quality assurance of the products in cattle and pigs”.
References
- BarpJ. AraujoA.S. FernandsT.R. RigattoK.V. LlesuyS. BellokleinA. SingalP. 2002. Myocardial antioxidant and oxidative stress changes due to sex hormones. Braz. J. Med. Biol. Res. 35:1075-1081.
- BauguilS.C. MaestreN. SegafredoC. GalinierA. GarciaJ. ProstM. PériquetB. PénicaudL. SalvayreR. CasteillaL. 2009. Evaluation of whole antioxidant defenses of human mononuclear cells by a new in vitro biological test: lack of correlation between erythrocyte and mononuclear cell resistance to oxidative stress. Clin. Biochem. 42:510-514.
- BertrandS. Alonso-AlvarezC. DeveveyG. FaivreB. ProstJ. SorciG. 2006. Carotenoids modulate the trade-off between egg production and resistance to oxidative stress in zebra finches. Oecologia 147:576-584.
- BlacheD. ProstM. RaffiJ.J. 1991. In vitro biological test of resistance to oxidation: application to identification of irradiated food. In: RaffiJ.J. BelliardoJ.J. ( eds.) Potential new methods of detection of irradiated food. Commission of the European Communities Publ., Luxembourg, pp 105- 116.
- BorrasC. SastreJ. Garcia-SalaD. LloretA. PallardoF.V. VinaJ. 2003. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radical Bio. Med. 34:546-552.
- BrambillaG. CivitarealeC. BalleriniA. FioriM. AmadoriM. ArchettiL.I. ReginiM. BettiM. 2002. Response to oxidative stress as a welfare parameter in swine. Redox Rep. 7:159-163.
- BurnettN. MathuraK. MetivierK.S. HolderR.B. BrownG. CampbellM. 2006. An investigation into haematological and serum chemistry parameters of rabbits in Trinidad. World Rabbit Sci. 14:175-187.
- CohleS.D. AbdusS. MakkouiD.E. 1981. Effects of storage on stability of haematological parameters. Am. J. Clin. Pathol. 76:67-69.
- GoxeB. PrunierA. RemyJ.J. SalesseR. 1993. Ontogeny of gonadal luteinizing hormone and follicle-stimulating hormone receptors in the fetal pig and related changes in gonadotropin and testosterone secretion. Biol. Reprod. 49:609-616.
- HalliwellB. GutteridgeJ.M.C. 1999. Free radicals in biology and medicine, 3rd ed. Oxford University Press, Oxford, UK.
- KhadijaA. AtiA. MohammedS. SaadA.M. MohamedH.E. 2009. Response of broiler chicks to dietary monosodium glutamate. Pak. Vet. J. 29:165-168.
- KoseogluM. HurA. AtayA. CuhadarS. 2011. Effects of hemolysis interference on routine biochemistry parameters. Biochem. Medica 21:79-85.
- LesgardsJ.F. DurandP. LassarreM. StockerP. LesgardsG. LanteaumeA. ProstM. Lehucher-MichelM.P. 2002. Assessment of lifestyle effects on the overall antioxidant capacity of healthy subjects. Environ. Health Persp. 110:479-486.
- LesgardsJ.F. Lehucher-MichelM.P. VidalN. ProstM. StockerP. 2005. Assessment of antioxidative activity of lipid-and watersoluble vitamins in human whole blood. Comparative analysis between a biological test and chemical methods. Int. J. Vitam. Nutr. Res. 75:11-18.
- LiveseyJ. H. DolamoreB. 2010. Stability of plasma adrenocorticotrophic hormone (ACTH): influence of hemolysis, rapid chilling, time, and the addition of a maleimide. Clin. Biochem. 43:1478-1480.
- LiuY. ZhangH. ZhangL. 2008. Evaluation of sex specificity on oxidative stress induced in lungs of mice irradiated by 12C6+ ions. Nucl. Sci. Tech. 19:17-21.
- LopaczyskiW. ZeiselS.H. 2001. Antioxidants, programmed cell death and cancer. Nutr. Res. 21:295-307.
- LykkesfeldtJ. SvendsenO. 2007. Oxidants and antioxidants in disease: oxidative stress. Vet. J. 173:502-511.
- MontuschiP. BarnesP.J. RobertsL.J. 2004. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 18:1791-1800.
- PastorelliG. RossiR. CannataS. CorinoC. 2009. Total antiradical activity in male castrated piglets blood: reference values. Ital. J. Anim. Sci. 8(Suppl.2):640-642.
- ProstM. 1989. Utilisation de générateur de radicaux libres dans le domaine des dosages biologiques. French patent n. 2,642,526. INPI Publ., Courbevoie Cedex, France.
- ProstM. 1992. Process for the determination by means of free radicals of the antioxidant properties of a living organism or potentially aggressive agents. US patent n. 5,135,850. US Patent and Trademark Office Publ., Washington, DC, USA.
- RossiR. CorinoC. PastorelliG. DurandP. ProstM. 2009. Assessment of antioxidant activity of natural extracts. Ital. J. Anim. Sci. 8(Suppl.2):655-657.
- RossiR. PastorelliG. CannataS. CorinoC. 2012. KRL test to objective evaluation of welfare: sensibility to housing conditions and dietary supplements. pp 259-264 in Proc. 7th Int. Symp. on the Mediterranean Pig, Zaragoza, Spain.
- SastreJ. BorrasC. Garcia-SalaD. LloretA. PallardoF.V. VinaJ. 2002. Mitochondrial damage in aging and apoptosis. Ann. NY Acad. Sci. 959:448-451.
- ShishehborM.H. HazenS.L. 2004. Inflammatory and oxidative markers in atherosclerosis: relationship to outcome. Curr. Atheroscler. Rep. 3:243-250.
- StockerP. LesgardsJ.F. VidalN. ChalierF. ProstM. 2003. ESR study of a biological assay on whole blood: antioxidant efficiency of various vitamins. Biochim. Biophys. Acta 1621:1-8.
- Taleb-SenouciD. GhomariH. KroufD. BouderbalaS. ProstJ. Lacaiile-DuboisM.A. BouchenakM. 2009. Antioxidant effect of Ajuga iva aqueous extract in streptozotocin induced diabetic rats. Phytomedicine 16:623-631.
- ThornC.E. 2000. Normal hematology of the pig. In: FeldmanB.F. ZinklJ.G. JainN.C. ( eds.) Schalm’s Veterinary Hematology, 5th ed., Blackwell Publ., Oxford, UK, pp 1089-1095.
- TsimikasS. 2008. In vivo markers of oxidative stress and therapeutic interventions. Am. J. Cardiol. 101:34D-42D.
- TudusP.M. 2000. Estrogen and gender effects on muscle damage, inflammation, and oxidative stress. Can. J. Appl. Physiol. 25: 274-287.