Abstract
Re-authorization of processed animal proteins (PAPs) in EU, derived from by-products of human food production, could increase manufacturing of proteins for feed ingredients and reduce the need of imported proteins mainly of plant origin. The PAPs production is largely done by the rendering process during which authorized animal by-products are heat treated to extract valuable protein and animal fat, ensuring sterilizing conditions of raw incoming materials. Proteins exposure to certain processing conditions induces two important chemical changes, racemization of amino acids and formation of cross-linked amino acid. These changes are associated with appreciable reduction of protein digestibility and nutritional value. The aim of this study was to verify the effect of heat treatment on amino acid racemization in processed animal proteins of poultry origin and related nutritional implications by evaluation of their in vitro digestibility. The results reported confirm the detection of racemized amino acids in processed animal proteins, especially D-aspartic acid, as realistic indicators of thermal treatments during PAP manufacturing. In our results, the severe (115°C) and prolonged heat treatment (180 minutes) revealed a D-Asp content of 28.1%.
Prolongation of temperature treatment (20, 30 and 180 min, at 115°C) significantly (P<0.05) affects in vitro protein digestibility, which decrease from 86.0%, in no-treated sample, to 78.3% and 79.1% after 20 and 30 min, respectively, and to 76.3% after 180 min. The processing conditions applied during PAPs preparations and the racemization of proteins amino acids may reasonably be involved in the loss of protein quality.
Introduction
The first ban concerning the use of mammalian protein in feedstuffs introduced in 1994 (European Commission, Citation1994) together with other measures adopted in the rendering system and the handling of specific risk material played a critical role in the bovine spongiform encephalopathy (BSE) eradication in Europe.European regulations (European Commission, Citation2002, Citation2009, Citation2011) have introduced new features concerning the fact that only category 3 materials from animals declared by veterinary inspection fit but not intended for human consumption, may be used in the production of processed animal proteins (PAPs), and established the ban of intra-specie recycling. Actually, possible uses of third category material provide different applications (European Food Safety Authority, Citation2011; European Commission, Citation2011): i) incineration and disposal; ii) co-incineration for energy production; iii) catalytic conversion into liquid fuels; iv) composting into a fertiliser used as v) organic fertilisers and soil improvers after adequate alkaline hydrolysis or vi) production of pet foods; vii) rendering for PAPs production. In the EU approximately 18 million metric tons per year of slaughter by-products are produced by meat industry, of which more than 14.5 million tonnes from animals declared fit for human consumption (EFPRA, Citation2013). In 2012, PAPs production in Europe stood at 2.86 million tons and Europe’s protein production covers only 30% of the consumption (EFPRA, Citation2014). Combinations of heat, time and pressure (known as rendering process) are the safest way to stabilize raw materials, allowing water evaporation and ensuring sterilizing conditions. However, the efficacy of the treatment could vary according to the state of desiccation and particle size of the product, and its lipid content; actually, most of rendering processes refer to high fat raw material. The PAPs production is largely done by a wet rendering process, during which most of the water and part of the fat present in the raw material are removed (Woodgate and Van der Veen, Citation2004). The European Commission (Citation2013; Regulation Citation2013/56/EU) reauthorized PAPs in EU derived from animal by-products, which are fit for human consumption at the point of slaughter, in feed for farmed fish. Ruminant PAPs remains banned and stringent controls, including species of origin testing, should ensure that only poultry and porcine PAP enter the feed chain. The PAP producers have had to comply with severe legal aspects to prevent involuntary cross contamination with prohibited species (i.e., raw material only from slaughter houses, cutting plants handling non-ruminant species).
