Abstract
An experiment was conducted to assess the optimal levels of dietary lysine (Lys) in Japanese quail from 7 to 21 days of age. A dose-response diet was formulated to be adequate in all amino acid concentrations with the exception of Lys. Different levels of supplemental L-Lysine.HCl were added to the dose-response diet at the expense of corn starch, sodium bicarbonate, and NaCl to create 6 levels of Lys ranged from 0.91% to 1.51% in diet. Optimal Lys for feed conversion ratio, breast meat yield, and thigh meat yield were estimated at 1.15%, 1.21%, and 1.16% of diet, respectively, based on linear broken-line regression. With quadratic broken-line regression, the Lys requirements for body weight gain, feed conversion ratio, thigh meat yield, and breast meat yield were estimated at 1.27%, 1.21%, 1.32%, and 1.34% of diet, respectively. Overall, Lys requirements of starting Japanese quail may be at least 1.34% of diet for optimizing carcass attributes fed low-CP dose-response diet.
Introduction
Amino acids as metabolism regulators affect the bird performance (Mehri et al., Citation2012) and protein turnover (Wu, Citation1998). Among indispensable amino acids (IAA) it has been elucidated that the lysine (Lys) content of breast meat is relatively higher than other amino acids (Munks et al., Citation1945); and it has the biggest effects on proteolysis (Tesseraud et al., Citation1996) and breast muscle development (Tesseraud et al., Citation1999). Therefore, the nutritional needs of Lys for optimum breast meat yield may be higher than gain and feed conversion ratio (Dozier, et al., Citation2008; Zaboli et al., Citation2011). Lysine is the second limiting amino acid in poultry diets and extensive works have been done to estimate Lys requirements of broiler chickens (Dozier et al., Citation2008, Citation2009, Citation2010; Han and Baker, Citation1993, Citation1994; Mehri et al., Citation2010) but such references on the Lys needs of quail chicks are limited (Hajkhodadadi et al., Citation2013). NRC (Citation1994) has published amino acid requirements for quail on the total amino acid basis. Genetic improvement during the decades leads to change nutritional requirements of the modern birds (Havenstein et al., Citation2003) so there is needed to update nutritional information of modern Japanese quail. In this regard, Hajkhodadadi et al. (Citation2013) reported that Lys recommendation of NRC (Citation1994) for quail chicks might be inadequate and modern quails may have higher amino acid requirements than older generations. Therefore, we aimed to estimate the Lys requirement of modern quail chicks in the present study.
Materials and methods
Bird management
Quail chicks were obtained from the hatchery of Research Center of Special Domestic Animals at the University of Zabol (RCSDA, Zabol, Iran) and fed starter diet according NRC (Citation1994) from 0 to 7 days of age. At d 7 of age and after 3 h starvation, the birds (15 male and female without sexing) were weighed and randomly assigned to 24 floor pens with similar body weight (36.8±0.09). The pens were equipped with feeder, drinker, and wood shaving. The room temperature during second and third weeks of age was 30ºC and 28ºC, respectively. The relative humidity was 55-60% throughout the study.
Experimental diets
A basal diet containing corn, wheat, soybean meal, and corn gluten meal was formulated to meet or exceed the requirements recommended by NRC (Citation1994) except for Lys (). Six graded levels of L-Lys.HCl were added to the basal diet at the expense of corn starch, sodium bicarbonate, and NaCl to generate six levels of Lys ranged from 0.91% to 1.51% of diet. Different proportions of sodium bicarbonate and NaCl help to keep dietary electrolyte balance (DEB) in the experimental diets. Before diet formulation, protein-containing ingredients were analyzed for crude protein (CP) and amino acid contents using near infrared reflectance spectroscopy (Fontaine et al., Citation2001, Citation2002). The analyzed values of amino acids in feed ingredients were used for diet formulations. Experimental diets were offered from 7 to 21 days of age and birds consumed feed (provided in whole mash form) and water on an ad libitum basis. At the end of experiment, 4 birds of each replicate were randomly selected and were dissected for carcass portions by cervical dislocation (Kim et al., Citation2012). The breast and thigh sections were harvested as skinless boned-meat.
Statistical analysis
In this experiment, gradient treatment structure was conducted as a completely randomized design. The 6 dose-response diets for Lys were fed to each pen with 4 replicates. All data were subjected to MIXED procedure using linear and quadratic responses to explain potential effects of Lys (SAS, Citation2002). Differences among means (P≤0.05) were separated using LSMEANS option of SAS (Citation2002). Both broken-line linear and broken-line quadratic models were used for estimation of Lys needs using NLIN procedure (Robbins et al., Citation2006).
