Abstract
Amiata donkeys are a native breed reared in central Italy. Safeguarding native donkey breeds represents an opportunity for the development of marginal areas, especially given that donkey milk is now appearing on the market due to its potential benefits for human health. To date, only a few studies have focused on the characteristics of the milk fat globules (MFGs) in the donkey species. The aim of this study was to assess the morphometric characteristics of the fat globules, gross composition and fatty acid classes in milk from Amiata donkeys reared according to the typical farming and feeding systems of the area of origin. Individual milk samples, collected from 28 Amiata donkeys between 90 and 150 days of lactation, showed the following average composition (g/100 mL): 9.47 dry matter, 1.63 protein, 0.78 casein, 0.53 fat, 7.12 lactose, and 0.36 ash. The unsaturated:saturated fatty acids ratio in milk was close to 1. The percentage of short chains was 12.29, and the percentage of long chain fatty acids was 47.64. The donkey MFGs showed an average diameter of 1.92 μm, and a number of 2.18*109/mL. Regarding MFG distribution, 70% of the globules donkey of milk are smaller than 2 μm. In conclusion, the gross composition and fatty acids of Amiata donkey milk showed similarities with milk from other Italian donkey breeds, with the exception of the monounsaturated fatty acid values which were slightly higher. Donkey MFGs had a smaller diameter and were fewer than in the ruminant species.
Introduction
Since the 19th century, Amiata donkeys have been reared in the Amiata mountains (42°53’00” N, 11°37’00” E), a large inactive volcano, in Toscana Region (central Italy). In 1993 the Anagraphic Register of the Amiata breed was established. Like most local European breeds the Amiata donkey has suffered a critical decrease in numbers, mainly as a consequence of agriculture industrialization.
Safeguarding this native donkey breed helps to protect biodiversity and is also an opportunity for developing marginal areas. Donkey rearing is also becoming increasingly common as an alternative productive option for farms, and for milk production. In fact milk from this species has been used for human consumption since ancient times, and currently is part of a new trend in alternative foods mostly aimed at consumer welfare (Salimei and Fantuz, Citation2012).
Donkey milk has been successfully used in clinical studies, in children with cow’s milk protein allergy (CMPA), and has good palatability (Monti et al., Citation2007; Dello Iacono and Limongelli, Citation2010). Its composition is more similar to human milk than ruminant milk, however it is poor in lipids, and an adequate lipid integration is needed for a toddler’s diet (D’Auria et al., Citation2011). Recently, the potential role of donkey milk has also been of increasing interest in the prevention of atherosclerosis and cardiovascular diseases (Tafaro et al., Citation2007).
To date, studies on milk fat globules (MFGs) have been conducted mainly on ruminants, as the milk from these species is commercially available. A recent review of the literature carried out by our research group (Martini et al., Citation2013b) on the macrostructure of milk lipids in ruminants reported a relationship between the dimensions of the milk fat globules and the nutritional quality of the milk. In fact, smaller globules have a larger amount of membrane per volume of fat compared to the larger globules. Thus, smaller globules provide a higher surface available for digestive enzymes, and this surface is also rich in beneficial components such as polyunsaturated fatty acids (PUFAs) (Martini et al., Citation2013a).
However, only a few studies have focused on the characteristics of fat globules in the donkey species, despite the fact that donkey milk is now commercially available due to its potential benefits for human health. The aim of this study was to assess the morphometric characteristics of milk fat globules, gross composition and fatty acid classes from Amiata donkeys reared according to the typical farming systems of the area of origin.
Materials and methods
Animals and sampling
Twenty eight individual milk samples of an average volume of 322.16±140.95 mL were collected from the morning milking of Amiata jennies. The animals were healthy, adult (from 6 to 10 years) and pluriparous, had an average live weight of 311±43 kg and a body condition score measured on a 0 to 8 scale (Pearson and Ouassat, Citation2000) of 5.1. All the jennies were semi-extensively reared in a farm in the province of Grosseto, following typical farming systems of the area of origin based on natural pasture (an area of about 50 ha were rotationally grazed by the donkeys) integrated with polyphite hay ad libitum. The hay composition was 91.53% of dry matter (DM) and on a DM basis: 12.23% of crude protein, 1.14% of ether extract, 42.19% of nitrogen free extract, 28.66% of crude fibre, 49.96% of neutral detergent fibre, 36.93% of acid detergent fibre; 9.55% of acid detergent lignine, 27.14% of cellulose, 13.06% of emicellulose and 7.30% of ash. Clean drinking water was always available.