Concerning destination of the animal byproducts, two main risks emerge from their use. The first risk is that the by-products, also after rendering procedures, would carry a residual TSE infectivity (Taylor et al., Citation1995). The second issue arises from the nutritive value of the treated material after the intense heat treatment, usually applied to the animal waste, especially for the production of protein hydrolyzates (Piva et al., Citation2001; Hendriks et al., Citation2002, Citation2006). Exposure to proteins to certain processing conditions induces two major chemical changes, racemization of amino acids and formation of cross-linked amino acid (Liardon and Hurrell, Citation1983; Man and Bada, Citation1987; Friedman, Citation1999). These changes are associated with appreciable reduction (2-7%) of protein digestibility, particularly the digestibility of cystine (16-26% reduction) and aspartic acid (7-11% reduction) (Miller, Citation2003). Kinetics of racemization obtained as a function of time, temperature and acid or alkaline treatment are described for the free amino acids and for several purified and natural proteins (Friedman and Masters, Citation1982; Liardon and Hurrell, Citation1983; Bada, Citation1984; Kaiser and Benner, Citation2005). The treatments in the above mentioned kinetics studies are not always comparable with conditions obtained in PAPs production industry. Therefore, to evaluate the reliability of D-amino acids content as an indicator of the severity of thermal treatments during PAP manufacturing and its significance to evaluate processing conditions applied, racemization data should be obtained on whole material subjected to physical conditions similar to those which may occur in PAPs production. In a previous work (Luzzana et al., Citation1999), it was demonstrated that thermal treatment and different rendering conditions affect aspartic acid racemization in fish meal. Processing temperature, pH and moisture content of the raw material are the major factors affecting the rate of racemization of aspartic acid.
The aim of this study was to verify the effect of heat treatment on amino acid racemization in processed animal proteins of poultry origin, obtained in laboratory under time-temperature controlled conditions and during rendering process in an industry plant. Evaluation of in vitro digestibility of PAPs was reported, aiming to consider the nutritional implications of reintroduction of PAPs in animal nutrition.
Materials and methods
Chemicals and materials
Chemicals
Chemicals and solvents were of analytical grade and all supplied from Sigma Chemicals (Sigma-Aldrich, St Louis, MO, USA). Derivatization and purification reagents for DL-amino acids HPLC analysis: o-phthaldialdehyde (OPA) and N-isobutyryl-D-cysteine (IBDC); cation exchange resin, Dowex-50w 200-400 mesh. Standard reference proteins: lyophilized powder of bovine -lactalbumin, bovine serum albumin, bovine casein, and lysozyme. Analytical enzymes for in vitro protein digestion: pancreatic porcine trypsin, bovine pancreatic chymotrypsin and porcine intestinal peptidase. HPLC solvents: methanol and acetonitrile (Sigma Chromasolv®). Ultrapure water, resistivity ≥18 MΩ (Elga Purelab Analytical system, Elga Ltd., High Wycombe, UK).
Poultry processed animal proteins samples from laboratory preparation
Poultry by-product meal (PBM) consists of ground, rendered, clean parts of the carcass of slaughtered poultry. Processed animal proteins mainly consist of necks, feet and intestines, exclusive of feathers, except in the amounts as might occur unavoidably in processing practices. Poultry materials, representing typical ingredients of poultry by product (mainly, necks, feet, backs), were purchased from commercial sources, finely cut, divided in six sub-samples and stored at minus 20°C before use.
Experimental processed animal proteins (EPAPs) were prepared in the laboratory under controlled conditions. The sterilizing-heating conditions were obtained in a laboratory autoclave (Vapor matic 770, Asal, Cernusco s/N, Italy). Poultry meat (about 250 g) was weighed in Pyrex glass bottle (2 L volume) and different time-temperature combinations applied; three different bottles (3 replicates) were prepared for each scheduled treatments listed in . Samples EPAP 2, EPAP3 and EPAP4 were processed by applying the same temperature (115°C), but modifying the time of exposure (20, 30, 180 min, respectively). Samples EPAP5 and EPAP6 were exposed to different temperature (133°C and 150°C, respectively), both for 20 min. The pressure Samples (EPAP2-EPAP6) recovered from autoclave were pressed for liquor discharge, dried in a ventilated oven at 50°C until constant weight was achieved. The freeze-dried samples (EPAP1) and dried EPAPs were then ground using a IKA model A11 grinder (IKA, Königswinter, Germany).
Poultry processed animal proteins samples from processing rendering system
From a rendering Italian company producing PAPs for pet food and feed industry, just of poultry origin, sampling design from crucial points of wet continuous processing system was organised. With the aim to evaluate the effect of temperature treatments on protein quality during processing, a number of 15 samples were obtained as describe in . Since the rendering plan worked as continuous system and at atmospheric pressure, sampling was organized to collect materials during one week of rendering activity; a number of three samples (3 replicates) were taken from each points as describe below. The first sampling stage concerned raw material after grinder size reduction; the second one was after heating (89°C for 30 min); the third consisted of wet graves, obtained after separation of fat fraction; the fourth and fifth obtained after drying process performed by low temperature dryer (89°C 140 min) and by high temperature dryer (110°C 140 min), respectively.
