Abstract
Phospholipid hydroperoxide glutathione peroxidase (PHGPx) is a selenoprotein, which protects biomembranes from oxidative damages, and it also accounts for almost the entire selenium content of mammalian testis. The present investigation was performed to localise PHGPx in the testis and in epididymal and ejaculated spermatozoa of the bull by using light and electron immunomicroscopy. The study also aimed to further clarify the possible functions of the protein in bull fertility. In the testis, spermatogenic cells of the adluminal tubular compartment showed cytoplasmatic immunostaining; whereas, in the epididymal and ejaculated spermatozoa immunostaining was specifically localised at the level of the head and mid-piece. Ultrastructural data revealed the presence of signals for PHGPx in different subcellular compartments of maturing and mature sperm (mitochondria, chromatin, nuclear envelope, acrosomes, cytoskeletal structures) suggesting that this enzyme plays versatile and important biological roles during spermatogenesis. The final localisation of the immunostaining at acrosomal level puts forward a new role of the protein which further emphasises its relevance in male reproduction: it is reported to anchor substrate of the sperm acrosome to the oocyte zona pellucida during the fertilisation process.
Keywords:
Introduction
Selenium (Se) deficiency has been linked to reproductive problems and reduced sperm quality in rats, mice, chickens, pigs, sheep, and cattle (Combs and Combs, Citation1986; Olson et al., Citation2004; Baiomy et al., Citation2009). Selenium supplementation to diets has been reported to improve reproductive performance in mice, sheep and cattle (Sanders, Citation1984; Tang et al., Citation1991; Van Ryssen et al., Citation1992; Aréchiga et al., Citation1998), while others have not found association between herd milk and blood Se concentrations and fertility parameters (Ropstad and Refsdal, Citation1987; Jukola et al., Citation1996).
The phospholipid hydroperoxide glutathione peroxidase (PHGPx) is an enzyme working as the deposit of almost the entire Se content in the mammalian testis. The enzyme is a member of the large subfamily of the glutathione peroxidase (GPx) selenoproteins, most of which are antioxidant enzymes that reduce hydroperoxides at the expense of glutathione (GSH). However, PHGPx has a number of unique features compared to other family members, making the role of this enzyme in the testis worth of particular interest. Among GPx, PHGPx is the least specific (Ursini et al., Citation1995, Citation1997; Maiorino and Ursini, Citation2002) reducing a broad spectrum of hydroperoxides and accepting different thiols, including protein thiols as reductants. By catalysing hydroperoxide reduction, PHGPx plays a key antioxidant function, controls inflammatory responses (Schnurr et al., Citation1996; Imai et al., Citation1998; Sakamoto et al., Citation2000), and inhibits apoptosis (Nomura et al., Citation2001). Additionally, in spermatogenesis, PHGPx plays a major role in oxidising specific protein thiols. Phospholipid hydroperoxide glutathione peroxidase is also represented by glutathione peroxidase 4 (GPx4) and three different isoforms are identified (Brigelius-Flohé and Maiorino, Citation2013): cytosolic (cGPx4), mitochondrial (mGPx4) and sperm nuclear GPx4 (snGPx4). Indeed, it is associated with the cytoplasm, mitochondria and nucleus of spermatogenic cells in rodent testis, suggesting that this enzyme plays versatile roles during the various phases of spermatogenesis (Tramer et al., Citation2002; Haraguchi et al., Citation2003). However, PHGPx – abundantly expressed in early spermatogenic cells as an active peroxidase – is transformed into an enzymatically inactive insoluble structural protein during the late phase of spermatogenesis (Ursini et al., Citation1999). Therefore, it makes up the keratin-like material, constituting the mitochondrial capsule that surrounds the helix of mitochondria in the sperm mid-piece. Hence, this function may explain the Se deficiency dependent alterations in sperm cells, suggesting a new approach to investigate male fertility dependency on Se. Previously, a reduced PHGPx content was reported in both infertile human males (Foresta et al., Citation2002) and bulls with impaired seminal characteristics and the normal motility and morphology of mature spermatozoa were demonstrated to be associated with a high PHGPx content (Stradaioli et al., Citation2009).
