Abstract
The present study aimed to investigate the effects of epigallocatechin-3-gallate (EGCG) on the in vitro development of porcine oocytes, parthenogenetic activation embryos (PA), and somatic cell nuclear transfer (SCNT) embryos. In Experiment 1, 0 (control), 10, 30, and 50 μg/mL EGCG were added to in vitro maturation (IVM) medium to explore the effect of EGCG on IVM of pig oocytes. The matured oocytes were then used to produce PA and SCNT embryos. Either for nuclear maturation of oocytes or for the rates of cleavage and blastocyst of PA and SCNT embryos, no significant difference was found among all groups. However, the total cell number per cloned blastocyst was significantly lower in blastocysts derived from oocytes matured in 50 μg mL EGCG (P<0.05) as compared with the other groups. In Experiment 2, we cultured pig SCNT and PA embryos in medium containing various concentrations of EGCG to examine the effect of EGCG on preimplantation development. The cleavage and blastocyst rates and the total cell number per blastocyst did not significantly differ between PA and SCNT embryos among all groups. However, the reactive oxygen species level was significantly lower in the PA embryos cultured in 10 μg mL EGCG than the other groups (P<0.05). Our results suggest that high doses of EGCG in IVM are harmful to the oocytes as evidenced by the decreased quality of SCNT embryos, and EGCG has no beneficial effects on in vitro development of pig cloned embryos.
Introduction
Somatic cell nuclear transfer (SCNT) is a useful technique for improving domestic animals, rescuing endangered species, and producing transgenic animals. The first successful production of porcine somatic cell clones was reported in Citation2000 by Polejaeva et al. Thereafter, many studies have successfully produced cloned pigs in the laboratory (Campbell et al., Citation2007). However, the efficiency of development of healthy offspring through SCNT remains low (Campbell et al., Citation2007). As SCNT technology includes different processes and oocytes of different species possess specific characteristics, SCNT involves various problems that need to be addressed, such as the requirement of an appropriate in vitro culture (IVC) system for oocytes nuclear recipient and SCNT embryo donor cells.
Electrically-activated porcine embryos are essential for the production of clone embryos, but the SCNT process results in increased levels of reactive oxygen species (ROS; Koo et al., Citation2008). Preimplantation mammalian embryos incur damage due to the ROS activity during IVC, which damages the organelles, particularly the mitochondria, and causes species-specific embryo developmental blocks and apoptosis (Nakano et al., Citation2012). Free radical scavengers such as hypotaurine, thioredoxin, betamercaptoethanol, glutathione, and cysteine have been found to offer protection to IVC cultured embryos against the oxidative stress induced by ROS (Nakano et al., Citation2012).
Green tea is a popular beverage consumed worldwide, and has attracted significant attention recently as a result of its health benefits. It has been demonstrated to act against cancer, neurodegenerative diseases, and inflammation, and it has been shown to improve cardiovascular function, decrease obesity, and facilitate weight loss (Zaveri, Citation2006). Epigallocatechin-3-gallate (EGCG) is the most abundant catechin in green tea and accounts for 65% of the total catechin content therein (Zaveri, Citation2006). It has been shown to have antioxidant properties that can prevent ROS-induced chromosomal damage in somatic cells (Sugisawa and Umegaki, Citation2002). Roth et al. (Citation2008) demonstrated that while hyperthermia disrupts the competence of follicle-enclosed oocytes, in vivo administration of EGCG improves the developmental competence and quality of porcine oocytes matured in vitro. Spinaci et al. (Citation2008) found that significantly fewer embryos developed to the blastocyst stage following parthenogenetic activation when 25 μg/mL EGCG was added to the in vitro maturation (IVM) medium. Yavari et al. (Citation2010) and Spinaci et al. (Citation2008) demonstrated that addition of EGCG to the maturation medium and IVC had no beneficial effects on the developmental ability of porcine oocytes and parthenogenesis. These findings suggest that supplementation of the culture medium with higher concentrations of EGCG may inhibit the developmental ability of ooctyes and embryos rather than enhance it.
In the present study, we examined the effect of EGCG on the IVM and culture of porcine oocytes and embryos, and evaluated the developmental potential of oocytes by parthenogenetic activation (PA) and SCNT embryos when EGCG was only added into IVM medium; and developmental ability of PA and SCNT embryos when EGCG was just added into IVC medium.