Proximate composition
Proximate composition analyses were carried in duplicate on raw poultry meat, on laboratory prepared EPAPs, and on samples from rendering plant, available as three replicates each, applying standard procedures (AOAC, Citation1996); for moisture (930.15), crud protein (976.05), by estimating the Kjeldahl nitrogen (Nx6.25) in a automated distillation unit (Buchi 339, Switzerland) and ash (942.05). Lipid extraction was done by Bligh and Dyer method (Bligh and Dyer, Citation1959).
DL-amino acid purification and separation analysis by reversed phase-high-performance liquid chromatography
Five hundred mg of each sample were defatted by methanol-chloroform extraction (Bligh and Dyer, Citation1959) than 100 mg hydrolysed in 6N HCl, under vacuum, at 110°C for 24 h (Liardon and Ledermann, Citation1981). Hydrolysis of standard proteins was carried out on 5 mg. After hydrolysis, the hydrochloric acid was removed under vacuum on a rotary evaporator (Buchi R-210, Switzerland) and the dried residue was dissolved in 0.1 M HCl (2 mL) and filtered. Hydrochloric acid 0.1 M was added up to a volume of 10 mL and the sample was purified passing through a cation-exchanger resin (Dowex-50w 200-400 mesh); the amino acids were eluted with 4 M aqueous ammonia (30 mL). The eluates were evaporated to dryness in a rotary evaporator, afterwards dissolved in 2 mL of ultra-pure water.
D and L amino acids content was determined by RP-HPLC with fluorescence detection (λEx 340 nm, λEm 445 nm) after a pre-column automatic derivatization with o-phthaldialdehyde (OPA) together with N-isobutyryl-D-cysteine (IBDC) (Brückner et al.Citation1991). Fifty µL of borate buffer, 10 µL of OPA/IBDC reagent (170 mM OPA and 260 mM IBDC, in 1 M potassium borate buffer pH 10.4) and 20 µL of sample or standard solution were drawn up by the syringe of the autosampler and mixed in a reaction vial. After 2 min, 20 µL of the reaction mixture were injected onto the HPLC system. The HPLC system consisted of two pumps (980-PU, Jasco Co., Tokio, Japan), an autosampler (AS-950, Jasco Co.) and a fluorescence detector (FP-920, Jasco Co.). Chromatograms were recorded and integrated by Empower Chromatography Software (Waters, Milford, MA, USA). A Hypersil ODS (5 mm 250 mm x 0.46 mm i.d) column was used (Shandon HPLC, Runcorn, UK). The gradient elution applied for separation varied linearly from sodium acetate buffer 40 mM, pH 6.0 to methanol (1% to 53.5% over 75 min). The flow rate was 1 mL/min.
Determination of in vitro protein digestibility
A multi-enzyme assay for estimation of in vitro apparent protein digestibility (DV) of EPAPs was applied according to the method described by Hsu et al. (Citation1977). The aqueous protein suspension (50 ml) prepared from extracted proteins (6.25 mg/mL) was adjusted to pH 8.0, while stirring in a water bath at 37°C. Fifty millilitres of the multi-enzymes solution (1.6 mg/mL pancreatic trypsin, 3.1 mg/mL bovine pancreatic chymotrypsin and 1.3 mg/mL porcine intestinal peptidase), maintained in ice and adjusted to pH 8.0, were added to the protein suspension and the mix stirred at 37°C. A rapid decline of pH occurred and the pH drop was recorded after ten minutes (pH10 min) digestion, using a pH meter (PHM 220, Radiometer analytical, Lyon, France). The regression equation used to calculate the percent in vitro protein digestibility was, Y = 210.464 – 18.103 pH10 min.
Statistical analysis
When appropriate, the analysis of variance (ANOVA) was calculated and values presented as mean ± SD; a P value of ≤0.05 was considered as significant. A Newman-Keuls’s posthoc test was used where significant differences were detected. Correlations between protein digestibility and D-amino acids contents were evaluated by Pearson’s correlation analysis. Statistical analysis was performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). Kinetics equations were obtained by linear regression analysis, using GraphPad Prism version 5.03 (GraphPad Software, San Diego, CA, USA).
Results and discussion
Proximate composition of raw materials and the means of proximate composition of laboratory prepared proteins from EPAP2 to EPAP6, and of samples from rendering plant were calculated and reported in . Fat content was higher in EPAPs samples (23.1 g/100 g) than samples from rendering (14.5 g/100 g) and protein content resulted lower (49.5 /100 g EPAPs vs 64.7 g/100 g rendering samples).
