Abstract
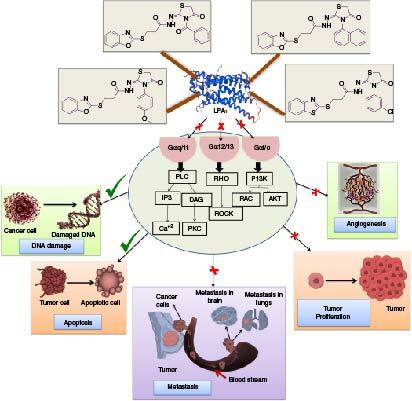
Aim: Breast cancer has been a leading cause of mortality among women worldwide in recent years. Targeting the lysophosphatidic acid (LPA)–LPA1 pathway using small molecules could improve breast cancer therapy. Materials & methods: Thiazolidin-4-ones were developed and tested on MCF-7 cancer cells, and active compounds were analyzed for their effects on apoptosis, migration angiogenesis and LPA1 protein and gene expression. Results & conclusion: Compounds TZ-4 and TZ-6 effectively reduced the migration of MCF-7 cells, and induced apoptosis. TZ-4, TZ-6, TZ-8 and TZ-14 significantly reduced the LPA1 protein, LPA1 and angiogenesis gene expression in treated MCF-7 cells. Molecular docking and molecular dynamic simulation studies reveal the ligand interactions and stability of the LPA1–ligand complex. Developed thiazolidin-4-ones showed great potential as an LPA1-targeted approach to combating breast cancer.
Plain language summary
Breast cancer is a major cause of death for women worldwide. Using small molecules to target the lysophosphatidic acid (LPA)–LPA1 pathway could improve breast cancer treatment. We tested a type of molecule called thiazolidin-4-ones on breast cancer cells in the lab. We looked at how these molecules affected cell death, movement, blood vessel growth and the activity of the LPA1 gene and protein. Some of these molecules, such as TZ-4 and TZ-6, reduced the movement of cancer cells and caused them to die. They also decreased the levels of LPA1 protein and gene activity in the cells. We used computer simulations to see how these molecules interacted with the LPA1 protein. Our findings suggest that thiazolidin-4-ones could be a promising treatment for breast cancer by targeting LPA1.
Tweetable abstract
New thiazolidin-4-ones were tested on MCF-7 cells; active compounds were analyzed for effects on apoptosis, migration, angiogenesis and LPA1 expression. TZ-4 and TZ-6 reduced MCF-7 migration and induced apoptosis.
The study focused on developing novel thiazolidin-4-one based lysophosphatidic acid receptor-1 (LPA1) inhibitors to target breast cancer.
Compounds TZ-4, TZ-6, TZ-8 and TZ-14 reduced LPA1 expression and were most effective against MCF-7 breast cancer cells, with low toxicity.
All the designed molecules showed potential and stable interactions for amino acids of LPA1 binding pocket with less binding energy compared to the co-crystal structure.
The compounds TZ-4, TZ-6, TZ-8 and TZ-14 show promise as potential candidates for breast cancer therapy with a focus on LPA1 modulation.
Author contributions
M Bhagyalalitha and GV Pujar conceptualized and designed the study. M. Bhagyalalitha conducted the experimental work. Prabhu A and Pavan SR were responsible for the biological activity investigations. AK Sethu, Akshatha HS and KG Pujar assisted in the computational aspects and data analysis. The manuscript was written by M Bhagyalalitha and GV Pujar and further reviewed by M Singh and KG Pujar. The final version of the manuscript received approval from all authors.
Acknowledgements
The authors thank the Principal, JSS College of Pharmacy, JSS Academy of Higher Education, and Research, Mysuru, India, for providing the necessary facilities. The authors also thank Yenepoya Research Centre, Yenepoya (Deemed to Be University) for providing the necessary facilities for the cell culture studies.
Financial disclosure
Authors thank the All India Council for Technical Education-Quality Improvement Program New Delhi for the QIP Scholar Fellowship award, No.19-13/RIFD/2018-19. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.