Abstract
A trial has been performed on 201 dairy cows from two Italian commercial herds in order to verify whether the mitigation of a recognized negative energy balance (NEB) by a therapeutic mean may influence the incidence of peri-partum diseases. All animals were tested for beta-hydroxybutyrate (β-HOB) and non-esterified fatty acids (NEFA) three times a week from 2 weeks before the expected due time to 2 weeks after calving. Animals whose blood levels were above β-HOB>1.2 or NEFA>0.5 mmol/L were declared POSITIVE and then split in two groups. Group T animals (n=57) were treated with a glycogenic treatment (ENERGAN KETOSIS, Virbac). The treatment was repeated daily as long as biochemical values remained abnormal. Group C animals (n=48) served as untreated controls. Animals with values within the physiological range over the study period were said NEGATIVE (n=96). This study confirmed that animals presenting excessive β-HOB or NEFA concentrations show a higher risk to get sick during the study period (P<0.05), the major risk being clinical ketosis (P<0.01) and in a lesser extend retention of the placenta (P=0.09). The application of a glycogenic treatment did not show an impact on blood metabolite levels due to huge individual differences. However, application of the treatment for an average duration of 5 days tends to reduce the incidence of all the diseases related to a NEB. Moreover, untreated control animals were more likely to get dislocation of the abomasum (P<0.05) than NEGATIVE animals whereas treated animals were not.
Introduction
During the past two decades, considerable progress has been made in understanding the physiology of the transition towards lactation in dairy cows (Drackley et al., Citation2006). During the transition period, and particularly during the two weeks following parturition, dairy cows are faced with an inevitable energy deficit (NEB), which will play a crucial role for the remainder of lactation (Wensing et al., Citation1997).
Relations have already been reported between the duration and severity of periods of NEB, on the one hand, and the incidence of certain diseases, on the other hand, particularly digestive disorders and lameness (Collard et al., Citation2000). Between 30 and 50% of cows may suffer from a metabolic or infectious disease just before or after calving (Ingvartsen et al., Citation2003; LeBlanc, Citation2010). The disorders associated with inadequate energy intake predispose cows to metabolic or infectious diseases such as milk fever (MF), endometritis (MET), acetoneamia (CK), displaced abomasum (DA) and retention of the placenta (RP) (Esposito et al., Citation2014). The reciprocal relations between the disorders referred to above have long since been demonstrated (Morrow, Citation1976; Curtis et al., Citation1985; Peeler et al., Citation1994; Heuer et al., Citation1999; Chapinal et al., Citation2011). Uncomplicated CK, RP MET and MF are risk factors for DA (Shaver, Citation1997).
It is now an accepted fact that an extreme rate of mobilisation of fatty tissue is related to a high incidence of metabolic diseases (Drackley, Citation1999). Such intense lipomobilisation leads to an elevation in the serum concentration of non-esterified fatty acids (NEFA) and then to their uptake by the liver, and to the accumulation of triglycerides in the latter. This accumulation of fatty acids in the liver predisposes the cow to the induction of ketosis accompanied by an elevation of serum beta-hydroxybutyrate (β-HOB). Various authors have already demonstrated that the blood levels of β-HOB, NEFA and calcium (Ca) are closely correlated with the incidence of production diseases (Hoedemaker et al., Citation2004; Goff, Citation2006; Stengärde et al., Citation2010; Chapinal et al., Citation2012, Citation2011).
The idea of compensating a NEB by the daily administration of propylene glycol or glycerol, starting from the last days of gestation and for 2 or 3 weeks after calving, is inconsistently practiced in modern dairy farms although it has already been experimented by various authors (Miettinen, Citation1995; Formigoni et al., Citation1996; Hoedemaker et al., Citation2004; Castañeda-Gutiérrez et al., Citation2009; Lomander et al., Citation2012; McArt et al., Citation2011). In addition to the practical and economic constraints involved, and although propane 1,2-diol is a permitted additive for animal feed in Europe (EU Regulation 892/2010) and in the United States, the adjunction of a non-natural additive to the ration of dairy cows may be questioned. It may be judicious to use propylene glycol sparingly in the form of a prescription drug. Some authors have already suggested the strategic use of blood tests for the detection of animals suffering from sub-clinical ketosis. The measurement of NEFA should be used during the last week of gestation and that of β-HOB during the first week post-partum (McArt et al., Citation2013).