During rendering process (), most of the fat was separated from the original raw incoming material, and treated as a different by-product. However, chemical composition of poultry meat purchased to prepare laboratory PAPs samples might represent the category 3 material used in the industrial plant considering that rendering industry aims to differentiate and offer a variety of products on the market of animal feeding. Nutritional requirements of different species (pet food, aquafeed) result the main scope to prepare PAPs with declared and certified nutritive peculiarities, but marketing strategies very often impose novel products, mostly for economic finalities.
As it was already studied and widely discussed by several authors, (Liardon and Ledermann, Citation1981; Liardon and Hurrell, Citation1983; Bada, Citation1984) the hydrolytic treatment (6N HCL, at 110° C, for 24 h) applied in sample preparation induced racemization that should be taken into account in a reliable determination and discussion of results. Induced racemization quantity depends on the nature of both amino acid and protein, and under acid hydrolysis conditions, proteins-bound amino acids may not racemize at the same rate as free amino acids. Racemization induced by 24 hours acid hydrolysis of protein-bound amino acids was verified and reported by measuring enantiomers formations, D/L ratio (%), in several purified proteins (Liardon and Ledermann, Citation1981; Kaiser and Benner, Citation2005). The inductive strength of R substituents of bound amino acids has been recognized to be the principal factor determining the relative racemization rate for peptide and the relative order of racemization has been described in proteins heated at elevated temperatures at neutral and basic pH (Bada, Citation1984; Friedman and Liardonn, Citation1985). In this study, D-amino acids formation in different purified standard protein after acid traditional hydrolysis (6 N HCl, 110°C, 24 h) was tested and results reported in . Rates of racemization () clearly confirmed how various proteins treated under same conditions produced different responses in the formation of D-amino acids enantiomers (Liardon and Hurrell, Citation1983; Liardon and Friedmann, Citation1987; Csapó et al., Citation2008). Particularly, for bovine serum albumin the rate of racemization of identified amino acids agree with results previously reported by Liardon et al. (Citation1981). D-isomers initially present in standard purified proteins could be assumed irrelevant or even absent as it was previously discussed and verified (Luzzana et al., Citation1996). Percentages of D-enantiomers identified in bovine serum albumin and lysozyme resulted higher than D-amino acids percentages reported by Kaiser and Benner (Citation2005) in the same proteins, but our hydrolysis conditions applied changed in heating time that was 24 h instead of 20 h of the Kaiser and Benner’s work. However, under traditional hydrolysis conditions (6 N HCl, 110°C 24 h) Csapó et al. (2008), reported percentages of amino acids racemization in standard proteins, especially for D-aspartic acid, comparable to our results. Racemization rates of amino acids produced under hydrolysis conditions appeared to be similar for all triplicate of standard proteins analysed, confirming the high reproducibility of hydrolysis conditions obtained. D amino acid formation from five amino acids, aspartic acid (Asp), glutamic acid (Glu), serine (Ser), alanine (Ala) and lysine. (Lys), has been considered in EPAPs samples and the enantiomers, expressed as [D/(D + L) 100], are reported in .
Literature data and previous experiments (Liardon and Ledermann, Citation1981; Bada, Citation1984; Luzzana et al., Citation1996, Citation1999) established that the lower limit for a correct determination of D/L (%) was 0.2-0.4 %, especially when the analyses concern a mixture of amino acids or proteins. Below this limit, a correct determination failed because of the residual interference of hydrolysis-induced recemization. Aspartic acid is the amino acid more prone to racemization, whose rate appeared strictly related to heat exposure. The racemization kinetics of aspartic acid under different temperature, moisture and pH conditions revealed that the content of D-aspartic acid is a function of heat exposure and may be considered a useful biomarker to predict the thermal processing undergone samples (Luzzana et al., Citation1996, Citation1999). Kinetics studies of racemization in peptide or protein treated even under controlled conditions, are very complex mainly because of the inductive strength of the side chain influences interactions between neighbouring amino acids, however in some cases it could not be considered as the main driving force of amino acids racemization. When the amino acid inversion results low (i.e., <1.5), the inversion rate constant k, follows the first-order reaction mechanism, described with the equation, In [1+D/L] – In [ 1-D/L] = k t, where t is the time of treatment. On the bases of D-amino acid contents in EPAPs samples, as reported in , first order rate plots for racemization of D-Asp, D-Glu and D-Lys are represented in . The linear regressions of concern results obtained from EPAP2, EPAP3 and EPAP4, processed at different heating times (20, 30, 180 min, respectively), but same temperature (115°C). Since the hydrolysis step induces racemization, the initial ratio D/D+L 100 obtained in freeze-dried EPAP1 sample (without any heat treatment) was not exactly zero (), and consequently the first order rate racemization plots do not through the origin. The equations of the lines plot are calculated by linear regression model and coefficients are reported in . The higher R2 values 0.9960, 0.9942, 0.9792 were obtained for lysine, aspartic acid and glutamic acid respectively. Linear regression of amino acid racemization rates was also calculated in EPAP samples prepared by applying different temperatures (EPAP2, EPAP5, EPAP6). Under these conditions, R2 values resulted certainly lower, with the higher value for D-Asp (0.6346), whose linear regression plot is represented in .