Materials and methods
Chemicals
Unless otherwise stated, all chemicals used in the present study were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA).
Collection and in vitro maturation of oocytes
The experiment was performed according to the protocol employed by Zhang et al. (Citation2007) and Cao et al. (Citation2012). Briefly, sow and peripubertal gilt ovaries were collected from abattoirs and transported to the laboratory within 1 h after slaughter in physiological saline solution containing antibiotics at 28 to 35°C. Cumulus-oocyte complexes (COCs) were aspirated from 3 to 6 mm follicles with an 18 gauge needle attached to a disposable syringe or using vacuum suction. Cumulus-oocyte complexes with at least three layers of compact cumulus investment and even cytoplasm were selected and washed twice in 4-(2-hydroxyethyl)-1-piperazine ethanesulphonic acid (HEPES)-buffered tissue culture medium (TCM)-199 containing 0.3% heparin, 5% amphotericin, and 10% calf serum (CS). Then, the selected COCs were matured in groups of 15 to 20 in 50 μL of maturation medium, consisting of TCM-199 (without HEPES) supplemented with 10% (v/v) porcine follicular fluid, 10% (v/v) fetal bovine serum (FBS), 10 U/mL human chorionic gonadotropin, 10 U/mL equine chorionic gonadotropin, 0.1 mg/mL L-cysteine, and 10 ng/mL epidermal growth factor. Various concentrations of EGCG (0, 10, 30, and 50 μg/mL; Sigma-Aldrich) were added to IVM medium. All the cultures were performed in a 38.5°C humidified incubator containing 5% CO2 in air for 42 to 44 h. After IVM, the COCs were transferred to 1 mg/mL hyaluronidase in Dulbecco’s phosphate-buffered saline without calcium or magnesium (Invitrogen, Carlsbad, CA, USA) to remove cumulus cells. Oocytes with intact cell membrane and extruded PB1 (first polar body) were selected for future experiments.
Production of parthenogenetically activated embryos
Denuded oocytes were washed three times followed by balancing in activation medium for 2 to 3 min. Approximately 20 to 30 meiosis II oocytes were placed in a fusion chamber filled with activation medium. Oocyte activation was stimulated by applying two direct current pulses of 1.56 kv/cm at 80 μsec with a 1-s interval with a CFS-150/B BLS fusion machine (BLS Ltd., Budapest, Hungary). The activation medium consisted of 0.30 M mannitol, 0.05 mM CaCl2, 0.10 mM MgSO4, 0.5 mM HEPES, and 0.01% (w/v) polyvinyl alcohol. The oocytes were washed three times and cultured in embryo culture medium, which consisted of porcine zygote medium (PZM; Yoshioka et al., Citation2002) supplemented with 2.78 mol/L inositol, 3 mg/mL BSA, 10 μg/mL of cytochalasin B (CB), and 10 μg/mL of cycloheximide for 4 h at 38°C in 5% CO2. Four hours later, the activated oocytes were washed three times and cultured in PZM3.
Production of cloned embryos
A porcine fetal fibroblast cell line was generated from Meishan pig fetuses as reported previously (Cao et al., Citation2012; Zhang et al., Citation2007). Before SCNT, 1- to 3-day-old fetal fibroblast cells exhibiting contact inhibition were detached, washed, centrifuged, and resuspended in cell culture medium to serve as nuclear donors. For the construction of SCNT embryos, mature oocytes and nuclear donor cells were placed in a drop of micromanipulation solution and incubated at 38.5°C in 5% CO2 and saturated humidity for 10 to 15 min. Then, a single 50-μL drop of micromanipulation medium containing TCM-199, 2% FBS, and 5 μg/mL CB was placed in the centre of the lid of a 60-mm culture dish and covered with mineral oil for observation under an inverted microscope (IX71; Olympus, Tokyo, Japan). Each group of 20 to 30 oocytes and nuclear donor cells was placed in this drop and incubated for 5 to 10 min. One oocyte was secured with a holding pipette (inner diameter: 25 to 35 μm; outer diameter: 100 to 120 μm). PB1 and 20% of the presumed adjacent cytoplasm containing the metaphase plate were aspirated using a beveled pipette (inner diameter: 15 to 25 μm). A globular, smooth, strongly refractive somatic cell with a diameter of approximately 20 μm was injected into the perivitelline space through the same slit. Reconstructed couplets were transferred into drops of HEPES-buffered TCM-199 with 2% CS and covered with mineral oil for recovery by incubation at 38.5°C for 0.5 h in 5% CO2 and 100% humidity until fusion and activation.