Results and discussion
Starting quail chicks fed gradient concentration of Lys showed significant (P<0.05) linear and quadratic trends for body weight (BW), BW gain, feed conversion ratio (FCR), chilled carcass, breast mass (BM) yield and the weight of thigh meat (TM) from 7 to 21 d of age ( and ). The quadratic effect of Lys levels was significant for feed intake (; P=0.007) and TM yield (; P=0.0433) but no linear trends were observed both parameters ( and ).
Two statistical methods including linear broken-line (LBL) and quadratic broken-line (QBL) models were fitted to estimate the Lys requirements of quail chicks. Lysine requirements for FCR, TM yield, and BM yield were estimated at 1.15%, 1.21%, 1.16% of diet, respectively, based on LBL model (; , , and ). The LBL model could not fit the data of BW gain. The nutritional needs of Lys were estimated at 1.27%, 1.21%, 1.32%, and 1.34% of diet, respectively, for BW gain, FCR, TM yield, and BM yield based on QBL model (; , , , and ). NRC (Citation1994) suggested the 1.3% Lys level for growing Japanese quail. The Lys requirement of quail chick with practical diets containing 25% protein has been reported as 1.35% of diet (Svacha et al., Citation1970). The estimated nutritional needs of Lys for BW gain was higher than FCR using quadratic broken-line model (1.27 %vs 1.21% of diet). Dozier et al. (Citation2010) reached to the same result with broiler chicken. However, some reports revealed that the Lys requirements for FCR might be higher than growth rate (Mehri et al., Citation2010; Zaboli et al., Citation2011; de Lima et al., Citation2013). Baker et al. (Citation2002) argued that as Lys exceeds the concentration for the requirement of BW gain, feed intake decreases without decline in BW gain, so resulting in higher Lys requirement for FCR. The method of statistical analysis had a profound effect on the estimated Lys requirement. Lysine requirements for FCR were 1.15% and 1.21% of diet using linear broken-line and quadratic broken-line models, respectively. Pesti et al. (Citation2009) stated that the linear ascending portion of linear broken-line regression might cause to underestimate the amino acid requirements because the bird response to incremental levels of dietary nutrient is nonlinear. The researchers came to the same result with broiler chicken and using the quadratic broken-line regression may overcome the underestimation (Dozier et al., Citation2008; Labadan et al., Citation2001; Mehri et al., Citation2010).
There is increasingly demand for breast meat of the chickens and interest to optimizing breast meat yield so considering the breast meat yield as a response criterion for determination of Lys requirements (Dozier et al., Citation2008, Citation2010; Zaboli et al., Citation2011). In the present study, the Lys requirements for carcass portions including breast meat and thigh meat yield were higher than quail performance. Considering the estimated values of quadratic broken-line model, the average Lys requirements for bird performance and processing yields were 1.24% and 1.33% of diet, respectively. Muscle accretion in poultry depends on protein degradation and protein synthesis. Tesseraud et al. (Citation1996) showed the critical effects of dietary Lys on proteolysis in breast muscle. Some studies revealed the higher Lys requirement for maximizing breast meat yield than bird performance (Dozier et al., Citation2008; Labadan et al., Citation2001) supporting the pivotal role of Lys in breast muscle development (Tesseraud et al., Citation1999). It should be noted that despite of genetic progress in the modern Japanese quail and higher nutritional needs of modern strains (Havenstein et al., Citation2003) the estimated Lys requirements reported herein are lower than suggested by Hajkhodadadi et al. (Citation2013). This phenomenon may related to the CP level of basal diet and statistical procedure in the current research. In the present study, we used a 23% CP dose-response diet and spline models for analyzing data. Hurwitz et al. (Citation1998) and Morris et al. (Citation1987) demonstrated that dietary CP could affect the Lys requirements of broiler chicks. The recommended CP requirements for quail chick by NRC (Citation1994) and Hyankova et al. (Citation1997) were 24 and 26% of diet, respectively; therefore, the lower CP level of dose-response diet of this experiment could be resulted in lower estimation of Lys requirement. In addition, Pesti et al. (Citation2009) stated that an appropriate model should be used to analyze dose-response data and regression models would be suitable for amino acid studies. Hajkhodadadi et al. (Citation2013) have used multiple range Duncan for determination of optimal Lys. As stated by Pesti et al. (Citation2009) the mean comparison method could not estimate accurately the amino acid requirements using experimental data.