After prolonged or severe heat treatments, a degree of racemization rate was reported (Liardonn and Hurrell, Citation1983; Bada, Citation1984; Friedman and Liardon, Citation1985; Luzzana et al. Citation1999). In our results, the prolonged heat treatment (115°C, 180 min) revealed a D-Asp content of 28.1%, that reasonably agree with previously reported D-Asp racemization rates on heated (121°C) chicken muscle protein, 22.4% and 26.3%, after 4 h and 8 h, respectively (Liardon and Hurrell, Citation1983). The D-isomer content of aspartic acid in freeze-dried sample (EPAP1) resulted 2.7% and even this value may be considered comparable to reported D-Asp content in not heated chicken muscle of 2.9%, assuming to be induced by hydrolysis sample preparation (Liardon and Hurrell, Citation1983). Similarly, other enantiomers were identified in EPAP1 freeze-dried sample (), with negligible amount for D-Ala (0.1%), but more significant for D-Glu (1%) and D-Lys (1.5%).
Concerning the processed poultry animal proteins sampled in a rendering plant at different stages of the process as showed in , we decided to approach just the determination of aspartic acid racemization rate, mainly for considerations that follow, i) aspartic acid is the more prone to racemization; ii) racemization of aspartic acid revealed a linear kinetics, verified in different processed animal proteins under different heat exposure; iii) differently from other amino acids, the behaviour of bound aspartic acid of different proteins is very reproducible with a progressive complete liberation of this amino acid after the applied hydrolysis conditions (Luzzana et al., Citation1996, Citation1999); iv) under chromatographic conditions described above aspartic acids enantiomers separation and elution are completed after 16 min (). Moreover, aiming to moderate the hydrolysis-induced racemization but to guarantee the breakage of peptide bond aspartic acid, the time of hydrolysis and temperature were reduced to 6 h and 100°C for analysis of samples from industrial rendering plan, maintaining the same acid condition (6 N HCl) (Luzzana et al., Citation1999). The D-aspartic content of sampling is reported in . Supporting our previous results obtained in fishmeal, where elevation of temperature from 95°C to 120°C increased the Kasp from 0.46 to 3.39x10-3 min-1, the lower dryer temperature of 89°C compared to higher of 110°C, significantly reduced D-aspartic acid formation, with beneficial for nutritional value in feed processed proteins. With some exception for ruminant species where rumen bacteria are able to metabolise D-amino acid since peptidoglycans of cell wall are rich in D-aspartic, D-glutamic and D-alanine (Man and Bada, Citation1987), dietary protein digestibility is negatively affected when significant racemization occurred. The normal proteolytic enzymes are unable to break a peptide bond involving D-amino acid residues and consequently a racemization in a residue next to essential L-amino acids may compromise the release of disposable free amino acid in vivo digestion. The products of hydrolysed proteins rich in racemized amino acid consist of small peptides containing D-amino acids (Friedman, Citation1999; Friedman et al., Citation1981; Man and Bada, Citation1987).