A simultaneous fusion and activation protocol was employed using 0.30 M mannitol supplemented with 0.05 mM CaCl2, 0.10 mM MgSO4, and 0.01% (w/v) polyvinyl alcohol. Reconstructed couplets were equilibrated in the activation solution for 2 min, and then groups of 10 couplets were placed in the fusion chamber filled with fusion solution. The couplets were manually aligned using a fine needle to make the contact plane parallel to the electrodes, and then two direct current pulses of 1.65 kv/cm were applied for 100 μsec with a 1-sec interval in a CFS-150/B fusion machine. The couplets were then washed three times and cultured in PZM3 with 10 μg/mL of CB and 10 μg/mL of cycloheximide. Four hours later, the results of the fusion were examined under a stereomicroscope.
In vitro culture and quality assessment of embryos
After parthenogenetic activation or SCNT, all embryos were transferred into IVC medium. The basic embryo culture medium was PZM supplemented with 2.78 mol/L inositol and 3 mg/mL BSA. Different concentrations (0, 10, 30, and 50 μg/mL) of EGCG were added into the IVC medium. The embryos were cultured at 39°C with 5% CO2 and 100% humidity. The cleavage (number of cleaved embryos/number of mature oocytes) and blastocyst (number of blastocyst embryos/number of mature oocytes) rates were determined under a stereomicroscope after 48 h and 144 to 168 h of IVC, respectively. Some blastocysts were stained with 20 μg/mL of Hoechst 33342 for 10 min, and the stained blastocysts were mounted onto glass slides under a cover slip and counted under an inverted microscope (Olympus), and photographed under UV light. Total cell numbers per blastocyst were counted using NIH ImageJ (NIH, Bethesda, MD, USA; http://rsb-web.nih.gov/ij/) software.
Measurement of intracellular reactive oxygen species
Intracellular ROS activity in the embryos was measured by 2-7-dichlorodihydrofluorescein diacetate (DCHFDA) fluorescence, using the method reported by Hashimoto et al. (Citation2000). Briefly, embryos were incubated with 10 μM of DCHFDA for 20 min at 38.5°C, washed three times in PBS-polyvinyl pyrrolidone (PBS-PVP) medium to remove traces of dye, and the intracellular fluorescent dichlorofluorescein was visualised under an epifluorescence microscope (Olympus) using excitation and emission wavelengths of 450 to 490 nm and 515 to 565 nm, respectively. A digital camera (Olympus) attached to the microscope was used to acquire images, and the mean gray value of the fluorescent oocytes was measured using ImageJ software. Background fluorescent values were subtracted from the final values before analysing for the statistical difference among the groups. The experiment was replicated three times with 10 to 20 oocytes in each replicate.
Experimental design
In Experiment 1, we examined the effect of 0, 10, 30, and 50 μg/mL EGCG on the maturation rate of oocytes and their ability to develop into blastocysts after parthenogenetic activation and SCNT.
In Experiment 2, we investigated the effect of EGCG on the developmental potential of PA and SCNT embryos. Parthenogenetic activation and SCNT embryos were cultured in medium supplemented with the same concentrations of EGCG used in Experiment 1. The ROS levels were measured after the oocytes reached the 4-cell stage following culture for 2 days in PZM3 supplemented with EGCG.
Statistical analysis
Data were expressed as the means±SEM and analysed using the chi-square test. All analyses were carried out using SPSS 11.0, and a P value of <0.05 was considered as statistically significant.
