Respiratory syncytial virus (RSV) remains the leading viral etiological agent of acute lower respiratory tract infection (ALRI) and a major cause of hospital admissions and death in young children worldwide Citation[1–3]. By the age of two, virtually all children have been infected by RSV at least once. Infants younger than 6 months, born prematurely or close to the annual RSV season and/or born with chronic heart or lung disease are at the highest risk of developing severe ALRI upon RSV infection . Children that suffer from severe RSV infection in the first year of life have a higher chance of developing recurrent wheezing or asthma later in life Citation[4,5].
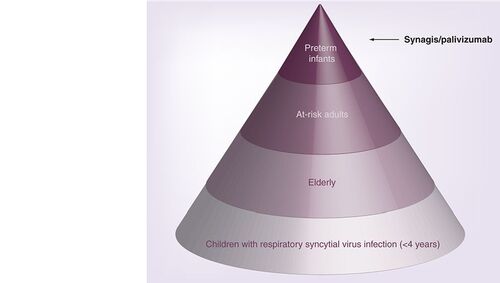
Different groups of respiratory syncytial virus patients who may seek specific respiratory syncytial virus therapy are represented in the pyramid, with the relative number of patients in each group (hospitalized and outpatients) increasing towards the base of the pyramid. Current therapeutic options are limited to passive immunization of premature infants with palivizumab. Preterm infants are less than 35 weeks of gestational age and include infants at high risk of developing severe respiratory syncytial virus disease. At-risk adults present immunocompromised patients including those with underlying pulmonary or cardiac disease. Elderly patients are more than 65 years of age.
In a recent retrospective study by Nair et al., global numbers of RSV-associated ALRIs in children under the age of five were estimated to be around 34 million, accounting for 22% of all ALRIs, with a mortality rate of 3–9% Citation[1]. This indicates that the global burden of RSV in children is still rising, particularly in countries with poor socio-economic conditions and with steep population growth such as sub-Saharan Africa or the Asian continent Citation[1]. At the UN Millennium Summit in 2000, the member states committed to Millennium Development Goal 4 whose aim was the reduction of under-five mortality by two-thirds by 2015, as compared with 1990 Citation[101]. Although significant progress has been made since then, based on the current trend, an estimated 13.2 million excess deaths will occur between 2010 and 2015 Citation[101]. For that matter, pneumonia remains the ‘number one killer’ of children under the age of five in every region of the world Citation[102] and recent studies have indicated Streptococcus pneumonia, Haemophilus influenza and RSV as the three main pathogens associated with childhood pneumonia Citation[2]. It is estimated that approximately 20–35% of pneumonia and viral bronchiolitis cases are caused by RSV Citation[1,6,7]. Yet, the virus remains the only deadly agent of these three for which no vaccine is available Citation[7].
In healthy adults, RSV infection usually provokes symptoms similar to the common cold, but in the elderly and immunocompromised adults (including those with underlying pulmonary or cardiac disease), the virus causes pneumonia and exacerbations of chronic obstructive pulmonary disease, contributing to significant morbidity and mortality during the winter season Citation[8,9]. In these patients, mortality can be as high as 20 and 70%, respectively Citation[9,10]. In the USA, it is estimated that approximately 170,000 hospitalizations and 10,000 deaths associated with RSV occur annually in patients older than 65 years Citation[8,9].
The increasing numbers of RSV infections demand new measures to decrease RSV burden in patients. New therapeutic opportunities to tackle RSV are currently being advanced via different strategic approaches, including development of new vaccine candidates, high-affinity antibodies, RNA interference-based agents and small-molecular chemical entities. However, each approach faces its own challenges and, consequently, RSV infection remains a high unmet medical need for most children and at-risk adults worldwide. In this article we will highlight some of these approaches and provide an update on the latest progress.
The only specific therapeutic option currently available for RSV is prophalytic intervention with the humanized murine monoclonal antibody palivizumab (®; MedImmune, Gaithersburg, MD). This drug (approved by the US FDA in 1998) is to be used for prophylaxis in those infants most at-risk for developing severe ALRI caused by RSV. Despite the significant reduction in hospital admissions in this patient group Citation[11], therapeutic costs are high and its use is therefore limited. Palivizumab patents will begin to expire from 2015 onwards, and the entrance of biogenerics is expected because of the worldwide market potential. The availability of cheaper biogenerics may represent a game changer, although their potential market impact remains unclear for now. For instance, will generic palivizumab ever be cheap enough for use in resource-poor countries?