Treated proteins may have significant presence of D-amino acids and their biological utilization depends on conversion in L-enantiomers by D-amino acids oxidase present in liver and kidney of most vertebrates. In mammals, the absorption of D-stereoisomer of essential amino acids is slower in the intestine and kidney, and they are mostly excreted with urine. D amino acids oxidases work at different rates and D-aspartic acid, one of the most present in the D-racemic form in nature, is a poor substrate for D- amino acid oxidase. However, in mammals a specific D-amino acid oxidase for D-aspartic acid is present, but no correlation has been found between susceptibility to racemization and of the amino acid and its fate to be rapidly oxidized in the corresponding -keto acid. Although the activity of D-amino acid oxidases allows the metabolism of D-enantiomers, this pathway does not result sufficient enough when large amount of D-amino acid are ingested and metabolism overcharged. Reduced digestibility is the most intuitive nutritional implication of amino acid racemization that concerns not only D-enantiomers, but also the protein itself since some neighbouring essential amino acids could be involved in a missed hydrolysis reaction.
In prediction of nutritional quality of protein sources, information on protein digestibility becomes useful. In , in vitro digestibility of EPAPs, measured by using a multi enzymes method base on the pH drop during the digestion, is reported. Good correlation (R>0.8) between this method and in vivo digestibility is described, especially with proteins of animal origin (Pedersen and Eggum, Citation1983; Butts et al., Citation2012). A direct relationship between the pH drop, protein hydrolysis and consequently digestibility is assumed by this method. The formation of cross-linkages, particularly lysinoalanime and lanthionine, together with the loss of enzymes attack sites as a consequence of amino acid destruction have been considered the main cause of decrease of protein digestibility (Friedman et al., Citation1981). However, a reduction in digestibility of proteins that undergone to a severe heating has been strictly related to the formation of D-enantiomers and consequently formation of L-D, D-L, D-D bonds, which are not available to proteolytic enzyme attack (Friedman et al., Citation1981). Our results confirm that prolongation of temperature treatment (from 20 to 180 min, at 115°C) significantly (P<0.05) affects in vitro digestibility (), which decrease from 86.0% of lyophilized EPAP to 78.3% and 79.1% after 20 and 30 min, respectively, and to 76.3% after 180 min. Pearson’s correlation coefficients calculated among D-amino acids content and in vitro protein digestibility resulted negative (D-Asp = - 0.932; D-Glu = - 0.914; D-Ala = - 0.954; D-Ser = -0.849; D-Lys = -0.928), but not at significant levels. The application of different temperature treatments (115, 133, 150 °C) maintained for 20 min did not produce significant differences in protein digestibility that resulted 78.3%, 78.7% and 79.1% in EPAP2, EPAP5 and EPAP6, respectively (). In a previous study (Shirley and Parsons, Citation2000) cooking (94°C) meat and bone meal for 20 min, without applying any pressure, did not affect amino acids digestibility, whereas the pressure processing (15 psi), for 20 min at 117°C produced a significant (P<0.05) reduction of most amino acids digestibility. The effect of pressure produced a significant cubic response on amino acids digestibility, with the exception of aspartic acid, whose response resulted linear.During laboratory preparation of PAPs, we applied the same pressure (15 psi), so reasonably we might expect to balance the effect of pressure variable on protein digestibility. The mechanism by which amino acid digestibility resulted affected by processing pressure has been already discussed and considered associated with racemization or cross-linking of amino acids (Butts et al., Citation2012).
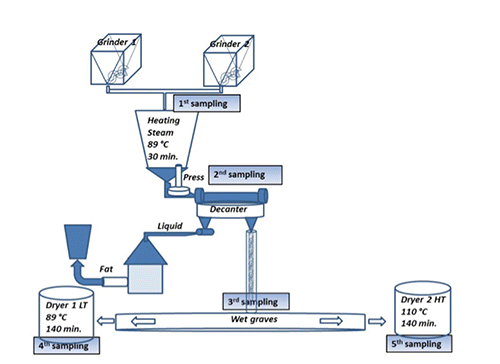
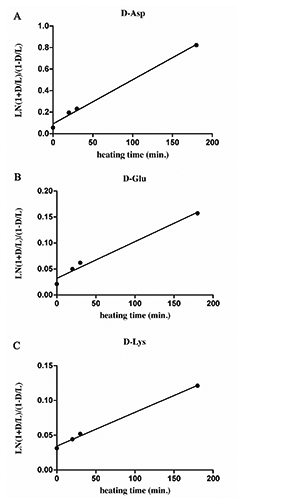
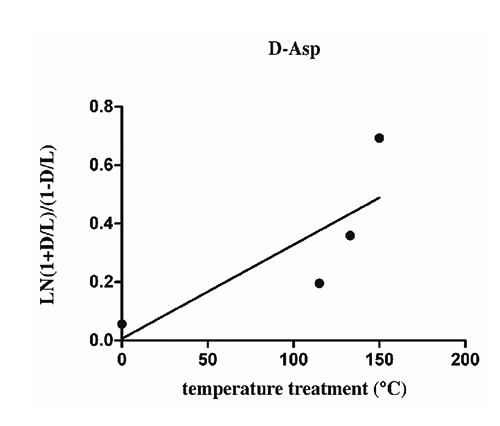
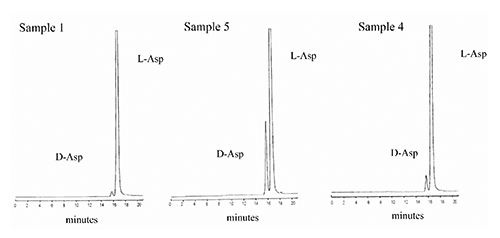
Table 1. Experimental processed poultry animal proteins obtained in laboratory under different temperature and time conditions.