The aim of this study was to localise subcellular PHGPx in spermatogenic cells and epididymal and ejaculated spermatozoa of the bull. Our results should help to clarify the biological significance of PHGPx in the fertilisation process, its clinical relevance and whether it has any role in improving bovine reproductive efficiency.
Materials and methods
Immunohistochemistry in light microscopy
Immunostaining was performed on bull testes and isolated epididymal and ejaculated spermatozoa. Samples of testes were obtained, immediately after slaughter, from 5 Chianina bulls clinically healthy aged 32±8 months. They were fixed by immersion in Bouin’s fixative for 24 h, dehydrated and embedded in paraffin wax. The paraffin sections were cut to 5-6 μm of thickness. The epididymides were rapidly isolated from the testes and divided into caput, corpus and cauda in order to collect the spermatozoa separately from the distinct regions as described by Seligman et al. (Citation1991). In brief, pieces of tissue were shaken in 0.1M phosphate buffered saline (PBS) at pH 7.4 and 37°C for 15 min to allow the release of spermatozoa and the epididymal fluid. The ejaculated spermatozoa were collected by means of an artificial vagina from 5 Chianina bulls of proven fertility aged 40±19 months. Epididymal and ejaculated spermatozoa were washed twice by centrifugation for 10 min at 600 g in 0.1M PBS at pH 7.4 and then smeared on double glass slides, air-dried and stored at-70°C until analysis. A series of smear preparations were treated with 6M guanidine-HCl, 10 mM GSH, 10 mM dithiothreitol (DTT) and 10 mM 2-mercaptoethanol (2-ME) for 30 min at room temperature (RT) and fixed in 4% paraformaldehyde for 30 min (Haraguchi et al., Citation2003). Subsequently, the testis sections and smear preparations, with and without the prior abovementioned treatments, were subjected to immunohistochemical procedures as previously described (Zerani et al., Citation2012, Citation2013a, Citation2013b; Colitti and Parillo, Citation2013; Parillo et al., Citation2013, Citation2014a, Citation2014b). Briefly, the testis sections were incubated with 3% H2O2 for 20 min to block the endogenous peroxidase activity. After rinsing in PBS containing 0.2% Triton X-100 and 0.1% bovine serum albumin (BSA), background labelling was prevented by incubating the slides with normal goat serum (NGS), diluted 1:10, for 30 min at RT. Then, the slides were incubated overnight in a humid chamber at 4°C with the primary antiserum, rabbit anti-PHGPx (supplied by Prof. Ursini, University of Padova), diluted 1:400. The next day, the slides were washed in PBS and incubated with biotin goat anti-rabbit IgG conjugate (Zymed 81-6140) diluted 1:200 for 30 min. After PBS washes, the slides were exposed to avidinbiotin complex (ABC kit; Vector Labs, Burlingame, CA, USA) for 30 min. The slides were rinsed again and the immunoreactive cells were visualised using a freshly prepared solution of 3,3’-diaminobenzidine tetrahydro-cloride-hydrogen peroxidase (DAB-H2O2 kit; Vector Labs). Finally, after washing in tap water, the slides were dehydrated through an ethanol series, cleared in xylene, and mounted in natural Canada balsam (BDH Chemicals, Radnor, PA, USA). Slides on which the primary antibody was omitted or substituted with rabbit IgG, were used as negative control of unspecific staining.