The asses were routinely machine milked. Foals were physically separated from the dams 3-3.5 h before the first milking, following Salimei and Fantuz (Citation2012). The pilot milking machine was a wheeled trolley and was set up according to Salimei et al. (Citation2004).
Sampling was carried out from the second half of September to the second half of October 2012 when the jennies were between 90 and 150 days of lactation. Milk was taken to the laboratory in tanks at 4°C; no preservatives were added. All the analyses were carried in duplicate.
Milk analysis
Milk gross composition
The milk samples were analysed for DM, fat and lactose by infrared analysis (Milkoscan, Italian Foss Electric, Padova, Italy); and proteins, caseins and ashes (AOAC, Citation1990). Nonfat dry matter was calculated from the difference between dry matter and fat content.
Morphometric analysis of milk fat globules
The diameter and the number of fat globules per mL of milk (μm) in each sample were measured by florescence microscopy following our direct method (Martini et al., Citation2013b).
Milk fatty acid profile
Milk fat extraction was performed on the samples following Rose-Gottlieb’s method modified by Secchiari et al. (Citation2003). Methyl esters of fatty acids were prepared using methanolic sodium methoxide according to Christie (Citation1982).
A Perkin Elmer Auto System (Perkin Elmer, Norwolk, CT, USA) equipped with a flame ionization detector and a capillary column (30 m × 0.25 mm; film thickness 0.25 mm; FactorFour Varian, Middelburg, Netherlands) were used. The helium carrier gas flow rate was 1 mL·min–1. The oven temperature program was as follows: level 1, 50°C held for 2 min, level 2, 50 to 180°C at 2°C·min–1 then held for 20 min, level 3, 180 to 200°C at 1°C·min–1 then held for 15 min, and finally level 4, 200 to 220°C at 1°C·min–1 then held for 30 min. The injector and detector temperatures were set at 270 and 300°C, respectively. The peak areas of individual fatty acids (FA) were identified using an FA standard injection (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and quantified as a percentage of the total FA.
Statistical analysis
The frequency distribution of the total counted and measured MFGs was evaluated according to their size: fat globule diameters were divided into ten classes of 1 μm class width, from 0 to >9 μm. For each milk sample, the percentage of MFGs within each size class was calculated. All ten classes were represented in all the milk samples evaluated. Each milk sample was thus characterised by a different percentage of MFGs, for each diameter size class. Subsequently, the ten classes were grouped, as reported in Martini et al. (Citation2010a), into three sizes of fat globules: small globules with a <2 μm diameter, medium-sized globules with a diameter from 2 to 5 μm, and large globules with a >5 μm diameter.
Milk fatty acids classes of saturated (SFA), monounsaturated (MUFA), PUFA, short chain (SCFA), medium chain (MCFA) and long chain (LCFA) were calculated as follows:
SFA=Σ C4:0, C6:0, C8:0, C10:0, C11:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, C21:0, C22:0, C23:0, C24:0; MUFA=Σ C14:1, C15:1, C16:1, C17:1, C18:1 t9, C18:1 t11, C18:1 c9, C20:1, C22:1, C24:1; PUFA=Σ C18:2 t9,12, C18:2 c9,12, C18:3 n6, C18:3 n3, C20:2, C20:3 n6, C20:4, C20:3 n3, C20:5, C22:2, C22:5, C22:6.
SCFA=Σ of the fatty acids from 4 to 10 C; MCFA=Σ of the fatty acids from 11 to 17 C; LCFA=Σ of the fatty acids from 18 to 24 C.
Means and standard deviation of the morphometric characteristics of the MGFs, milk gross composition and milk fatty acid classes were carried out using JMP (Citation2002) software.
Results and discussion
shows means and standard deviations of the chemical composition of Amiata donkey milk. The gross composition of the milk was in agreement with the review by Polidori et al. (Citation2009) on milk from the same species. The protein content was lower than the values reported for domestic ruminants (Park et al., Citation2007), but similar to the protein content of human milk in early lactation. Thus the protein content is near to the dietary needs of children and contributes to the low renal load of solute in children drinking donkey milk (Lönnerdal, Citation2003; Salimei and Fantuz, Citation2012). From a nutritional point the ash content also contributes to the renal load of solute. Ash in Amiata donkey milk showed values between human milk and cow milk, and was consistent with other values for donkey breeds (Salimei et al., Citation2004; Guo et al., Citation2007).