Results and discussion
We examined the effect of 0, 10, 30, and 50 μg/mL of EGCG on the maturation rate of oocytes and their ability to develop into blastocysts after parthenogenetic activation. shows the effects of EGCG on the maturation of porcine oocytes and the developmental potential of PA embryos from these oocytes. The proportion of matured oocytes and 2-cell embryos after parthenogenetic activation did not significantly differ between the EGCG-treated and the control groups. The oocytes treated with 10 μg/mL EGCG showed a tendency to develop into more blastocysts (76.06±7.17 vs 60.72±3.94) and had a high blastocyst cell number (46.50±6.72 vs 37.50±7.10) compared with the control; however, these values did not differ from those of the controls (P>0.05).We tested the ability of oocytes to develop into blastocysts after SCNT following the addition of EGCG to maturation medium at the same concentrations used in Experiment 1. As shown in , the number of blastocysts (51.67±14.40 vs 22.67±11.57) and the blastocyst cell number (41.75±10.52 vs 34.0±3.41) tended to be slightly higher in the 10 μg mL EGCG-treated group, although these values did not significantly differ from those of the controls (P>0.05). The blastocyst cell number was significantly decreased in the 50 μg mL EGCG group (P<0.05) compared with the other groups. As shown in and , EGCG supplementation of the IVC medium did not increase the cleavage rate, blastocyst rate, and the blastocyst cell number of PA and SCNT embryos compared to the controls. On day 2, the ROS levels in the porcine PA embryos were significantly decreased in the 10 μg mL EGCG group than in the other groups ( and ). On day 2, the ROS levels in the porcine SCNT embryos between the EGCG group and the other groups were not significantly different ( and ; P>0.05). With numerous reports of oxygen toxicity and its deleterious effects on preimplantation embryos, the importance of protecting embryos from ROS in vitro is being increasingly recognised as a key factor in improving IVC conditions for embryos (Choi et al., Citation2008).
During IVC, oocytes and embryos are inevitably more exposed to light and high oxygen concentrations than those cultured in vivo; these factors can favour increased production of ROS (superoxide anions [O2–], hydrogen peroxide, and highly reactive hydroxyl radicals). Reactive oxygen species can damage proteins, lipids, and nucleic acid components causing mitochondrial alterations, embryo cell blocks, and apoptosis (Guerin et al., Citation2001). Therefore, the addition of antioxidants to the IVM or IVC medium could improve the developmental competence of embryos (Choi et al., Citation2008; You et al., Citation2010, Citation2012). Epigallocatechin-3-gallate is a potent natural antioxidant. It is the major and most abundant polyphenol component of green tea, with apparent low toxicity (Zaveri, Citation2006). In our study, the ROS levels in the 4-cell embryos developed from parthenogenetically-activated oocytes were significantly lower in the EGCG group than in the control. However, the addition of low doses of EGCG to maturation medium and IVC medium had no significant positive effects on the developmental competence and quality of PA embryos. The parthenogenetic blastocyst rate was slight increased when 10 μg/mL of EGCG was added to the IVM medium. This result is in agreement with Yavari et al. (Citation2010). Yavari et al. found that treatment with low concentrations of EGCG during IVC did not influence the developmental competence of porcine PA embryos. In contrast, Wang et al. (Citation2007) reported that the blastocyst rate in cow embryos significantly (P<0.05) increased after in vitro fertilisation (IVF) following addition of 15 μM of green tea polyphenol (GTP) to the IVM medium. Recently, Wang et al. (Citation2013) reported that addition of 15 μM of GTPs during IVM and IVC improved the pregnancy rate in cows. Green tea polyphenol consists of different polyphenolic catechins such as epicatechin (EC), epicatechin-3-gallate (ECG), epigallocatechin (EGC), EGCG, catechin, and gallocatechin (GC) (Zaveri, Citation2006). Their order of effectiveness as radical scavengers is ECG>EGCG> EGC>EC> catechin. It is suggested that some other substance present in GTPs may play a role in improving the embryonic development potential. In our study, we found that supplementation of IVC and IVM media with EGCG did not increase the developmental potential of porcine SCNT embryos. Yet, the ROS levels slightly decreased in SCNT embryos after 48 h of IVC with 10 μg mL of EGCG as compared to the control. However, the beneficial effects of EGCG supplementation on the ROS levels were not reflected by the developmental potential of SCNT embryos to the blastocyst stage. Spinaci et al. (Citation2008) found that supplementation of medium with 10 μg/mL EGCG during IVF significantly increased the fertilisation rate while higher EGCG concentrations (25 μg/mL) decreased the percentage of fertilised oocytes. Similar results were reported by Nakano et al. (Citation2012), who found that the preimplantation development of oocytes and SCNT embryos did not increase when using the other well-documented antioxidant, melatonin. In contrast, some groups have reported that melatonin can increase the developmental rate of embryos and oocytes (Rodriguez-Osorio et al., Citation2007; Papis et al., Citation2007; Choi et al., Citation2008). In the present study, the number of blastocysts and the blastocyst cell number did not increase following addition of EGCG to the IVM and IVC media. However, it must be noted that only three concentrations (10, 30, and 50 μg/mL) of EGCG were used to treat SCNT embryos; these may be not the optimum concentrations for embryo development. Hence, we believe that further research is required to investigate the optimum concentration of EGCG for improving the developmental potential of SCNT embryos.