The purpose of this study was to verify whether systematic screening for sub-clinical ketosis during the transition period (2 weeks before and after calving) via the measurement of the blood levels of β-HOB and NEFA, followed by ad hoc treatment with propylene glycol, calcium propionate, betaine, niacin and molasses and by selective treatment with calcium for as long as necessary to restore the normal values of these metabolites could have an impact on the morbidity and incidence of MF, RP, MET and DA during the immediate post-partum period.
Materials and methods
The study was conducted in specialised dairy farms in the region of Milano (Italy) who had volunteered to take part in the trial. The farms were monitored by the same veterinary practice and were located close to the laboratory. All samplings points aimed to monitor the transition period disturbances and were performed under informed consent of the breeders. Blood (10 mL) were taken from all the animals during or just after the morning meal as animals were restrained for feeding, placed in plain tubes and immediately delivered to the laboratory. After centrifugation (2200 g X 10 min), serum was separated and analysed soon after. Concentration of NEFA, β-HOB and calcium were measured by means of an automated spectrophotometer (ILAB 300 plus; Instrumentation Laboratory Spa, Milano, Italy) using commercially available kits: NEFA (Acetyl CoA synthetase colorimetric method, Randox Laboratories Ltd., Crumlin, Co. Antrium, UK) and β-HOB (D-3-Hydroxybutyrate dehydrogenase method, Randox Laboratories Ltd.), calcium (orthocre-softaleine method; Instrumentation Laboratory Spa).
The investigating veterinarian was responsible for evaluating the annual incidence of DA, RP, MF, MET and CK, one of which must exceed 5, 15, 10, 10, 10% respectively.
Monitored animals were gestating dairy cows. The size of the population was set at about 200 animals. Animals were recruited according to their foreseeable due date. No animal was excluded a priori if gestation was confirmed. Animals were maintained in permanent open housing, with individual bedding cubicles. Towards the end of gestation, the animals were fed a diet of corn silage, straw and concentrates. Glucogenic treatments were prohibited and their use was grounds for exclusion.
Follow-up protocol
The animals were included starting at 2 weeks before the presumed date of parturition. The study was continued until 15 days after calving. A computerised data-collection form indicated, for each animal, the dates of the blood samples and the tests to be performed. Sampling on day d±1 was tolerated, according to the day of the week on which the animals were included. Overall, blood samples were taken 3 times a week from the animals monitored. The results from the laboratory were available late in the afternoon. The sampling plan was as follows:
First week of the study (14-7 days pre-partum): all the animals had samples taken for the measurement of β-HOB and NEFA.
Second week of the study (7 days pre-partum up to the date of parturition), all the animals had samples taken for the measurement of β-HOB, NEFA and Ca.
Third week of the study (first week post-partum): all the animals were monitored for β-HOB and Ca.
Fourth week (final sample) of the study (7-15 days post-partum): all the animals were monitored for β-HOB.
Treatment given to the animals
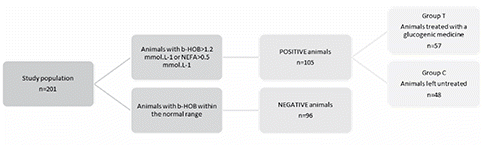
Animals showing levels of β-HOB>1.2 mmol/L or of NEFA>0.5 mmol/L for the first time were considered as POSITIVE animals. As soon as results were available (within 8 to 12 hours), positive animals with an odd-numbered ear tag (last digit) were included in the treatment group (group T) and were then drenched on a daily basis, with a glucogenic medication containing propylene glycol (122 g), calcium propionate (11 g), betaine (7 g), niacin (5 g) and molasses (~150 g) until the biological values returned to normal (ENERGAN KETOSIS; Virbac S.A., Carros, France). Other POSITIVE animals (even-numbered ear tag) were left untreated and served as controls (group C). Animals with β-HOB and NEFA within the physiological range all over the study period were declared NEGATIVE (). Hypocalcemia, even if mild, was judged to be a potential bias, liable in itself to jeopardise the possible beneficial effect of the glucogenic treatment. In an attempt to control it, calcium was therefore administered selectively at the time of parturition. The effects of the two treatments were therefore merged. Positive animals were thus equipped with a parturition detector (VEL’PHONE, Medria, Châteaubourg, France), in order to be able to treat them with calcium in due time. When the parturition detector made it possible to predict the hour at which calving would occur, the animals involved received 41.4 g of elemental calcium 12 h prior to parturition and 12 hours after it, in the form of a commercial preparation containing calcium propionate and chloride associated with disodium phosphate and magnesium oxide (ENERGAN CALCIUM, Virbac).
