Abstract
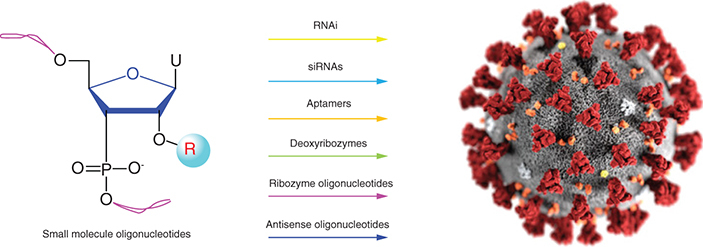
Small-molecule oligonucleotides could be exploited therapeutically to silence the expression of viral infection-causing genes, and a few of them are now in clinical trials for the management of viral infections. The most challenging aspect of these oligonucleotides’ therapeutic success involves their delivery. Thus medicinal chemistry strategies are inevitable to avoid degradation by serum nucleases, avoid kidney clearance and improve cellular uptake. Recently small-molecule oligonucleotide design has opened up new avenues to improve the treatment of drug-resistant viral infections, along with the development of COVID-19 medicines. This review is directed toward the recent advances in rational design, mechanism of action, structure–activity relationships and future perspective of the small-molecule oligonucleotides targeting viral infections, including COVID-19.
Tweetable abstract
Small-molecule oligonucleotides as antiviral agents.
Graphical Abstract
Author contributions
Kar and Dhar contributed most in preparing this manuscript.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Data sharing statement
For studies reporting the original results of a clinical trial or the secondary analysis of clinical trial data, authors should include a data sharing statement, as described on the ICMJE website: www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html. Authors are asked to specify whether their manuscript reports either the original results of a clinical trial, or the secondary analysis of clinical trial data that have been shared with them, and in either case include a suitable data sharing statement. Further information, and example wording, can be found in the Author Guidelines on our website.