Table 2. Proximate composition of raw materials, experimental processed poultry animal proteins and samples obtained from rendering plant.
Table 3. Racemization of amino acids in standard proteins under acid hydrolysis conditions (6 N HCl, 110°C, 24 h).
Table 4. Contents of D-amino acids [D/(D+L) 100] and in vitro digestibility of experimental processed animal proteins of poultry origin. Mean standard deviations in brackets (n=3).
Table 5. Equations of the first-order plots represented in .
Table 6. Racemization of aspartic acid in samples from rendering plant. Mean standard deviations in brackets (n=3).
Conclusions
PAPs are valuable for a wide range of purposes, but as a feed ingredient it maintains high quality nutritive value because of the presence of essential amino acids, minerals such as calcium and phosphorus, and vitamins. It is realistic to expect that the European Commission intention is to re-authorized the use of poultry PAPs in pig diets, as well as pig PAPs in poultry feed by the end of the next year. The safety of PAPs, derived from category 3 material, has been already proved by the authorities and the rendering industry has already adopted necessary changes to deliver species specific sources of PAPs. The results reported in our study confirm the detection of racemized amino acids in processed animal proteins, especially D-aspartic acid, as realistic indicators of thermal treatments during PAP manufacturing and approach considerations concerning nutritional implications. In vitro protein digestibility significantly (P<0.05) decreased in respect of the intensity of heating treatment applied during PAPs preparations and the racemization of proteins amino acids may reasonably be involved in the loss of protein quality.
References
- AOAC, 1996. Official methods of analysis, 16th ed. Association of Official Analytical Chemists, Gaithersburg, MI, USA.
- BadaJ.L. 1984. In vivo racemization in mammalian proteins. In: WoldF. MoldaveK. (eds.) Methods in enzymol. Elsevier, Amsterdam, The Netherlands, pp 98-115.
- BlighE.G. DryerW.Y. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 37:911-917.
- BrücknerH. WittnerR. GodelH. 1991. Fully automated high-performance liquid chromatographic deparation of DL-amino acids derivatized with o-phtaldialdehyde together with N-isobutyryl-cysteine. Application to food samples. Chromatographia 32:383-388.
- ButtsC.A. MonroJ.A. MoughanP.J. 2012. In vitro determination of dietary protein and amino acid digestibility for humans. Br. J. Nutr. 108:282-287.
- CsapóJ. Csapó-KissZs. AlbertCs. LókiK. 2008. Hydrolysis of proteins performed at high temperatures and for short times with reduced racemisation, in order to determine the enantiomers of D-and L-amino acids. Acta Univ. Sapientiae Alimentaria 1:31-48.
- EFPRA, 2013. EFPRA welcomes publication in the EU official of the use of non ruminant PAPs in aquafeed and of approval of PCR test. Available from: www.efpra.eu/Objects/3/Files/EFPRA%2024%2001%202013%20PR.doc
- EFPRA, 2014. PAPs highly beneficial to EU aquaculture sector. Available from: www.efpra.eu/Objects/3/Files/interview-alm260514.pdf
- European Food Safety Authority, 2011. Scientific opinion on the revision of the quantitative risk assessment (QRA) of the BSE risk posed by processed animal proteins (PAPs). EFSA Panel on Biological Hazards (BIOHAZ). EFSA J. 9:1947.
- European Commission, 1994. Commission decision of 27 June 1994 concerning certain protection measures with regard to bovine spongiform encephalopathies and the feeding of mammalian derived protein, 94/381/EC. In: Official Journal, L 172, 07/07/1994, pp 23-24.