Postembedding immunoelectron microscopy
Samples of testes, isolated epididymal (from caput, corpus and cauda epididimys) and ejaculated sperms were also processed for immunogold microscopy. Samples of testes were fixed with 4% paraformaldehyde and 0.25% gluteraldehyde in 0.1M cacodylate buffer (pH 7.4) for 1.30 h at 4°C. Aliquots of epididymal and ejaculated spermatozoa from each bull were diluted in PBS, centrifuged twice (600xg) and the resulting pellets were fixed as above. The samples were washed several times in a cacodylate buffer, dehydrated in a graded series of ethanol up to absolute and embedded in Bioacryl resin (Bio-optica, Milan, Italy) (Parillo et al., Citation2003, Citation2005). The ultrathin sections (90 nm) were mounted on 200-mesh nickel grids, treated with 1% BSA in 0.1M tris buffered saline (TBS) pH 7.4 for 5 min at RT, and then incubated overnight in a humid chamber at 4°C with a solution of 0.1M TBS containing 1% BSA, 1% NGS, 0.1% Tween-20 and anti-PHGPx rabbit polyclonal antibody (diluted 1:400). After several washes in TBS to remove the excess antibody, the sections were incubated for 20 min at RT with the goat anti-rabbit IgG secondary 10 nm gold-conjugated antibody (Sigma Chemicals, St. Louis, MO, USA) diluted 1:10 in 0.1M TBS pH 7.4 plus 1% BSA and 0.05% Tween 20. As controls, some grids were treated with the incubation mixture without the primary antibody and then processed as described above. Finally, after counterstaining with uranyl acetate, the sections were examined with a Philips EM 208 electron microscope (Philips, Amsterdam, The Netherlands).
Results
Immunohistochemistry in light microscopy
In the testis sections, specific immunostaining by antiserum against PHGPx was evident in the apical compartment of the seminiferous tubules where it was localised in the cytoplasm of maturing sperm cells; whereas there was any apparent immunostaining in the basal compartment of the tubules (). In epididymal (caput, corpus, cauda) and ejaculated spermatozoa, weak staining was localised in the head and mid-piece portion (), whereas the tails resulted constantly negative. Treatment with 6M guanidine-HCl, 10 mM DTT as well as with 10 mM 2-ME strongly enhanced the reactivity of the sperm mid-piece (), while treatment with 10 mM GSH strongly increased immunostaining of the sperm head (). The positive staining was missing in control sections (). Results are summarised in .
Postembedding immunoelectron microscopy
Only at electron microscopy it was possible to detect the presence of gold particles for PHGPx in different subcellular structures of sperm cells at various phases of the spermatogenic cycle.
In spermatogonia, PHGPx labelling was evidenced in the matrix of the mitochondria, cytosol and euchromatin ().
In round spermatids, characteristic PHGPx reactivity appeared in the acrosomal vesicles; the number of gold particles gradually increased as the acrosomes developed (,). Furthermore, PHGPx signals were observed in the matrix, in the outer membrane of the mitochondria and the cytosol (). In the nuclei of these cells, PHGPx positivity was localised in the heterochromatin and over the nuclear envelope ().
In elongated spermatids, the mitochondria surrounded the flagellar axoneme to form a spiral structure; PHGPx labelling was located in the matrix and mainly in the outer membrane (,). Signals for PHGPx were also observed in the condensed chromatin and over the nuclear envelope, in the residual cytoplasm () and the acrosome (,). At this stage of germ cell development, a few gold particles for PHGPx were also noted in the outer dense fibres of mid-piece cytoskeletal structures ().
Epididymal and ejaculated spermatozoa showed very similar immunogold signals; in particular, gold labelling for PHGPx was detected in the acrosome, in the condensed chromatin and the nuclear envelope. In the midpiece, PHGPx signals were closely associated with the outer mitochondrial membrane and concentrated in the outer dense fibres, which surround the flagellar axoneme (). An immunocytochemical response was also seen in the main piece of the tail at the level of both the outer dense fibres and the fibrous sheet, which underlies the plasma membrane (). In addition, gold particles were observed in the cytoplasmic droplets of spermatozoa (). There was no immunostaining in the control sections.
