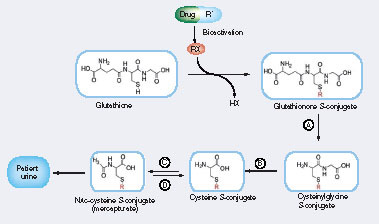
(A) γ-glutamyltranspeptidase, (B) dipeptidase: cysteineglycine dipeptidase and aminopeptidase, (C) cysteine conjugate N-acetyltransferase and (D)N-deacetylase.
Glu: Glutamate; Gly: Glycine; RX: Reactive species.
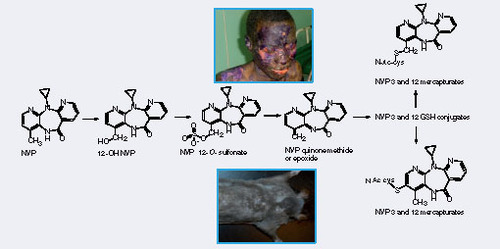
NVP: Nevirapine.
A common mechanism for detoxification of toxic electrophilic compounds occurs via glutathione (GSH) conjugation Citation[1]. The GSH conjugates can be further processed into breakdown products such as mercapturate conjugates and excreted in urine Citation[2]. Therefore, mercapturate conjugates are potentially biomarkers of drug or compound bioactivation that are not dependent upon cell death to be released into an accessible compartment (e.g., urine) Citation[3]. The levels of mercapturate conjugates in human urine are an indirect measure of exposure of the liver to reactive electrophilic intermediates. GSH conjugates are unsuitable biomarkers of metabolic activation in vivo because they are only very rarely eliminated in urine and, seemingly, only at very low concentrations.
Human urinary mercapturates have been recognized for many years as particularly useful biomarkers in assessments of human exposure to chemically diverse environmental and biogenic electrophiles Citation[3,4]. Recently, through the employment of advanced MS techniques, there has been a revival of interest in the bioanalytical possibilities of mercapturates. Mercapturate biosynthesis has been modeled as an interorgan pathway, with the liver serving as the major site of GSH conjugation and the kidney as the primary site for conversion of GSH conjugates to cysteine conjugates Citation[5]; however, in some cases, it can be an entirely intrahepatic process, even in rats Citation[6]. The GSH adducts of bendamustine are metabolized completely in human liver, with only the mercapturates and further metabolites appearing in bile Citation[7]. The cysteine adduct is the sole thioether metabolite of acetylpara-aminophenol (APAP) eliminated in human bile, with the APAP cysteine adduct and mercapturate being excreted in urine Citation[8]. Any drug mercapturate produced extrahepatically might also be eliminated in bile: rat liver possesses an efficient mechanism for the uptake and biliary excretion of circulating mercapturates Citation[9]. Although the metabolism of GSH adducts can yield numerous S-linked products via modifications of the tripeptide moiety Citation[7], as in the case of carbamazepine Citation[10,11], mercapturates are not always found amongst the excreted metabolites and there is evidence for a species-selective absence of carbamazepine mercapturates from human urine Citation[10,12]. When taken with the frequent observation that a compound can be metabolized to several GSH adducts, these findings commend careful analysis of a drug’s metabolites to identify a thioether derivative suitable for use as a biomarker of metabolic activation.
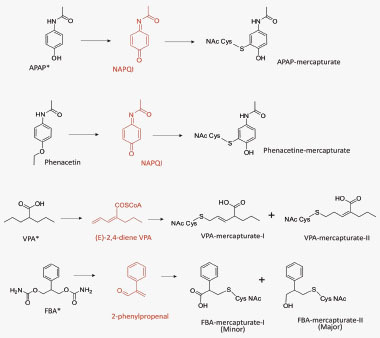
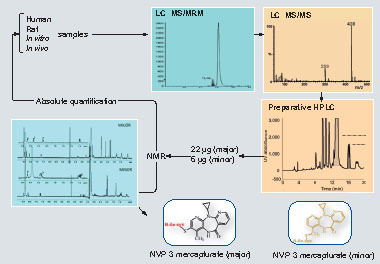
S-phenyl mercapturic acid and N-methylcarbamoyl mercapturic acids have been proposed by the American Conference of Governmental Industrial Hygienists (2001) as biomarkers for purposes of evaluating exposure to benzene and N,N-dimethylformamide, respectively. Among the human pharmaceuticals, the mercapturate conjugates of APAP Citation[8], phenacetin, felbamate Citation[13], valproic acid Citation[14] and nevirapine (NVP) Citation[15] have been used for the quantitative assessment of in vivo bioactivation. Clearance of APAP and phenacetin to urinary metabolites of the mercapturic acid pathway, which, in both cases, are derived from the glutathione adduct of N-acetyl-p-benzoquinone imine , has been determined Citation[8]. Metabolic activation of felbamate, an antiepileptic associated with idiosyncratic aplastic anemia and hepatotoxicity, can also be assessed in patients by monitoring (two) urinary mercapturates , in this instance derived from an α,β-unsaturated aldehyde Citation[13]. Valproic acid, an antiepileptic linked with idiosyncratic clinical hepatotoxicity, is metabolized via a reactive diene to two isomeric urinary mercapturates that are evidence of bioactivation and are associated with risk factors of drug-induced toxicity Citation[14]. Recently, we have shown that NVP, an antiretroviral drug associated with idiosyncratic hepatotoxicity and skin rash, is bioactivated in patients by characterizing novel NVP mercapturates, potentially formed from multiple reactive metabolites Citation[15]. An integrated NMR and MS approach was used to quantify the levels of urinary NVP mercapturates in HIV patients Citation[15]. Here, we isolated NVP mercapturates from rat bile