Abstract
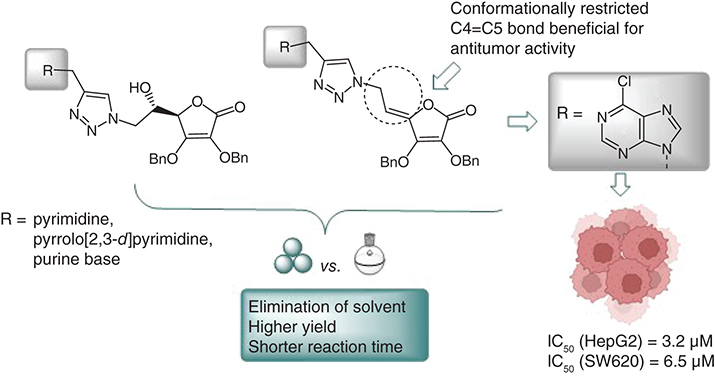
Aim: The authors' aim was to improve the application of copper-catalyzed azide-alkyne cycloaddition in the synthesis of hybrids containing biologically significant nucleobases and L-ascorbic acid scaffolds by introducing an environmentally friendly and waste-free ball mill. Results: Two series of hybrids with a purine, pyrrolo[2,3-d]pyrimidine or 5-substituted pyrimidine attached to 2,3-dibenzyl-L-ascorbic acid via a hydroxyethyl- (15a–23a) or ethylidene-1,2,3-triazolyl (15b–23b) bridge were prepared by ball milling and conventional synthesis. The unsaturated 6-chloroadenine L-ascorbic acid derivative 16b can be highlighted as a lead compound and showed strong antiproliferative activity against HepG2 (hepatocellular carcinoma) and SW620 (colorectal adenocarcinoma) cells. Conclusion: Mechanochemical synthesis was superior in terms of sustainability, reaction rate and yield, highlighting the advantageous applications of ball milling over classical reactions.
Graphical Abstract
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at:www.tandfonline.com/doi/full/10.2217/epi-2016-0184
Financial & competing interests disclosure
Financial support from the Croatian Science Foundation under the project HRZZ-IP-2018-01-4682 is gratefully acknowledged. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