Discussion
Specific immunostaining for PHGPx was revealed in the apical region of the seminiferous tubules with a light microscope. In epididymal and ejaculated sperm, immunostaining for PHGPx was more evident if the sperm smears had been treated with chaotropic or reducing agents. The enhancement of immunoreactivity following some pre-treatments suggests that the access of antibody to PHGPx in ejaculated sperm is hindered. It is possible, as suggested by Roveri et al. (Citation1992), that in spermatozoa the protein could be embedded in a complex matrix, hampering its interaction with the antibody. It is clear that only chaotropic treatments are capable of rendering more protein from the heads and mitochondria of the midpiece accessible.
More detailed information on the presence of PHGPx signals in bull testes and isolated spermatozoa were obtained with immunogold electron microscopy, which allows also some considerations about the functional role of this protein. phospholipid hydroperoxide glutathione peroxidase immunostaining was evident at the mitochondria, chromatin, cytosol and acrosome levels; indeed, these localisation sites were similar to those previously described in the rat (Tramer et al., Citation2002; Haraguchi et al., Citation2003) and the mouse (Nayernia et al., Citation2004).
Mitochondria resulted positive throughout the differentiation process although changes in the localisation of PHGPx labelling during the various phases of sperm cell maturation were evidenced. In fact, PHGPx signals were localised in the matrix of the mithocondria during spermatogonia developmental stage, evidenced both in the matrix and in the mitochondrial outer membrane during round spermatids stage, and were strongly associated to the outer membrane in elongated spermatids and in mature sperm. The translocation of PHGPx signals from the matrix to the peripheral region of the mitochondria was previously reported in the rat (Haraguchi et al., Citation2003). It has been hypothesised that the re-localisation of PHGPx in the mitochondrial outer membrane may indicate the switch of PHGPx from a soluble active enzyme, involved in the reduction of H2O2 generated in the mitochondria, to an enzymatically inactive, oxidatively crosslinked and insoluble protein that performs a structural role in forming the mitochondrial capsule (Ursini et al., Citation1999).
Cytosolic PHGPx positivity was also evident in all maturation stages; in fact, the enzyme plays a crucial role in reducing intracellular increases in the concentration of H2O2 dispersed in the cytoplasm matrix, reflecting the need to protect these cells from oxidative damage caused by hydroperoxides (Arai et al., Citation1999).
The aforementioned localisation is likely due to the change in substrate availability of the enzyme; in fact, the most striking observation related to PHGPx substrate specificity was that protein thiols can take over the function of GSH as reductants when the latter becomes limited. This has been shown for chromatin (Godeas et al., Citation1996), for sperm mitochondria associated cysteine-rich protein (Maiorino et al., Citation2005) and even for GPx4 itself (Ursini et al., Citation1999; Mauri et al., Citation2003). Thus, depending on the availability of GSH, GPx4 can act either as a GSH peroxidase or as a thiol peroxidase.
Chromatin positivity could indicate an important role of PHGPx in the maturation process of sperm cell nuclei by protecting them from mutagens and chromatin from autolysis and, in general, by protecting the nucleus from external stress before fertilisation. In fact, it is well known that sperm maturation in testes ends with nucleus compaction achieved by protamines replacing nuclear histones. Through this, the sperm haploid genome is compacted to ca. 1/10 the size of the nucleus of other somatic cells (Miller et al., Citation2010). This process does not end with the entry of spermatozoa into epididymal lumen when they are still functionally immature, namely, they are not yet able to move appropriately in female genital tract and to recognise, bind to and penetrate oocytes. Indeed, it has been demonstrated that, apart from a protective role against DNA peroxidative damage, PHGPx acts as a protamine thiol peroxidase, responsible for the formation of cross-linked protamine disulfide (Pfeifer et al., Citation2001), thus contributing to the structural stability of sperm chromatin (Conrad et al., Citation2005). The positivity of the spermatid’s and mature sperm’s nuclear envelope may have a role as a structural protein similar to the PHGPx associated with the mitochondrial outer membrane (Haraguchi et al., Citation2003). In fact, it has been recently demonstrated that mGPx4 knockout male mice are sterile as they produce spermatozoa with structural abnormalities, such as bent sperm, sperm heads detached from the mid-piece, sliding mitochondria along the mid-piece, i.e. with characteristics reminding of long-term Se deficiency (Schneider et al., Citation2009).