in order to optimize the MS conditions, develop preparative HPLC conditions and characterize the structures by NMR. In addition, we use NMR for the absolute quantification of the isolated mercapturates. NMR offers advantages over HPLC and LC–MS because the isolated metabolites can be reliably quantified using a standard curve generated with the parent drug or even structurally unrelated compounds. Calibration curves can be created to quantitate an unknown because the proton signals do not constitute unique fingerprints of a molecule Citation[16]. In this study, paracetamol was used as the reference standard to generate a standard curve for the quantification of NVP mercapturates. This assay has been used successfully to determine the concentration of NVP mercapturates in HIV patients’ urine. The assay was found to be sufficiently sensitive for the quantification of the two NVP mercapturates with limit of quantitations of 16 and 19 ng/ml, respectively. We are currently applying this chemistry to a Brown Norway rat model of NVP skin toxicity Citation[17], allowing modulation of the rodent drug metabolism to mimic that seen within patients with serious adverse reactions to NVP .
The applicability of NVP mercapturates as clinical biomarkers requires detailed verification, but it should be noted that only a low proportion of mercapturate might be found in urine, even if there is extensive bioactivation, if bioactivation coincides with glutathione depletion, possibly due to other patient factors such as chronic hepatitis, co-administration of other drugs, progression of HIV, diet and malnutrition.
Chemically reactive drug metabolites have been implicated hypothetically as essential causal factors in many drug-associated clinical toxicities Citation[1]. However, to date there are no robust models for predicting the idiosyncratic toxicities of new pharmaceuticals. As a consequence, minimizing metabolic activation via structural modification is now a significant precautionary objective in pharmaceutical optimization programmes Citation[18]. Considerable efforts have been made to develop screening systems for reactive drug metabolites, which are usually based on subcellular liver fractions Citation[19–22] or isolated hepatocytes Citation[19]. These systems, through measurements of either irreversible binding or thioether adduct production, have shown that many drugs associated with clinical toxicities do undergo bioactivation. However, the two biochemical metrics can be poorly correlated even in a series of analogues Citation[23]. These systems are able to give an indication of the chemical hazard posed through the structure of the compound, they do not give any indication as to the downstream biology affected. This perturbed biology is a function of the susceptible individual (one in over 1000 patients). The relatively high frequency of false-positive results has revealed limitations for predicting the hepatotoxicity of new drug candidates. Nevertheless, the ability of microsomal preparations to predict metabolic activation in vivo, in the form of irreversible binding of radiolabeled material, has been tested in rats with some success Citation[21]. What is currently lacking is a noninvasive, generic and quantitative method for assessing bioactivation of drugs in experimental animals, human volunteers and patients. The integration of NMR and MS creates early opportunities for assaying newly characterized metabolites that are of potential biological/toxicological importance but impractical targets for chemical synthesis. Wider availability of powerful spectrometers is likely to result in increasingly frequent exploitation of NMR to authenticate and quantify complex isolated metabolites that can be used as reference standards for high-throughput LC-based quantitative analyses. The present study can serve as a template for future applications of this method to biomarkers of the metabolic activation of drugs in humans.
Acknowledgements
Thanks to Abhi Srivastava for all your hard work during your PhD. Also, Kevin Park, James Maggs and Lu-Yun Lian for their scientific input.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- Park BK , KitteringhamNR, MaggsJL, PirmohamedM, WilliamsDP. The role of metabolic activation in drug-induced hepatotoxicity. Annu. Rev. Pharmacol. Toxicol. 45, 177–202 (2005).
- Jan BMD , NicoNMC, VermeulenPE. Mercapturic acids as biomarkers of exposure to electrophilic chemicals: applications to environmental and industrial chemicals. Biomarkers3, 239–303 (1998).
- Kuiper HC , MirandaCL, SowellJD, StevensJF. Mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites are in vivo markers of oxidative stress. J. Biol. Chem. 283, 17131–17138 (2008).