Data collection and processing
The occurrence of DA, MF, RP, CK and MET during the study period was recorded for all the animals in order to compare the incidence of those diseases in each group.
While 80% of calving in artificially inseminated Holstein cows occur 272±10 days after insemination (Matthews and Morton, Citation2012), predicting the exact date of parturition in an individual remains problematic. As a result, and without even taking into account the recording mistakes regarding the actual date of insemination, the date of the animals’ incorporation into the follow-up may be somewhat distant from the date of calving. In addition, since blood samples were taken in each animal about 1 out of every 3 days (d±1), the number of values able to be processed for each of the three groups and for each day prior to calving may be expected to possibly be small. Moreover, in cows, the physiological distribution of β-HOB serum concentrations is very broad (<0.4 to >1.2 mmol/L) (Enjalbert et al., Citation2001). The combination of such phenomena was expected to lead to a significant loss of statistical power over the pre-partum period. However, to do justice to the important work of blood sample collection and testing, blood chemistry results of experimental groups has been processed for a preliminary comparison. Pre-partum samples from 3 consecutive days were thus grouped together beginning on day - 29 (±1 day) to day -2 (±1 day), resulting in 10 periods of 3 days. Cows that calved more than 30 days after inclusion or with no blood tests during the pre-partum period were excluded from this exploratory analysis.
The data were analysed at DIVET using the Analyse-it software (Analyse-it Ltd, Leeds, UK). The relative incidence of the diseases monitored were compared via a chi-2 test.
Results
Two dairy farms and 201 animals were included in this trial.
Application of glucogenic treatments
Fifty-seven animals received glucogenic treatment during the study. The average treatment duration was 4.6 days, involving one dose per day of the commercial product. All treatment took place before parturition. Forty-three animals (75%) did not require any other treatment. Ten animals relapsed and had to be treated again for a mean duration of 6.2 days. The median day for the application of the glucogenic treatment was 6 days before calving (IQR=10 days).
Prevalence of the diseases monitored
During the study period 57 (28.3%) experimental animals got one of the monitored diseases (). The most common disease was CK (18.9%) then RP (9.9%), DA (4.5%) and MF (2.0%). No puerperal metritis (MET) was reported in any group. All cases were diagnosed or confirmed by the veterinary surgeon responsible for the two farms. RP and MF diagnostic is regarded as obvious for an experienced veterinarian. CK diagnosis is based on lack of appetite, lack of rumination, milk yield decrease, depression and positive result to urine or milk test. DA diagnostic relies on similar observations and a positive left flank auscultation (typical ping). All DA were left displacement of abomasum.
Table 1. Statistical comparison (χ2) of the distribution of cows affected by monitored diseases (sick or with DA, MF, RP, CK) or by a specific condition in NEGATIVE animals (b-HOB<1.2 and NEFA<0.5 mmol/L, all tests ) or in POSITIVE and treated animals (group T) or in POSITIVE and untreated controls (group C).
A comparison between NEGATIVE and control animals on one side, and NEGATIVE and treated animals on the other side, revealed the following points:
Positive animals (test+) were significantly (P<0.01) more SICK than NEGATIVE animals (test-), showing significantly more CK (P<0.01) and were possibly at increased risk to show RP (P=0.093).
Animals belonging to group C (tests+, untreated) were significantly (P<0.01) more SICK than NEGATIVE animals (test-), presented significantly more CK (P<0.01), more DA (P<0.05) and were possibly at increased risk to show RP (P=0.099).
Animals belonging to group T (tests+, treated) were significantly (P<0.01) more SICK than NEGATIVE animals and presented significantly more CK (P<0.05). Regarding DA and other conditions, differences were not significant.
There was no significant difference between group C and group T animals for any of the monitored conditions.
The median day (and IQR) for the diagnostic of DA and CK were 11 (IQR=7) and 5 (IQR=7) days after calving respectively.
Blood chemistry results
Dispersion of calving
The median time elapsing between the inclusion of the animals and calving was 18 days, 50% of cows having calved between 15 and 21 days after enrolment. The rest of the animals calved between 49 to 22 and 14 to 0 days after inclusion.