In addition, the fact that PHGPx has also been localised in the acrosomal granules and membranes of both spermatids and mature spermatozoa leads us to hypothesise an additional effect of male gamete PHGPx, released during acrosomal reaction and the early steps of fertilisation. We suggest that acrosomal PHGPx could be part of the insoluble matrix which persists after acrosome reaction allowing stabile binding of the sperm head to the oocyte zona pellucida during the early steps of the fertilisation process (Bedford, Citation2014). This could be a further functional explanation of Se deficiency related sterility.
Finally, the presence of PHGPx in the outer dense fibres and fibrous sheet seems to suggest that PHGPx plays an important function in stabilising cytoskeletal structures.

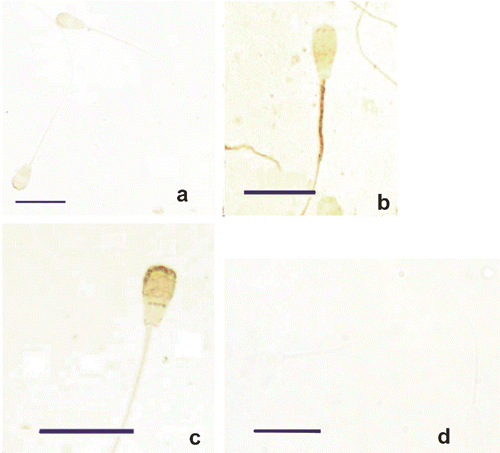
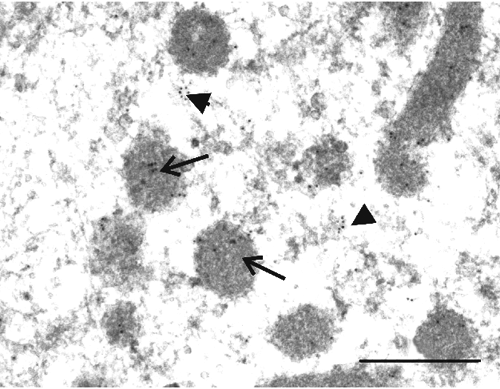
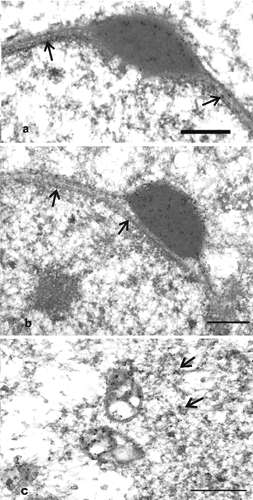
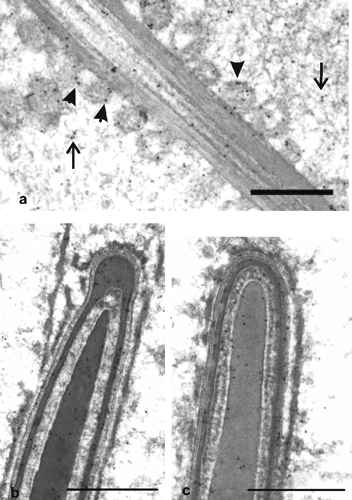
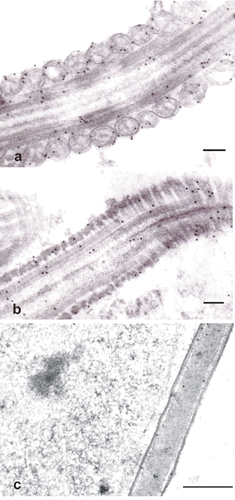
Table 1. Localisation of phospholipid hydroperoxide glutathione peroxidase immunosignals at light microscopy.