Caseins constituted 48% of the nitrogen fraction of donkey milk, with similar percentage to human milk (Lönnerdal, Citation2003), but less than ruminant milk, in which caseins account for 75-80% of the total nitrogen fraction (Park et al., Citation2007; Salimei and Fantuz, Citation2012); the casein:whey protein ratio was 0.92.
The good tolerability of donkey milk in children suffering from CMPA (Monti et al., Citation2007) could thus be due to the levels of its major allergenic milk components, in fact its low casein content and casein:whey protein ratio play an important role in the sensitization capacity of the milk (Lara-Villoslada et al., Citation2005). Other factors may also help to explain the good tolerability of donkey milk, for example the number of casein fractions, the primary structure of the milk proteins, and the differences in digestibility of potential milk allergens, factors which have not yet been analysed in depth (Salimei and Fantuz, Citation2012).
The analysed milk showed an average similar lactose content to the data reported in the literature for donkeys, a higher content than cow’s milk (7.1 vs 4.6 g/100 mL), but similar to that reported for humans (6.6 g/100 mL) (Salimei et al., Citation2004; Guo et al., Citation2007). Lactose is a source of fast energy and gives donkey milk a good palatability (Innocente et al., Citation2011). In addition, the lactose content represents a substrate for the development of intestinal microbiota with health-promoting properties (Coppola et al., Citation2002). On the other hand, the majority of adults and certain ethnic groups exhibit intolerance to milk sugar (Guo et al., Citation2007).
Our results regarding the Amiata milk fat content were in agreement with other studies on two Italian donkey breeds - Ragusana and Martina Franca (Salimei et al., Citation2004; Martemucci and D’Alessandro, Citation2012). In our study the fat was also lower than values reported by Oftedal and Jenness (Citation1988). This difference may be due above all to the breed, the stage of lactation (Guo et al., Citation2007) as well as the milking technique (Salimei and Fantuz, Citation2012).
The lower fat content in donkey milk compared to human and cow’s milk (3.1% and 3.7%, respectively) (Saarela et al., Citation2005; Guo et al., Citation2007) could be a limiting factor in its use in infant nutrition in a diet exclusively based on milk, thus an appropriate lipid integration should be introduced (Iacono et al., Citation2006; D’Auria et al., Citation2011). On the other hand it is encouraging for studies on the possible use of donkey milk in dietotherapy.
The unsaturated fatty acid (UFA) percentage () was in agreement with studies on Martina Franca donkeys fed on fresh grass (Chiofalo et al., Citation2005), and semi-extensively farmed (Martemucci and D’Alessandro, Citation2012), although our MUFA values were slightly higher than those reported in previous studies.
Donkey milk consumption leads to a lower intake of SFA than bovine and small ruminant milk (Dewhurst et al., Citation2006; Park et al., Citation2007). From a nutritional point of view, despite being rich in UFA, donkey milk provides a limited amount of fat, thus the total intake of UFA per 100 mL of milk is lower (251 mg) than whole cow’s milk (1110 mg) (cow data derived from Dewhurst et al., Citation2006).
The UFA/SFA ratio in the donkey milk analysed was 1, closer to the values reported for donkeys in the review of Salimei and Fantuz (Citation2012) and to human milk (1.4) (Francois et al., Citation2003) than for ruminant milk (0.4) (Dewhurst et al., Citation2006). Similar to human milk (Yuhas et al., Citation2006), donkey milk was characterized by low amounts of short chains and high quantities of long chain fatty acids.
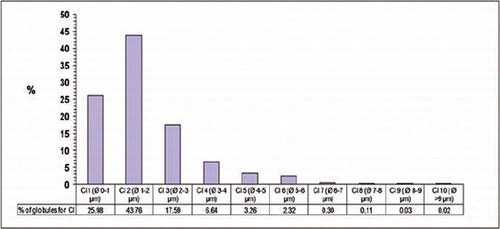
The analysis of the morphometric characteristics of the milk fat globules () highlighted that the average diameter of globules in donkeys (1.9 μm) was considerably lower than other dairy species. In fact, average diameters reported in the literature for ruminants are 3.5 and 5.5 μm for cows; 2.79 and 4.95 μm for sheep; and 2.2 and 2.5-2.8 μm for goats (Martini et al., Citation2013b). Donkey MFGs are also smaller than horse MFGs (2-3 μm) (Welsch et al., Citation1988). Regarding human MFGs, larger dimensions have also been found (4 μm) (Michalski et al., Citation2005b).