- Seutter-Berlage F , van Dorp HL, Kosse HG, Henderson PT. Urinary mercapturic acid excretion as a biological parameter of exposure to alkylating agents. Int. Arch. Occup. Environ. Health39, 45–51 (1977).
- Inoue O , KannoE, KasaiK, UkaiH, OkamotoS, IkedaM. Benzylmercapturic acid is superior to hippuric acid and o-cresol as a urinary marker of occupational exposure to toluene. Toxicol. Lett. 147, 177–186 (2004).
- Hinchman CA , BallatoriN. Glutathione conjugation and conversion to mercapturic acids can occur as an intrahepatic process. J. Toxicol. Environ. Health41, 387–409 (1994).
- Teichert J , SohrR, HennigLet al. Identification and quantitation of the N-acetyl-L-cysteine S-conjugates of bendamustine and its sulfoxides in human bile after administration of bendamustine hydrochloride. Drug Metab. Dispos. 37, 292–301 (2009).
- Siegers CP , LoeserW, GieselmannJ, OltmannsD. Biliary and renal excretion of paracetamol in man. Pharmacology29, 301–303 (1984).
- Hinchman CA , RebbeorJF, BallatoriN. Efficient hepatic uptake and concentrative biliary excretion of a mercapturic acid. Am. J. Physiol. 275, G612–G619 (1998).
- Amore BM , KalhornTF, SkilesGLet al. Characterization of carbamazepine metabolism in a mouse model of carbamazepine teratogenicity. Drug Metab. Dispos. 25, 953–962 (1997).
- Madden S , MaggsJL, ParkBK. Bioactivation of carbamazepine in the rat in vivo. Evidence for the formation of reactive arene oxide(s). Drug Metab. Dispos. 24, 469–479 (1996).
- Maggs JL , PirmohamedM, KitteringhamNR, ParkBK. Characterization of the metabolites of carbamazepine in patient urine by liquid chromatography/mass spectrometry. Drug Metab. Dispos. 25, 275–280 (1997).
- Dieckhaus CM , Fernandez-MetzlerCL, KingR, KrolikowskiPH, BaillieTA. Negative ion tandem mass spectrometry for the detection of glutathione conjugates. Chem. Res. Toxicol. 18, 630–638 (2005).
- Gopaul S , FarrellK, AbbottF. Effects of age and polytherapy, risk factors of valproic acid (VPA) hepatotoxicity, on the excretion of thiol conjugates of (E)-2,4-diene VPA in people with epilepsy taking VPA. Epilepsia44, 322–328 (2003).
- Srivastava A , LianLY, MaggsJLet al. Quantifying the metabolic activation of nevirapine in patients by integrated applications of NMR and mass spectrometries. Drug Metab. Dispos. 38(1), 122–132 (2010).
- Espina R , YuL, WangJet al. Nuclear magnetic resonance spectroscopy as a quantitative tool to determine the concentrations of biologically produced metabolites: implications in metabolites in safety testing. Chem. Res. Toxicol. 22, 299–310 (2009).
- Shenton JM , TeranishiM, bu-AsabMS, YagerJA, UetrecJP. Characterization of a potential animal model of an idiosyncratic drug reaction: nevirapine-induced skin rash in the rat. Chem. Res. Toxicol. 16, 1078–1089 (2003).
- Kumar S , KassahunK, Tschirret-GuthRA, MitraK, BaillieTA. Minimizing metabolic activation during pharmaceutical lead optimization: progress, knowledge gaps and future directions. Curr. Opin. Drug Discov. Devel. 11, 43–52 (2008).
- Bauman JN , KellyJM, TripathySet al. Can in vitro metabolism-dependent covalent binding data distinguish hepatotoxic from nonhepatotoxic drugs? An analysis using human hepatocytes and liver S-9 fraction. Chem. Res. Toxicol. 22, 332–340 (2009).
- Obach RS , KalgutkarAS, SogliaJR, and Zhao SX. Can in vitro metabolism-dependent covalent binding data in liver microsomes distinguish hepatotoxic from nonhepatotoxic drugs? An analysis of 18 drugs with consideration of intrinsic clearance and daily dose. Chem. Res. Toxicol. 21, 1814–1822 (2008).
- Takakusa H , MasumotoH, YukinagaHet al. Covalent binding and tissue distribution/retention assessment of drugs associated with idiosyncratic drug toxicity. Drug. Metab. Dispos. 36, 1770–1779 (2008).
- Teichert J , SohrR, HennigLet al. In vitro screening of 50 highly prescribed drugs for thiol adduct formation-comparison of potential for drug-induced toxicity and extent of adduct formation. Chem. Res. Toxicol. 22, 690–698 (2009).
- Levesque JF , DaySH, ChauretNet al. Metabolic activation of indole-containing prostaglandin D2 receptor 1 antagonists: impacts of glutathione trapping and glucuronide conjugation on covalent binding. Bioorg. Med. Chem. Lett. 17, 3038–3043 (2007).