Conclusions
Our immunocytochemical study clearly evidenced the presence of PHGPx in different subcellular compartments of bull sperm together with dynamic changes occurring to the protein in the cells during the maturative processes. These findings confirm biochemical data suggesting that PHGPx is required for a correct development and function of mammalian spermatozoa (Ursini et al., Citation1999). The structural function ascribed to the protein may therefore explain the Se-deficiency-dependent alterations in sperm cells. Indeed, ultrastructural analyses of spermatozoa from Se deficient animals have identified structural irregularities in the mid-piece mitochondria where the enzyme is amply present (Olson et al., Citation2004). In addition, in both humans and bulls failure of the expression of mitochondrial PHGPx in spermatozoa has been reported to be one of the possible causes of oligoasthenozospermia (Foresta et al., Citation2002; Stradaioli et al., Citation2009).
Acknowledgements
The present research was supported by an Italian Ministry of Education (MIUR) grant (PR1N-2007M3E2T2).
References
- AraiM. ImaiH. KoumuraT. YoshidaM. EmotoK. UmedaM. ChibaN. NakagawaY., 1999. Mitochondrial phospholipid hydroperoxide glutathione peroxidase plays a major role in preventing oxidative injury to cells. J. Biol. Chem. 274:4924-4933.
- AréchigaC.F. Vázquez-FloresS. OrtizO. Hernandez-CerónJ. PorrasA. McDowellL.R. HansenP.J., 1998. Effect of injection of beta-carotene or vitamin E and selenium on fertility of lactating dairy cows. Theriogenology 50:65-76.
- BaiomyA.A. MohamedA.E.A. MottelibA.A., 2009. Effect of dietary selenium and vitamin E supplementation on productive and reproductive performance in rams. pp 43-46. in Proc. 14th Int. Congr. on Animal Hygiene, Vechta, Germany.
- BedfordJ.M., 2014. Singular features of fertilization and their impact on the male reproductive system in the eutherian mammals. Reproduction 147:43-52.
- Brigelius-FlohéR. MaiorinoM., 2013. Glutathione peroxidases. Biochim. Biophys. Acta 1830:3289-3303.
- ColittiM. ParilloF., 2013. Immunolocalization of estrogen and progesterone receptors in ewe mammary glands. Microsc. Res. Techniq. 76:955-962.
- CombsG.F.Jr. CombsS.B., 1986. The role of selenium in nutrition. Academic Press, San Diego, CA, USA.
- ConradM. MorenoS.G. SinowatzF. UrsiniF. KölleS. RoveriA. BrielmeierM. WuretW. MaiorinoM. BornKammG.W., 2005. The nuclear form of phospholipid hydroperoxide glutathione peroxidase is a protein thiol peroxidase contributing to sperm chromatin stability. Mol. Cell. Biol. 25:7637-7644.
- ForestaC. FlohèL. GarollaA. RoveriA. UreiniF. MaiorinoM., 2002. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol. Reprod. 67:967-971.
- GodeasC. TramerF. MicaliF. RoveriA. MaiorinoM. NisiiC. SandriG. PanfiliE., 1996. Phospholipid hydroperoxide glutathione peroxidase (PHGPx) in rat testis nuclei is bound to chromatin. Biochem. Mol Med. 59:118-124.
- HaraguchiC.M. MabuchiT. HirataS. ShodaT. YamadaA.T. HoshiK. YokotaS., 2003. Spatio-temporal changes of levels of a moonlighting protein, phospholipid hydroperoxide glutathione peroxidase, in subcellular compartments during the spermatogenesis in the rat testis. Biol. Reprod. 69:885-895.
- ImaiH. NarashimaK. AraiM. SakamotoH. ChibaN. NakagawaY., 1998. Suppression of leukotriene formation in RBL-2H3 cells that overexpressed phospholipid hydroperoxide glutathione peroxidase. J. Biol. Chem. 273:1990-1997.