In donkey milk the distribution of globule percentages in diameter classes () was different from all the other dairy species. In fact, donkey milk has the highest percentage of globules smaller than 2 μm, approximately 70% of the total measured MFGs. In contrast, only 40% of the globules are smaller than 2 μm in goat and about 36% in sheep (Martini et al., Citation2010b, Citation2012). The fat is secreted in the mammary epithelial cells as triglyceride droplets which are excreted in milk enveloped by the cell membranes. Thus our findings seem to confirm the hypothesis that when low fat is synthesized, a higher amount of mammary epithelial cell membrane is available to envelop the milk fat globules, resulting in the secretion of smaller globules (Martini et al., Citation2013b).
The average size of donkey MFGs may be important for milk digestibility. Although there are no explanatory results in the literature, it seems that the diameter of the MFGs has a different effect on the way in which fat is digested and metabolized (Michalski et al., Citation2005a). In fact, some authors have speculated that smaller native MFGs may have the best digestive parameters due to the larger surface available for the lipase action (Raynal-Ljutovac et al., Citation2008). Differences between donkey and other dairy species have also been found regarding the number of MFGs/mL of milk. In fact, the number of donkey MFGs has been found to be lower than that found in cows, goats and sheep (Martini et al., Citation2013a).
Conclusions
The gross and fatty acid composition of Amiata donkey milk showed similarities with the milk from other Italian donkey breeds. In addition has slightly higher monounsaturated fatty acid values. The average morphometric characteristics of milk fat globules highlighted a smaller diameter and a lower number of globules per mL than species that are typically used to produce milk for human consumption.
Acknowledgments
The authors would like to thank the Regional Forestry Agricultural Complex Bandite di Scarlino (Grosseto, Italy).
Table 1. Gross composition and fatty acid classes of Amiata donkey milk.
Table 2. Morphometric characteristics of milk fat globules.
Additional information
Funding
References
- AOAC, 1990. Official methods of analysis. 15 th ed. Association of Official Analytical Chemist, Arlington, VA, USA.
- ChiofaloB. PolidoriM. CostaR. SalimeiE. 2005. Fresh forage in dairy ass’s ration: effect on milk fatty acid composition and flavours. Ital. J. Anim. Sci. 4(Suppl.2):433-435.
- ChristieW.W. 1982. A simple procedure of rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid. Res. 23: 1072-1075.
- CoppolaR. SalimeiE. SucciM. SorrentinoE. NanniM. RanieriP. BelliblanesR. GraziaL. 2002. Behaviour of Lactobacillus rhamnosus strains in ass’s milk. Ann. Microbiol. 52:55-60.
- D’AuriaE. MandelliM. BallistaP. Di DioF. GiovanniniM. 2011. Growth impairment and nutritional deficiencies in a cow’s milk-allergic infant fed by unmodified donkey’s milk. Case Rep. Pediatr. 2011:1-4.
- Dello IaconoI. LimongelliM.G. 2010. Impiego del latte di asina nel bambino con allergia alle proteine del latte vaccino: nuovi contributi. Riv. Immunol. Allergol. Pediatr. 4:10-15.
- DewhurstR.J. ShingfieldK.J. LeeM.R.F. ScollanN.D. 2006. Increasing the concentrations of beneficial polyunsaturated fatty acids in milk produced by dairy cows in high-forage systems. Anim. Feed Sci. Tech. 131:168-206.
- FrancoisC.A. ConnorS.L. BolewiczL.C. ConnorW.E. 2003. Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am. J. Clin. Nutr. 77:226-233.
- GuoH.Y. PangK. ZhangX.Y. ZhaoL. ChenS.W. DongM.L. RenF.Z. 2007. Composition, physiochemical properties, nitrogen fraction distribution and amino acid profile of donkey milk. J. Dairy Sci. 90:1635-1643.
- IaconoG. ScaliciC. D’AmicoD. D’AmicoA. Di PrimaL. PirroneG. AmbrosianoG. CarroccioA. IannolinoG. 2006. Il latte di asina nel trattamento della stipsi allergica in età pediatrica: efficacia e valore nutrizionale. pp 108-110 in Proc. 2nd Nat. Congr. on Donkey, Palermo, Italy.
- InnocenteN. ParpinelM. RinaldiA. BiasuttiM. 2011. Composition and nutritional value of donkey milk. Available from: http://www.fil-idf.org/Public/Download. php?media=39335
- JMP, 2002. User’s guide, version 5.0. SAS. Inst. Inc., Cary, NC, USA.
- Lara-VillosladaF. OlivaresM. Xaus.J. 2005. The balance between caseins and whey proteins in cow’s milk determines its allergenicity. J. Dairy Sci. 88:1654-1660.
- LönnerdalB. 2003. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 77:1537-1543.
