Abstract
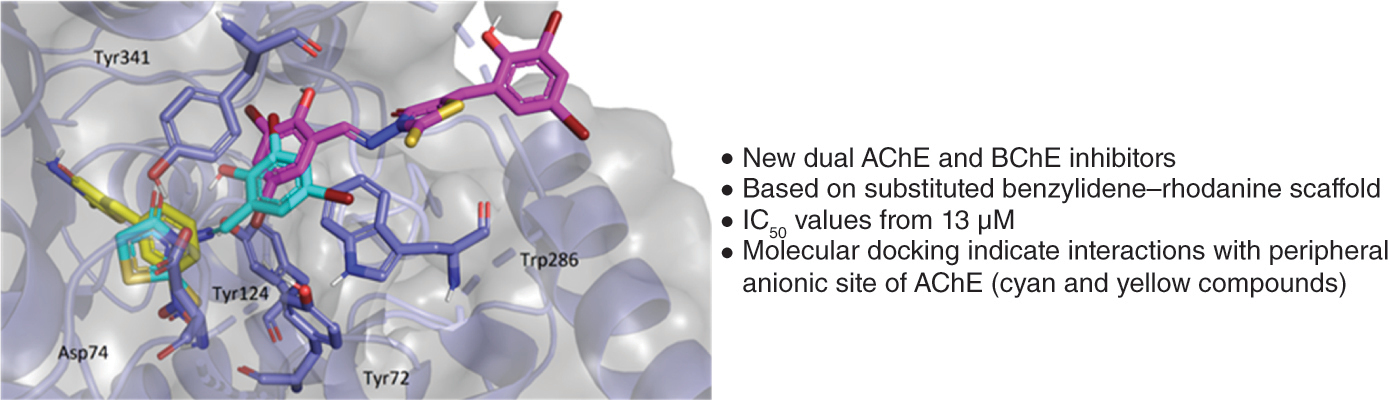
Aim: 2-Thioxothiazolidin-4-one represents a versatile scaffold in drug development. The authors used it to prepare new potent acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors that can be utilized, e.g., to treat Alzheimer’s disease. Materials & methods: 3-Amino-2-thioxothiazolidin-4-one was modified at the amino group or active methylene, using substituted benzaldehydes. The derivatives were evaluated for inhibition of AChE and BChE (Ellman’s method). Results & conclusion: The derivatives were obtained with yields of 52–94%. They showed dual inhibition with IC50 values from 13.15 μM; many compounds were superior to rivastigmine. The structure–activity relationship favors nitrobenzylidene and 3,5-dihalogenosalicylidene scaffolds. AChE was inhibited noncompetitively, whereas BChE was inhibited with a mixed type of inhibition. Molecular docking provided insights into molecular interactions. Each enzyme is inhibited by a different binding mode.
Graphical abstract
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.future-science.com/doi/suppl/10.4155/fmc-2023-0268
Author contributions
Conceptualization: M Krátký; methodology: Š Štěpánková, M Švarcová and M Krátký; investigation: Š Štěpánková, K Nováčková, K Svrčková, M Švarcová and M Krátký; writing – original draft preparation: Š Štěpánková, M Švarcová and M Krátký; writing – review and editing: Š Štěpánková, K Svrčková and M Krátký; supervision: Š Štěpánková and M Krátký; funding acquisition: M Krátký. All authors have read and agreed to the published version of the manuscript.
Financial disclosure
This work was supported by the Charles University (SVV 260 661). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
Writing disclosure
No writing assistance has been used in the creation of this manuscript.