- JukolaE. HakkarainenJ. SaloniemiH. SankariS., 1996. Blood selenium, vitamin E, vitamin A and ß-carotene concentrations and udder health, fertility treatments and fertility. J. Dairy Sci. 79:838-845.
- MaiorinoM. RoveriA. BenazziL. BoselloV. MauriP. ToppoS. TosattoS.C. UrsiniF., 2005. Functional interaction of phospholipid hydroperoxide glutathione peroxidase with sperm mitochondrion-associated cysteine-rich protein discloses the adjacent cysteine motif as a new substrate of the selenoperoxidase, J. Biol. Chem. 280:38395-38402.
- MaiorinoM. UreiniF., 2002. Oxidative stress, spermatogenesis and fertility. Biol. Chem. 383:591-597.
- MauriP. BenazziL. FlohéL. MaiorinoM. PiettaP.G. PilawaS. RoveriA. UrsiniF., 2003. Versatility of selenium catalysis in PHGPx unraveled by LC/ESI–MS/MS. Biol. Chem. 384:575-588.
- MillerD. BrinkworthM. IlesD., 2010. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 139:287-301.
- NayerniaK. DiaconuM. AumUllerG. WennemuthG. SchwandtI. KleeneK. KuehnH. EngelW., 2004. Phospholipid hydroperoxide glutathione peroxidase: expression pattern during testicular development in mouse and evolutionary conservation in spermatozoa. Mol. Reprod. Dev. 67:458-464.
- NomuraS.Y. ImaiH. KoumuraT. NakagawaY., 2001. Involvement of mitochondrial phospholipid hydroperoxide glutathione peroxidase as an antiapoptotic factor. Biol. Signal. Recept. 10:81-92.
- OlsonG.E. WinfreyV.P. HillK.E. BurkR.F., 2004. Sequential development of flagellar defects in spermatids and epididymal spermatozoa of selenium-deficient rats. Reproduction 127:335-342.
- ParilloF. BoitiC. MaranesiM. Dall’AglioC. GaleatiG. BrecchiaG. ZeraniM. González-MariscalG., 2014a. Ovarian hormones and fasting differentially regulate pituitary receptors for estrogen and gonadotropin-releasing hormone in rabbit female. Microsc. Res. Techniq. 77:201-210.
- ParilloF. CatoneG. CapezzoneC. ZeraniM., 2013. Presence of immunoreactive cyclooxygenases in the ductuli efferentes and epididymis of prepubertal and adult alpaca (Lama pacos). Vet. Arhiv 83:677-684.
- ParilloF. Dall’AglioC. Verini SuppliziA. CeccarelliP. GargiuloA.M., 2003. Immunogold study on lectin binding in the porcine zona pellucida and granulosa cells. Eur. J. Histochem. 47:353-358.
- ParilloF. MaranesiM. BrecchiaG. GobbettiA. BoitiC. ZeraniM., 2014b. In vivo chronic and in vitro acute effects of di(2-ethylhexyl) phthalate on pseudopregnant rabbit corpora lutea: possible involvement of peroxisome proliferator-activated receptor gamma. Biol. Reprod. 90:41.
- ParilloF. ZelliR. Verini SuppliziA. FagioliO. GargiuloA.M., 2005. Topographical localisation of glucidic residues and their variations in the canine zona pellucida during folliculogenesis. J. Mol. Histol. 36:131-137.
- PfeiferH. ConradM. RoethleinD. KyriakopoulosA. BrielmeierM. BornakammG.V. BehneD., 2001. Identification of a specific sperm nuclei selenoenzyme necessary for protamine thiol cross-linking during sperm maturation. FASEB J. 15:1236-1238.
- RopstadE. RefsdalA.O., 1987. Herd reproductive performance related to urea concentration in bulk milk. Acta Vet. Scand. 28:55-63.