Motavizumab, a next-generation affinity-matured variant of palivizumab is expected to give additional protection to the upper respiratory tract and is aimed at reducing the number of observed treatment failures to prophylaxis with palivizumab Citation[12]. At the end of 2009, MedImmune sent a reply to the FDA’s ‘complete response letter’, but the recent FDA advisory committee raised concerns about hypersensitivity issues (allergic skin rash occurring within two days of dosing) as a primary safety alarm during the risk-benefit assessment of motivizumab Citation[13]. At the same time, motavizumab is being explored for treatment of young children up to 1 year of age infected with RSV, in an effort to expand the antibody’s indications Citation[103].
Infants in their first 2 months of life are considered the preferred patient population for vaccination. Developing a vaccine for very young RSV-naive infants however, poses a challenge both from a development and heightened-safety perspective Citation[14–16]. Repeated vaccination may be required because the virus is able to evade the host immune response and, therefore, immune protection after natural infection is only limited in time and re-infection with RSV is common in all stages of life. Moreover, it has been shown that formalin-inactivated RSV vaccines exacerbate pulmonary disease due to failure to activate a proper RSV-specific CD8+ T cell response and to induce an aberrant CD4+ T cell response Citation[17,18]. Analysis of lung tissue from two vaccine recipients who later died of RSV infection demonstrated immunopathology not characteristic of naturally occurring RSV lower respiratory tract infection Citation[19]. These observations have been linked recently to the failure to activate pattern recognition receptors (i.e., Toll like receptor 7) by the formalin-inactivated virus Citation[20]. Effective clearance of the virus may require the induction of a balanced Th1/Th2 adaptive immune response, able to promote production of RSV-neutralizing antibodies together with induction of IFNγ-secreting cytotoxic CD8+ T-cells Citation[21,22]. Therefore, novel vaccine strategies may include addition of Toll-like receptor-stimulating adjuvants that may help find a vaccine that elicits an appropriate immune response, which is more robust and prolonged than the immune response against the natural infection. Furthermore, the strain coverage of candidate vaccines, the genetic variability and the post-translational processing of some of the viral proteins must be taken into account. In addition, a vaccine candidate should not reduce the safety or efficacy of the other eight vaccines that are administered in current routine childhood vaccination strategies. To avoid these challenges, an alternative vaccine strategy could be to target healthy children between 6 months and 5 years of age or at-risk adults in order to promote herd immunity and to eventually indirectly lower the risk of RSV infection in very young infants. The two most advanced vaccine candidates are being developed by MedImmune and have now entered into Phase I–IIa clinical trials in healthy children up to 2 years of age and in the elderly. MEDI-534 is a human–bovine chimeric parainfluenza virus expressing the RSV fusion protein, and MEDI-559 is a vaccine candidate based on a cold-passaged, live-attenuated virus strain. Nevertheless, approval of the first RSV vaccine is not expected before the end of 2020.
Respiratory syncytial virus therapy will likely extend to patient groups other than the at-risk infants over the next decade (e.g., the at-risk adults and children suffering from acute upper respiratory tract infection), not only because therapeutic options with antibodies may expand beyond the very young infants, but also due to ongoing development programs of small-molecule and small-interfering RNA (siRNA) agents that may be more suitable for treating patient groups not eligible for antibody therapy. One of the agents that has progressed considerably in the last few years is ALN-RSV01, an siRNA molecule targeting the N-protein of RSV. The agent has been evaluated recently in a randomized, placebo-controlled Phase IIa trial of inhaled ALN-RSV01 in healthy adults challenged with RSV Citation[23]. Patients were treated once daily for 2 days before and 3 days after RSV inoculation with a nasal spray containing either ALN-RSV01 or placebo, resulting in an approximately 38% decrease in the number of infections detected by quantitative culture of patients that received the investigational agent as compared with patients receiving placebo. Currently, the agent is being evaluated in lung transplant patients with a confirmed RSV infection. Hence, siRNA as a treatment option for the at-risk adult population may become a reality in a couple of years.
The last decade has also been marked by the discovery of multiple small-molecule RSV inhibitors with several very potent molecules progressing fast through the preclinical phase Citation[24,25]. The most advanced molecule is RSV-604 Citation[26], an oral benzodiazepine targeting the viral N-protein, which has recently undergone Phase II evaluation. The safety and efficacy of this compound was tested in a multicenter trial that included RSV-infected adult bone marrow transplant patients. Although initiated in 2005, the study was only completed at the beginning of this year and data from the trial are expected to be released later this year. In addition, RSV-604 is also undergoing Phase I evaluation with pediatric formulations. Another promising target against RSV is the virus–host fusion process Citation[24]. Enveloped viruses such as RSV, HIV-1 or influenza need to fuse with a host cell in order to deposit their genome and to initiate their replication cycle Citation[27]. In RSV, fusion is facilitated by the fusion (F) protein, and the market approval of palivizumab has validated F as a clinically relevant target. Since then, research has been focusing on this protein as a target for developing next-generation therapeutics including small-molecule inhibitors. Recent structural information demonstrating how small-molecules bind to a six-helix bundle target site in F has fully elucidated this drug-binding site and has contributed in a better understanding of how to inhibit the viral fusion process Citation[28,29]. Although relatively few small-molecule fusion inhibitors have so far entered clinical evaluation, active development programs are ongoing and it can be expected that new molecules will enter clinical development over the next few years.