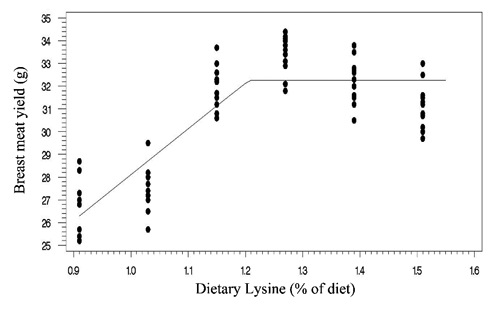
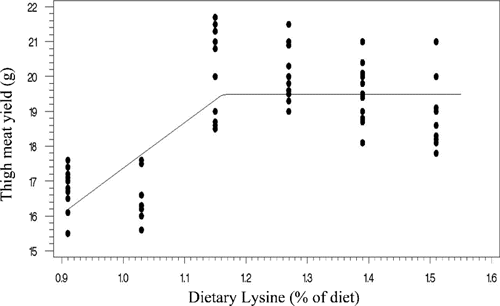
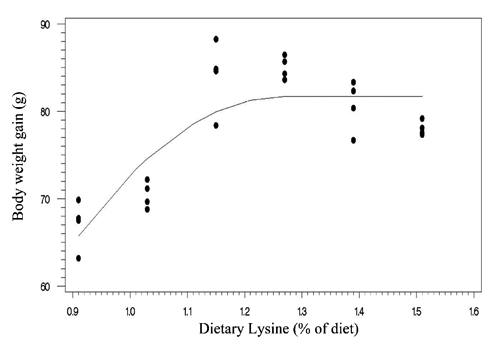
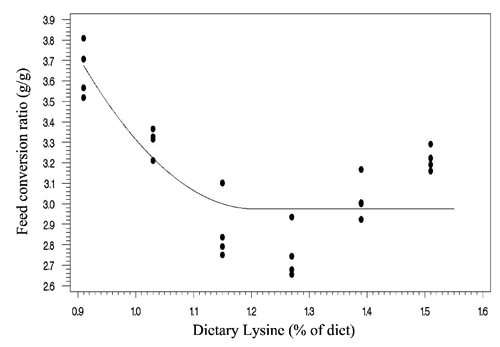
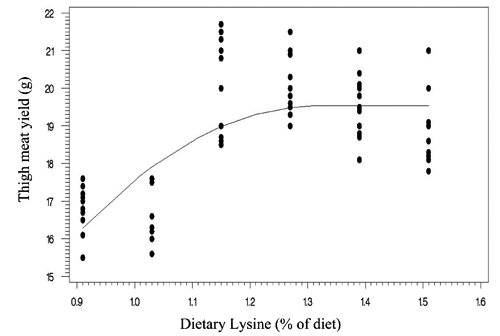
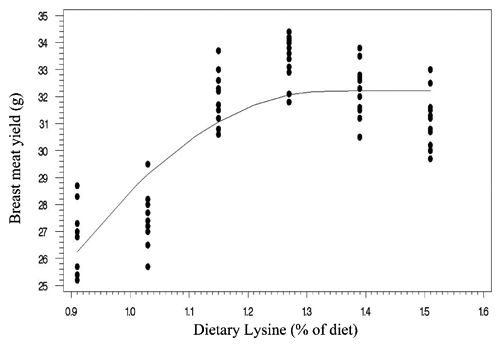
Table 1. Composition of basal diet.
Table 2. Growth performance of Japanese quail fed gradient levels of dietary lysine from 7 to 21 days of age.Footnote°
Table 3. Processing yield of Japanese quail fed gradient levels of dietary lysine from 7 to 21 days of age.Footnote°
Table 4. Estimated optimal dietary lysine in Japanese quail based on linear broken-line analyses.
Table 5. Estimated optimal dietary lysine in Japanese quail based on quadratic broken-line analyses.
Conclusions
In conclusion, the estimated Lys requirement of quail chick fed low-CP diet may be at least 1.34% of diet; and Lys requirement for processing yield was approximately 7% higher than growth performance. More studies may be needed to address the amino acid requirements of modern Japanese quail for different classes of age.
References
- BakerD. BatalA. ParrT. AugspurgerN. ParsonsC. 2002. Ideal ratio (relative to lysine) of tryptophan, threonine, isoleucine, and valine for chicks during the second and third weeks posthatch. Poultry Sci. 81:485-494.
- de LimaM.R. CostaF.G.P. GuerraR.R. da SilvaJ.H.V. RabelloC.B.V. MiglinoM.A. LobatoG.B.V. NettoS.B.S. da Silva DantasL. 2013. Threonine: lysine ratio for Japanese quail hen diets. J. Appl. Poultry Res. 22:260-268.
- DozierW.A.3rd CorzoA. KiddM. SchillingM. 2008. Dietary digestible lysine requirements of male and female broilers from forty-nine to sixty-three days of age. Poultry Sci. 87:1385-1391.
- DozierW.A.3rd CorzoA. KiddM. TillmanP. BrantonS. 2009. Digestible lysine requirements of male and female broilers from fourteen to twenty-eight days of age. Poultry Sci. 88:1676-1682.
- DozierW.A.3rd CorzoA. KiddM. TillmanP. McMurtryJ. BrantonS. 2010. Digestible lysine requirements of male broilers from 28 to 42 days of age. Poultry Sci. 89:2173-2182.