Dispersion of blood chemistry results
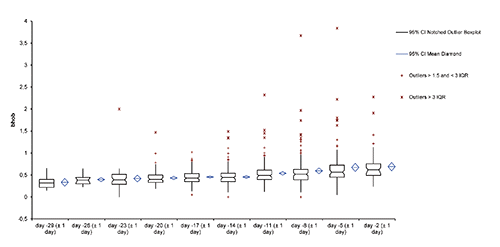
Although 2459 blood tests were performed on the animals, the number of samples available for each day prior to calving ranged from 59 (NEFA, day -12) to 0 (Ca, day -27). Pre-partum period samples were grouped together as explained in the Materials and methods section. A table indicating the final number of animals for which statistical analysis is possible may be consulted in . From d-29 (±1d) to d-14 (±1d) most of the animals remained NEGATIVE (data not shown) whereas few POSITIVE cows were spit into treated and non-treated animals.
Table 2. Number of cases included in the statistical comparison of results β-HOB grouped by 3 consecutive days from day -29 to day -2 pre-partum.
The β HOB blood concentration mean and median (3 d periods) gently increased over the pre-partum period (), always remaining below the action threshold (1.2 mmol/L). In contrast dispersion of results increased dramatically over the pre-partum period.
Calving dispersion and blood result dispersion have been judged being a major difficulty for a correct statistical analysis, regardless of validity conditions of tests such as ANOVA, and thus no relevant conclusion was expected. Therefore, authors gave up to further inappropriate statistical comparison between experimental groups. Additionally, analysis of NEFA and Ca results leaded to similar conclusions with even smaller groups for Ca interpretation. Although 4 milk fever have been recorded, no worrying hypocalcaemia was detected and not any cow was given a prophylactic treatment with calcium.
Discussion
This study was conducted in order to verify whether it would be possible to decrease the incidence of metabolic related diseases in dairy cows showing metabolic abnormalities during the transition period (altered blood concentration of β-HOB, NEFA and to a lesser extent Ca), by administering a commercial product containing molasses, propylene glycol, calcium propionate and niacin and selective treatment with calcium at the time of calving.
The main result of this study shows that animals that always had levels of NEFA<0.5 and of β-HOB<1.2 mmol/L (NEGATIVE animals) during the pre-partum period are significantly (P<0.01) less likely to develop one of the diseases monitored (DA, MF, RP or CK) than POSITIVE animals. Group T animals tended to be less sick and also tended to experience a lower incidence of DA, MF, RP and CK than those belonging to group C. Moreover, in the case of the animals presenting a distinct elevation of β-HOB or NEFA at any time prior to calving, the daily administration of a propylene glycol and calcium propionate-based treatment (group T) allowed a permanent return to normal values in 75% of them within 4 to 5 days. It was generally not possible to observe significant difference in biochemical results between the various groups of animals. This difficulty is ascribed to the great variability of the individual data and to the small size of each group, for each period.
The incidences of the four diseases monitored are those usually reported by the literature (Goff, Citation2006; Mulligan et al., Citation2006; Parker Gaddis et al., Citation2012; Vergara et al., Citation2014), except in the case of clinical ketosis (CK). For the NEGATIVE animals the incidence of CK is slightly higher than those reported in the literature (0 to 5%), but for the other groups the incidence is more comparable to that reported for sub-clinical ketosis (20 to 40% or even more) (McArt et al., Citation2011; Citation2013, Esposito et al., Citation2014).
Various blood parameters have been used as advanced indicators of an energy imbalance (Stengärde et al., Citation2010; Hailemariam et al., Citation2014). However, NEFA and β-HOB measurements are among the most convenient, as blood β-HOB can also be measured next to the cow by means of small portable devices (Free Style Optium Neo, Abbott Laboratories, CHICAGO, IL, USA) (Voyvoda and Erdogan, Citation2010). The threshold values chosen for this study are the same as those used by others (LeBlanc et al., Citation2005; McArt et al., Citation2011, Citation2012, Citation2013; Mulligan et al., Citation2006). NEFA and β-HOB levels provide two measures of the severity of energy imbalance. Although postpartum diseases often share risk factors, and these factors may trigger a cascade of other diseases (Vergara et al., Citation2014), it is now well known that this imbalance affects both cows at the start of lactation and dry cows at the end of gestation and predisposes them to LDA, RP, dystocia, fatty liver, CK and other problems (Mulligan et al., Citation2006). LeBlanc (LeBlanc, Citation2010) reports that NEFA>0,4 mmol/L during the 7 to 10 days before calving multiplies the risk of LDA by 2 to 4 fold and the risk of RP by 2 fold. Likewise, β-HOB>1,2 mmol/L after calving multiplies the risk of LDA by 3 to 8 fold and the risk of CK by 4 to 6 fold. The observations reported here fully agree with those findings, since the NEGATIVE animals were less (or significantly less) affected than the POSITIVE animals.