Conclusions
The present study revealed that supplementation of IVM with 50 μg/mL of EGCG is harmful to oocyte maturation, as evidenced by the decreasing quality of SCNT embryos, and that EGCG did not exert any beneficial effects on the in vitro development of pig embryos.
Acknowledgments
This work was supported by grants from the National High Technology Research and Development Program of China (2011AA100307-4), and the National Natural Science Foundation of China (31272442).
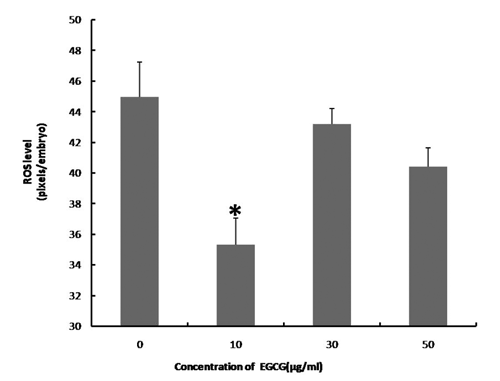
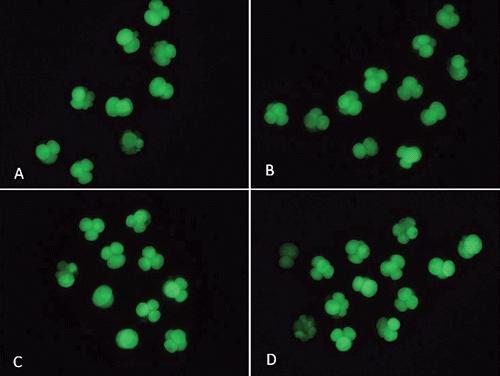
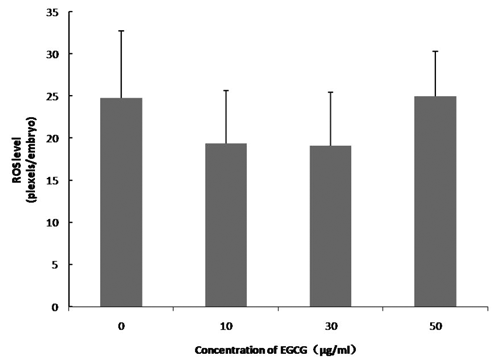
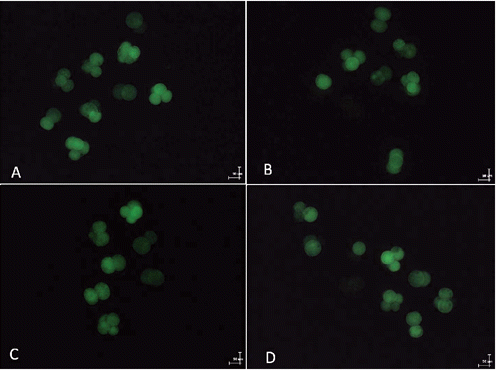
Table 1. Effect of epigallocatechin-3-gallate addition during in vitro maturation on the preimplantation development of parthenotes (five replicates).
Table 2. Effect of epigallocatechin-3-gallate addition during in vitro maturation on the preimplantation development of cloned embryos (three replicates).
Table 3. Effect of epigallocatechin-3-gallate addition during embryo in vitro culture on preimplantation developmental competence of porcine parthenogenetic embryos (five replicates).
Table 4. Effect of epigallocatechin-3-gallate addition during embryo in vitro culture on preimplantation development of porcine cloned embryos (five replicates).
References
- CampbellK.H.S. FisherP. ChenW.C. ChoiI. KellyR.D.W. LeeJ.H. XhuJ. 2007. Somatic cell nuclear transfer: past, present and future perspectives. Theriogenology 68:214-231.
- CaoZ.B. SuiL.C. LiY.S. JiS.F. ZhangX.R. ZhangY.H. 2012. Effects of chemically defined medium on early development of porcine embryos derived from parthenogenetic activation and cloning. Zygote 20:229-236.