- European Commission, 2002. Regulation of the European Parliament and of the Council of 3 October 2002 laying down health rules concerning animal by-products not intended for human consumption, 2002/1774/EC. In: Official Journal, L 273, 10/10/2002, pp 1-95.
- European Commission, 2009. Regulation of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No1774/2002 (Animal byproducts Regulation), 2009/1069/EC. In: Official Journal, L 300, 14/11/2009, pp 1-33.
- European Commission, 2011. Regulation of the European Parliament of 23 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive, 2011/142/EC. In: Official Journal, L 54, 26/02/2011, pp 1-254.
- European Commission, 2013. Commission Regulation of 16 January 2013 amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies, 2013/56/EU. In: Official Journal, L 21, 24/01/2013, pp 3-16.
- FriedmanM. 1999. Chemistry, nutrition and microbiology of D-amino acids. J. Agric. Food Chem. 47:3457-3479.
- FriedmanM. LiardonR. 1985. Racemization kinetics of amino acid residues in alkalitreated soybean protein. J. Agric. Food Chem. 33:666-672.
- FriedmanM. MastersP.M. 1982. Kinetics of racemization of amino acid residues in casein. J. Food Sci. 47:760-764.
- FriedmanM. ZahnleyJ.C. MastersP. 1981. Relationship between in vitro digestibility of casein and its content of Iysinoalanine and D-amino acids. J. Food Sci. 46:127-131.
- HendriksW.H. ButtsC.A. ThomasD.V. JamesK.A.C. MorelP.C.A. VerstegenM.W.A. 2002. Nutritional quality and variation of meat and bone meal. Asian-Austral. J. Anim. 15:1507-1516.
- HendriksW.H CottamY.H. ThomasD.V. 2006. The effect of storage on the nutritional quality of meat and bone meal. Anim. Feed Sci. Tech. 127:151-160.
- HsuW.H. VavakD.L. SatterleeL.D. MillerG.A. 1977. A multienzyme technique for estimating protein digestibility. J. Food Sci. 42:1269-1273.
- KaiserK. BennerR. 2005. Hydrolysis-induced racemization of amino acids. Limnol. Oceanogr.-Meth. 3:318-325.
- LiardonR. FriedmanM. 1987. Effect of peptide bond cleavage on the racemisation of amino acids residues in proteins. J. Agric. Food Chem. 35:661-667.
- LiardonR. HurrellR.F. 1983. Amino acid racemization in heated and alkali-treated proteins. J. Agric. Food Chem. 31:432-437.
- LiardonR. LedermannS. OttU. 1981. Determination of D-amino acids by deuterium labelling and selected ion monitoring. J. Chromatogr. 203:385-395.
- LuzzanaU. MentastiT. MorettiV.M. AlbertiniA. ValfreF. 1996. Aspartic acid racemization in fish meal as induced by thermal treatment. Aquaculture Nutr. 2:95-99.
- LuzzanaU. MentastiT. OpstvedtJ. NygardE. MorettiV.M. ValfreF. 1999. Racemization kinetics of aspartic acid in fish material under different conditions of moisture, pH, and oxygen pressure. J. Agric. Food Chem. 47:2879-2884.
- ManE.H. BadaJ.L. 1987. Dietary D-amino acids. Ann. Rev. Nutr. 7:209-225.
- MillerE.L. 2003. Protein nutrition requirements of farmed livestock and dietary supply. Available from: http://www.fao.org/docrep/007/y5019e/y5019e06.htm
- PedersenB. EggumB.O. 1983. Prediction of protein digestibility by in vitro enzymatic PH-stat procedure. Z. Tierphysiol. Tierer. 49:265-277.
- PivaG. MoschiniM. FiorentiniL. MasoeroF. 2001. Effect of temperature, pressure and alkaline treatments on meat meal quality. Anim. Feed Sci. Tech. 89:59-68.
- ShirleyR.B. ParsonsC.M. 2000. Effect of pressure processing on amino acid digestibility of meat and bone meal for poultry. Poultry Sci. 79:1775-1781.
- TaylorD. WoodgateS. AtkinsonM. 1995. Inactivation of the bovine spongiform encephalopathy agent by rendering procedures. Vet. Rec. 137:605-610.
- WoodgateS.L. Van Der VeenJ. 2004. The role of fat processing in the European Union animal production industry. Biotechnol. Agron. Soc. Environ. 8:283-294.