The early detection of sub-clinical ketosis on the basis of measurements of β-HOB and NEFA, for the purpose of instituting corrective treatment and subsequently reducing the incidence of metabolic related diseases, has already been proposed by other authors (Hoedemaker et al., Citation2004; Overton and Waldron, Citation2004; Lomander et al., Citation2012; McArt et al., Citation2012). While it is possible, generally speaking, to have an effect on the serum concentrations of β-HOB and NEFA, the results in regards the cows’ health, are however, diverse. Some have ascribed the lack of effectiveness of such treatments to the fact that the studies are conducted on herds of high-producing cows, an objective that can be attained only thanks to excellent management of the stock, and the cows can then go through the NEB period without any particular consequences (Hoedemaker et al., Citation2004). Overton et Waldron (Overton and Waldron, Citation2004) have stated that, in the absence of proven positive benefits, the routine administration of propylene glycol is not recommended.
Conclusions
This study confirms that an aggressive monitoring of pre-partum blood level would help to identify animals at increased risk to develop a production disease in the early post-partum. The proposed on-demand glycogenic treatment results in mitigating clinical differences between treated and NEGATIVE animals whereas differences remain between control and NEGATIVE animals. Animals with biochemical abnormalities show a noticeable decrease in the incidence of DA, CK and, in a lesser extent, RP when treated. However, other studies involving more aggressive therapeutic diets and more animals would be worth conducting.
Acknowledgments
The authors thank the cattle farmers for their availability throughout the duration of the study. This study was funded by VIRBAC S.A., Carros, France.
References
- Castañeda-GutiérrezE. PeltonS.H. GilbertR.O. ButlerW.R., 2009. Effect of peripartum dietary energy supplementation of dairy cows on metabolites, liver function and reproductive variables. Anim. Reprod. Sci. 112:301–315.
- ChapinalN. CarsonM. DuffieldT.F. CapelM. GoddenS. OvertonM. SantosJ.E.P. LeBlancS.J., 2011. The association of serum metabolites with clinical disease during the transition period. J. Dairy Sci. 94:4897–4903.
- ChapinalN. LeBlancS.J. CarsonM.E. LeslieK.E. GoddenS. CapelM. SantosJ.E.P. OvertonM.W. DuffieldT.F., 2012. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J. Dairy Sci. 95:5676–5682.
- CollardB.L. BoettcherP.J. DekkersJ.C. PetitclercD. SchaefferL.R., 2000. Relationships between energy balance and health traits of dairy cattle in early lactation. J. Dairy Sci. 83:2683–2690.
- CurtisC.R. ErbH.N. SniffenC.J. SmithR.D. KronfeldD.S., 1985. Path analysis of dry period nutrition, postpartum metabolic and reproductive disorders, and mastitis in Holstein cows. J. Dairy Sci. 68:2347–2360.
- DrackleyJ. DonkinS. ReynoldsC., 2006. Major advances in fundamental dairy cattle nutrition. J. Dairy Sci. 89:1324–1336.
- DrackleyJ.K., 1999. Biology of dairy cows during the transition period: the final frontier? J. Dairy Sci. 82: 2259–2573.
- EnjalbertF. NicotM.C. BayourtheC. MoncoulonR., 2001. Ketone bodies in milk and blood of dairy cows: relationship between concentrations and utilization for detection of subclinical ketosis. J. Dairy Sci. 84:583–589.
- EspositoG. IronsP.C. WebbE.C. ChapwanyaA., 2014. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 144, 60–71.
- FormigoniA. CornilM.C. PrandiA. MordentiA. RossiA. PortetelleD. RenavilleR., 1996. Effect of propylene glycol supplementation around parturition on milk yield, reproduction performance and some hormonal and metabolic characteristics in dairy cows. J. Dairy Res. 63:11–24.
- GoffJ., 2006. Major advances in our understanding of nutritional influences on bovine health. J. Dairy Sci. 89:1292–1301.
- HailemariamD. MandalR. SaleemF. DunnS.M. WishartD.S. AmetajB.N., 2014. Identification of predictive biomarkers of disease state in transition dairy cows. J. Dairy Sci. 97:, 2680–2693.