- MartemucciG. D’AlessandroA.G. 2012. Fat content, energy value and fatty acid profile of donkey milk during lactation and implications for human nutrition. Lipids Health Dis. 11:113.
- MartiniM. AltomonteI. SalariF. 2012. Relationship between the nutritional value of fatty acid profile and the morphometric characteristics of milk fat globules in ewe’s milk. Small Ruminant Res. 105:33-37.
- MartiniM. AltomonteI. SalariF. 2013a. Evaluation of the fatty acid profile from the core and membrane of fat globules in ewe’s milk during lactation. Lebensm. Wiss. Technol. 50:253-258.
- MartiniM. LiponiG.B. SalariF. 2010a. Effect of forage:concentrate ratio on the quality of ewe’s milk, especially on milk fat globules characteristics and fatty acids composition. J. Dairy Res. 77: 239-244.
- MartiniM. SalariF. AltomonteI. 2013b. The macrostructure of milk lipids: the fat globules. Crit. Rev. Food Sci. (In press).
- MartiniM. SalariF. AltomonteI. RignaneseD. ChessaS. GigliottiC. CaroliA. 2010b. The Garfagnina goat: A zootechnical overview of a local dairy population. J. Dairy Sci. 93:4659-4667.
- MichalskiM.C. BriardV. DesageM. GeloenA. 2005a. The dispersion state of milk fat influences triglyceride metabolism in the rat - A 13CO2 breath test study. Eur. J. Nutr. 44:436-444.
- MichalskiM.C. BriardV. MichelF. TassonF. PoulainP. 2005b. Size distribution of fat globules in human colostrums, breast milk, and infant formula. J. Dairy Sci. 88:1927-1940.
- MontiG. BertinoE. MuratoreM.C. CosciaA. CresiF. SilvestroL. FabrisC. FortunatoD. GiuffridaM.G. ContiA. 2007. Efficacy of donkey’s milk in treating highly problematic cow’s milk allergic children: an in vivo and in vitro study. Pediatr. Allergy Immu. 18:258-264.
- OftedalO.T. JennessR. 1988. Interspecies variation in milk composition among horses zebras and asses (Perissodactyla: Equidae). J. Dairy Res. 55:57-66.
- ParkY.W. JuárezM. RamosM. HaenleinG.F.W. 2007. Physico-chemical characteristics of goat and sheep milk. Small Ruminant Res. 68:88-113.
- PearsonR.A. OuassatM. 2000. A guide to live weight estimation of body condition scoring of donkey. Centre for tropical veterinary medicine, University of Edinburgh. Available from: http://www.cd3wd.com/cd3wd_40/LSTO CK/001/Equines/Condition%20Scoring%20Donkeys/Condition%20Scoring%20Donkeys.pdf
- PolidoriP. BeghelliD. MarianiP. VincenzettiS. 2009. Donkey milk production: state of the art. Ital. J. Anim. Sci. 8(Supl.2):677-683.
- Raynal-LjutovacK. LagriffoulG. PaccardP. GuilletI. ChilliardY. 2008. Composition of goat and sheep milk products: an update. Small Ruminant Res. 79:57-72.
- SaarelaT. KokkonenJ. KoivistoM. 2005. Macronutrient and energy contents of human milk fractions during the first six months of lactation. Acta Paediatr. 94:1176-1181.
- SalimeiE. FantuzF. 2012. Equid milk for human consumption. Int. Dairy J. 24:130-142.
- SalimeiE. FantuzF. CoppolaR. ChiofaloB. PolidoriP. VariscoG. 2004. Composition and characteristics of ass’s milk. Anim. Res. 53:67-78.
- SecchiariP. AntongiovanniM. MeleM. SerraA. BuccioniA. FerruzziG. PaolettiF. PetacchiF. 2003. Effect of kind of dietary fat on the quality of milk fat from Italian Fresian cows. Livest. Prod. Sci. 83:43-52.
- TafaroA. MagroneT. JirilloF. MartemucciG. D’AlessandroA.G. AmatiL. JirilloE. 2007. Immunological properties of donkey’s milk: its potential use in the prevention of atherosclerosis. Curr. Pharm. Design 13:3711-3717.
- WelschU. BuchheimW. SchumacherU. SchinkoI. PattonS. 1988. Structural, histochemical and biochemical observations on horse milk-fat-globule membranes and casein micelles. Histochemistry 88:357-365.
- YuhasR. PramukK. LienE.L. 2006. Human milk fatty acid composition from nine countries varies most in DHA. Lipids 41:851-857.