- RoveriA. CasascoA. MaiorinoM. Daleanp CalligaroA. UrsiniF., 1992. Phospholipid hydroperoxide glutathione peroxidase of rat testis: gonadotropin dependency and immunocytochemical identification. J. Biol. Chem. 267:6142-6146.
- SakamotoH. ImaiH. NakagawaY., 2000. Involvement of phospholipid hydroperoxide glutathione peroxidase in the modulation of prostaglandin D2 synthesis. J. Biol. Chem. 275:40028-40035.
- SandersD.E., 1984. Use of selenium in problem cattle herds. Mod. Vet. Pract. 65:136-138.
- SchneiderM. ForsterH. BoersmaA. SeilerA. WehnesH. SinowatzF. NeumullerC. DeutschM.J. WalchA. Hrabe de AngelisM. WuretW. UreiniF. RoveriA. MaleszewskiM. MaiorinoM. ConradM., 2009. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 23:3233-3242.
- SchnurrK. BelknerJ. UrsiniF. ScheweT. KuhnH., 1996. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxigenase with complex substrates and preserves the specificity of the oxygenation products. J. Biol. Chem. 271:4653-4658.
- SeligmanJ. ShalgiK. OschryY. KosowerN.S., 1991. Sperm analysis by cytometry using the fluorescent thiol labeling agent monobromobimane. Mol. Reprod. Dev. 29:276-281.
- StradaioliG. SyllaL. MonaciM. MaiorinoM., 2009. PHGPx in bull spermatozoa provides a unique marker in the quest for semen quality analysis. Theriogenology 72:91-98.
- TangC.C. ChenH.N. RuiH.F., 1991. The effects of selenium on gestation, fertility, and offspring in mice. Biol. Trace Elem. Res. 30:227-231.
- TramerF. MicaliF. SandriG. BertoniA. LenziA. GandiniL. PanfiliE., 2002. Enzymatic and immunochemical evaluation of phospholipid hydroperoxide glutathione peroxidase (PHGPx) in testes and epididymal spermatozoa of rats of different ages. Int. J. Androl. 25:72-83.
- UreiniF. HeimS. KiessM. MaiorinoM. RoveriA. WissingJ. FlohéL., 1999. Dual function of the selenoprotein PHGPx during sperm maturation. Science 285:1393-1396.
- UreiniF. MaiorinoM. Brigelius-FlohéR. AumannK.D. RoveriA. SchomburgD. FlohéL., 1995. The diversity of glutathione peroxidases. Method. Enzymol. 252:38-53.
- UrsiniF. MaiorinoM. RoveriA., 1997. Phospholipid hydroperoxide glutathione peroxidase (PHGPx): more than an antioxidant enzyme? Biomed. Environ. Sci. 10:327-332.
- Van RyssenJ.B. BradfieldmG.D. Van MalsenS. De VilliersJ.F., 1992. Response to selenium supplementation of sheep grazing cultivated pastures in the natal midlands. J. S. Afr. Vet. Assoc. 63:148-155.
- ZeraniM. CatoneG. BettiG. ParilloF., 2013a. Immunopresence and functional activity of prostaglandin-endoperoxide synthases and nitric oxide synthases in bovine corpora lutea during diestrus. Folia Morphol. 72:36-40.
- ZeraniM. CatoneG. MaranesiM. GobbettiA. BoitiC. ParilloF., 2012. Gonadotropin-releasing hormone 1 directly affects corpora lutea lifespan in mediterranean buffalo (Bubalus bubalis) during diestrus: presence and in vitro effects on enzymatic and hormonal activities. Biol. Reprod. 87:1-8.
- ZeraniM. MaranesiM. BrecchiaG. GobbettiA. BoitiC. ParilloF., 2013b. Evidence for a luteotropic role of peroxisome proliferator-activated receptor : expression and in vitro effects on enzymatic and hormonal activities in corpora lutea of pseudopregnant rabbits. Biol. Reprod. 88:1-9.