- HeuerC. SchukkenY.H. DobbelaarP., 1999. Postpartum body condition score and results from the first test day milk as predictors of disease, fertility, yield, and culling in commercial dairy herds. J. Dairy Sci. 82:295–304.
- HoedemakerM. PrangeD. ZerbeH. FrankJ. DaxenbergerA. MeyerH.H.D., 2004. Peripartal propylene glycol supplementation and metabolism, animal health, fertility, and production in dairy cows. J. Dairy Sci. 87:2136–2145.
- IngvartsenK. DewhurstR. FriggensN., 2003. On the relationship between lactational performance and health: is it yield or metabolic imbalance that cause production diseases in dairy cattle? A position paper. Livest. Prod. Sci. 83:277–308.
- LeBlancS., 2010. Monitoring metabolic health of dairy cattle in the transition period. J. Reprod. Dev. 56:S29–S35.
- LeBlancS.J. LeslieK.E. DuffieldT.F., 2005. Metabolic predictors of displaced abomasum in dairy cattle. J. Dairy Sci. 88:159–170.
- LomanderH. FrösslingJ. IngvartsenK.L. GustafssonH. SvenssonC., 2012. Supplemental feeding with glycerol or propylene glycol of dairy cows in early lactation-effects on metabolic status, body condition, and milk yield. J. Dairy Sci. 95:2397–2408.
- MatthewsB.J. MortonJ.M., 2012. Accuracy of predicted calving dates in Holstein-Friesian dairy cows based on fetal ages estimated using manual rectal palpation. N. Z. Vet. J. 60:234–240.
- McArtJ.A.A. NydamD.V OetzelG.R., 2012. A field trial on the effect of propylene glycol on displaced abomasum, removal from herd, and reproduction in fresh cows diagnosed with subclinical ketosis. J. Dairy Sci. 95:2505–12.
- McArtJ.A.A. NydamD. V OetzelG.R. OvertonT.R. OspinaP.A., 2013. Elevated non-esterified fatty acids and -hydroxybutyrate and their association with transition dairy cow performance. Vet. J. 198:560–570.
- McArtJ.A.A. NydamD. V OspinaP.A. OetzelG.R., 2011. A field trial on the effect of propylene glycol on milk yield and resolution of ketosis in fresh cows diagnosed with subclinical ketosis. J. Dairy Sci. 94:6011–6020.
- MiettinenP.V.A., 1995. Prevention of bovine ketosis with glucogenic substance and its effect on fertility in Finnish dairy cows. Berl. Munch. Tierarztl. Wochenschr. 108:14–19.
- MorrowD.A., 1976. Fat cow syndrome. J. Dairy Sci. 59:1625–1629.
- MulliganF.J. O’GradyL. RiceD.A. DohertyM.L., 2006. A herd health approach to dairy cow nutrition and production diseases of the transition cow. Anim. Reprod. Sci. 331–353.
- OvertonT.R. WaldronM.R., 2004. Nutritional Management of Transition Dairy Cows: Strategies to Optimize Metabolic Health. J. Dairy Sci. 87:E105–E119.
- Parker GaddisK. ColeJ. ClayJ. MalteccaC., 2012. Incidence validation and relationship analysis of producer-recorded health event data from on-farm computer systems in the United States. J. Dairy Sci. 95:5422–5435.
- PeelerE. OtteM. EsslemontR., 1994. Interrelationships of periparturient diseases in dairy cows. Vet. Rec. 134:129–132.
- ShaverR., 1997. Nutritional risk factors in the etiology of left displaced abomasum in dairy cows: a review. J. Dairy Sci. 80:2449–2453.
- StengärdeL. HolteniusK. TråvénM. HultgrenJ. NiskanenR. EmanuelsonU., 2010. Blood profiles in dairy cows with displaced abomasum. J. Dairy Sci. 93:4691–4699.
- VergaraC.F. DöpferD. CookN.B. NordlundK.V. McArtJ.A.A. NydamD.V. OetzelG.R., 2014. Risk factors for postpartum problems in dairy cows: Explanatory and predictive modeling. J. Dairy Sci. 97:4127–4140.
- VoyvodaH. ErdoganH., 2010. Use of a handheld meter for detecting subclinical ketosis in dairy cows. Res. Vet. Sci. 89:344–351.
- WensingT. KruipT. GeelenM.J.H. WentinkG.H. van den TopA.M., 1997. Postpartum fatty liver in high-producing dairy cows in practice and in animal studies. The connection with health, production and reproduction problems. Comp. Haematol. Int. 7:167–171.