- ChoiJ. ParkS.M. LeeE. KimJ.H. JeongY.I. LeeJ.Y. ParkS.W. KimH.S. HosseinM.S. JeongY.W. KimS. HyunS.H. HwangW.S. 2008. Anti-apoptotic effect of melatonin on preimplantation development of porcine parthenogenetic embryos. Mol. Reprod. Dev. 75:1127-1135.
- GuerinP. El MouatassimS. MenezoY. 2001. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 7:175-189.
- HashimotoS. MinamiN. TakakuraR. YamadaM. ImaiH. KashimaN. 2000. Low oxygen tension during in vitro maturation is beneficial for supporting the subsequent development of bovine cumulus-oocyte complexes. Mol. Reprod. Dev. 57:353-360.
- KooO.J. JangG. KwonD.K. KangJ.T. KwonO.S. ParkH.J. KangS.K. LeeB.C. 2008. Electrical activation induces reactive oxygen species in porcine embryos. Theriogenology 70:1111-1118.
- NakanoM. KatoY. TsunodaY. 2012. Effect of melatonin treatment on the developmental potential of parthenogenetic and somatic cell nuclear-transferred porcine oocytes in vitro. Zygote 20:199-207.
- PapisK. PoleszczukO. Wenta-MuchalskaE. ModlinskiJ.A. 2007. Melatonin effect on bovine embryo development in vitro in relation to oxygen concentration. J. Pineal Res. 43:321-326.
- PolejaevaI.A. ChenS.H. VaughtT.D. PageR.L. MullinsJ. BallS. DaiY.F. BooneJ. WalkerS. AyaresD.L. ColmanA. CampbellK.H.S. 2000. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature 407:86-90.
- Rodriguez-OsorioN. KimI.J. WangH. KayaA. MemiliE. 2007. Melatonin increases cleavage rate of porcine preimplantation embryos in vitro. J. Pineal Res. 43:283-288.
- RothZ. AroyoA. YavinS. AravA. 2008. The antioxidant epigallocatechin gallate (EGCG) moderates the deleterious effects of maternal hyperthermia on follicle-enclosed oocytes in mice. Theriogenology 70:887-897.
- SpinaciM. VolpeS. De AmbrogiM. TamaniniC. GaleatiG. 2008. Effects of epigallocatechin-3-gallate (EGCG) on in vitro maturation and fertilization of porcine oocytes. Theriogenology 69:877-885.
- SugisawaA. UmegakiK. 2002. Physiological concentrations of (-)-epigallocatechin-3-O-gallate (EGCg) prevent chromosomal damage induced by reactive oxygen species in WIL2-NS cells. J. Nutr. 132:1836-1839.
- WangZ.G. FuC.Q. YuS.D. 2013. Green tea polyphenols added to IVM and IVC media affect transcript abundance, apoptosis, and pregnancy rates in bovine embryos. Theriogenology 79:186-192.
- WangZ.G. YuS.D. XuZ.R. 2007. Effect of supplementation of green tea polyphenols on the developmental competence of bovine oocytes in vitro. Braz. J. Med. Biol. Res. 40:1079-1085.
- YavariM. NaoiH. KaedeiY. TaniharaF. NamulaZ. VietV.L. OtoiT. 2010. Effects of epigallocatechin-3-gallate on the developmental competence of parthenogenetic embryos in the pig. Ital. J. Anim. Sci. 9:e73.
- YoshiokaK. SuzukiC. TanakaA. AnasI.M. IwamuraS. 2002. Birth of piglets derived from porcine zygotescultured in a chemically defined medium. Biol. Reprod. 66:112-119.
- YouJ. KimJ. LimJ. LeeE. 2010. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology 74:777-785.
- YouJ. LeeJ. HyunS.H. LeeE. 2012. L-carnitine treatment during oocyte maturation improves in vitro development of cloned pig embryos by influencing intracellular glutathione synthesis and embryonic gene expression. Theriogenology 78:235-243.
- ZaveriN.T. 2006. Green tea and its polyphenolic catechins: medicinal uses in cancer and non-cancer applications. Life Sci. 78:2073-2080.
- ZhangY.H. LiJ. VillemoesK. PedersenA.M. PurupS. VajtaG. 2007. An epigenetic modifier results in improved in vitro blastocyst production after somatic cell nuclear transfer. Cloning Stem Cells 9:357-363.
