Abstract
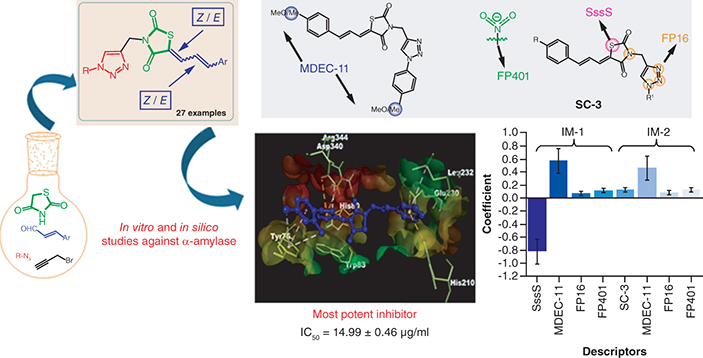
Aim: The primary objective of this investigation was the synthesis, spectral interpretation and evaluation of the α-amylase inhibition of rationally designed thiazolidinedione–triazole conjugates (7a–7aa). Materials & methods: The designed compounds were synthesized by stirring a mixture of thiazolidine-2,4-dione, propargyl bromide, cinnamaldehyde and azide derivatives in polyethylene glycol-400. The α-amylase inhibitory activity of the synthesized conjugates was examined by integrating in vitro and in silico studies. Results: The investigated derivatives exhibited promising α-amylase inhibitory activity, with IC50 values ranging between 0.028 and 0.088 μmol ml-1. Various computational approaches were employed to get detailed information about the inhibition mechanism. Conclusion: The thiazolidinedione–triazole conjugate 7p, with IC50 = 0.028 μmol ml-1, was identified as the best hit for inhibiting α-amylase.
Plain Language Summary
Graphical abstract
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at:www.tandfonline.com/doi/full/10.2217/epi-2016-0184
Acknowledgments
The Council of Scientific and Industrial Research (CSIR), New Delhi, India, provided financial assistance to Rahul Singh and Meena Devi in the form of Senior Research Fellowships (SRF). We would like to extend our thanks to Chemistry Department of Kurukshetra University for providing the facilities necessary to conduct our laboratory work. We thank DST FIST for granting funding under the FIST programme (No. SR/FST/CS-I/2017/12(C)) to set up an NMR apparatus in the department. Dr. Paola Gramatica and the QSARINS development team deserve credit for supplying the QSARINS software.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