- FontaineJ. HörrJ. SchirmerB. 2001. Near-infrared reflectance spectroscopy enables the fast and accurate prediction of the essential amino acid contents in soy, rape-seed meal, sunflower meal, peas, fishmeal, meat meal products, and poultry meal. J. Agr. Food Chem. 49:57-66.
- FontaineJ. SchirmerB. HörrJ. 2002. Near-infrared reflectance spectroscopy (NIRS) enables the fast and accurate prediction of essential amino acid contents. 2. Results for wheat, barley, corn, triticale, wheat bran/middlings, rice bran, and sorghum. J. Agr. Food Chem. 50:3902-3911.
- HajkhodadadiI. ShivazadM. MoravvejH. Zare-ShahnehA. 2013. Effect of dietary lysine on performance and immunity parameters of male and female Japanese quails. Afr. J. Agr. Res. 8:113-118.
- HanY. BakerD.H. 1993. Effects of sex, heat stress, body weight, and genetic strain on the dietary lysine requirement of broiler chicks. Poultry Sci. 72:701-708.
- HanY. BakerD.H. 1994. Digestible lysine requirement of male and female broiler chicks during the period three to six weeks posthatching. Poultry Sci. 73:1739-1745.
- HavensteinG. FerketP. QureshiM. 2003. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poultry Sci. 82:1500-1508.
- HurwitzS. SklanD. TalpazH. PlavnikI. 1998. The effect of dietary protein level on the lysine and arginine requirements of growing chickens. Poultry Sci. 77:689-696.
- HyankovaL. AkedkováL.D. KnížetováH. KleckerD. 1997. Responses in growth, food intake and food conversion efficiency to different dietary protein concentrations in meat type lines of Japanese quail. Brit. Poultry Sci. 38:564-570.
- KimC.H. KimG.B. ChangM.B. BaeG.S. PaikI.K. KilD.Y. 2012. Effect of dietary supplementation of Lactobacillus-fermented Artemisia princeps on growth performance, meat lipid peroxidation, and intestinal microflora in Hy-line Brown male chickens. Poultry Sci. 91:2845-2851.
- LabadanM.Jr. HsuK. AusticR. 2001. Lysine and arginine requirements of broiler chickens at two-to three-week intervals to eight weeks of age. Poultry Sci. 80:599-606.
- MehriM. DavarpanahA.A. MirzaeiH.R. 2012. Estimation of ideal ratios of methionine and threonine to lysine in starting broiler chicks using response surface methodology. Poultry Sci. 93:771-777.
- MehriM. Nassiri-MoghaddamH. Kerman -shahiH. Danesh-MesgaranM. 2010. Digestible lysine requirements of straight-run broiler chickens from fifteen to twenty-eight days of age. J. Anim. Vet. Adv. 9:2321-2324.
- MorrisT. Al AzzawiK. GousR. SimpsonG.L. 1987. Effects of protein concentration on responses to dietary lysine by chicks. Brit. Poultry Sci. 28:185-195.
- MunksB. RobinsonA. BeachE.F. WilliamsH.H. 1945. Amino acids in the production of chicken egg and muscle. Poultry Sci. 24:459-464.
- National Research Council, 1994. Nutrient Requirements for Poultry, 9th rev. ed. National Academy Press, Washington, DC, USA.
- PestiG.M. VedenovD. CasonJ.A. BillardL. 2009. A comparison of methods to estimate nutritional requirements from experimental data. Brit. Poultry Sci. 50:16-32.
- RobbinsK. SaxtonA. SouthernL. 2006. Estimation of nutrient requirements using broken-line regression analysis. J. Anim. Sci. 84:E155-E165.
- SAS, 2002. SAS/STAT 9.1 User’s Guide. SAS Inst. Inc., Cary, NC, USA.
- SvachaA. WeberC. ReidB. 1970. Lysine, methionine and glycine requirements of Japanese quail to five weeks of age. Poultry Sci. 49:54-59.
- TesseraudS. Le Bihan-DuvalE. PeressonR. MichelJ. ChagneauA. 1999. Response of chick lines selected on carcass quality to dietary lysine supply: live performance and muscle development. Poultry Sci. 78:80-84.
- TesseraudS. PeressonR. LopesJ. ChagneauA.M. 1996. Dietary lysine deficiency greatly affects muscle and liver protein turnover in growing chickens. Brit. J. Nutr. 75:853-866.
- WuG. 1998. Intestinal mucosal amino acid catabolism. J. Nutr. 128:1249-1252.
- ZaboliG. JalilvandG. DavarpanahA.A. MehriM. 2011. Estimation of standardized ileal digestible lysine requirement of starting broiler chicks fed soybean-and cottonseed meal-based diets. J. Anim. Vet. Adv. 10:1278-1282.