The fusion process of the respiratory syncytial virus with a host cell is driven by the dramatic refolding of its surface fusion protein. The fusion protein contains two hydrophobic heptad-repeat regions (HR1 and HR2), which during fusion need to refold into a six-helix bundle in order to complete the fusion process. It has been shown that small-molecule fusion inhibitors of the respiratory syncytial virus such as TMC353121, bind to a hydrophobic cavity present in the central trimeric coiled-coil region of the six-helix bundle and, as such, block the process of viral entry in host cells Citation[28,29].
![Figure 2. The fusion protein of respiratory syncytial virus as a validated target for small-molecule fusion inhibitors.The fusion process of the respiratory syncytial virus with a host cell is driven by the dramatic refolding of its surface fusion protein. The fusion protein contains two hydrophobic heptad-repeat regions (HR1 and HR2), which during fusion need to refold into a six-helix bundle in order to complete the fusion process. It has been shown that small-molecule fusion inhibitors of the respiratory syncytial virus such as TMC353121, bind to a hydrophobic cavity present in the central trimeric coiled-coil region of the six-helix bundle and, as such, block the process of viral entry in host cells Citation[28,29].](/cms/asset/c40af09a-b867-45e9-b4b4-4511616900ce/ifmc_a_12362339_f0002.jpg)
After adverse responses to formalin-inactivated RSV vaccination were reported, it was assumed that RSV disease following infection is driven by an exuberant pathogenic immune response Citation[18,19,30,31]. Recent data, however, illustrate that, at least in children, severe forms of RSV disease are related to inadequate rather than hyper-responsive immune reactions Citation[22,32,33]. Infant RSV pathogenesis seems to be driven mainly by a rapid and profound viral replication and the ineffectiveness of an adaptive immune response to limit the infection. Moreover, treatment of RSV disease in infants with corticosteroids seems to be ineffectual Citation[34,35] and a correlation has been demonstrated between the quantity of RSV in the respiratory tract of infants and disease severity Citation[36,37]. For drug developers and clinicians it is imperative that such aspects of RSV pathology are studied well and understood in all patient populations because they ultimately drive the decision as to what type of RSV therapeutics will need to be developed and whether patients will benefit from treatment with direct antivirals, immunomodulators or a combination of both.
It is encouraging to see that many different strategies are being employed in order to bring new therapeutic options to patients who are most at risk of developing severe ALRI caused by RSV, with each of them offering unique opportunities. The availability of prophylaxis with palivizumab has already significantly reduced intensive-care admission within at-risk infants in the industrialized world and, owing to the intensity of pharmaceutical RSV drug-discovery research employing various strategies from vaccines to RNA interference over the last decade, RSV can be considered a pharmaceutically prioritized target.
However, the global burden of RSV is still rising, and based on the available data, this increase is disproportionate in resource-poor nations Citation[1,38]. Patients at highest risk for severe RSV disease in these countries unfortunately have the least access to current therapy. Moreover, RSV therapies presently under development will not reach the market before 2015 and will, therefore, not contribute to reducing the global RSV burden or the under-five worldwide mortality rate within the UN’s Millennium Development Goal 4 timeframe. As such, RSV is still a neglected target with high unmet medical need and every effort or strategy in the pharmaceutical armament needs to be explored in order to develop new therapies for tackling this important viral pathogen.
Respiratory syncytial virus
Non-segmented negative strand RNA virus (order Mononegavirales) of the Paramyxoviridae family, Pneumovirinae subfamily. The RSV genome codes for 11 proteins, of which three (SH, G and F) are embedded in the envelope of the virus. The N-protein is a major nucleocapsid protein that binds (anti)genomic RNA.
Small-molecule inhibitor
compounds with a molecular mass < 500 Da.
Six-helix bundle
Formed in the F protein of RSV when F refolds in its postfusion conformation during the final stage of the viral fusion process. Six-helix bundle formation is an essential process for successful fusion to take place and disturbing the natural six-helix bundle formation has been demonstrated to inhibit viral fusion.
Financial & competing interests disclosure
The authors are employees of Tibotec-Virco Virology BVBA’ The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
Bibliography
- Nair H , NokesJD, GessnerBDet al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375(9725), 1545–1555 (2010).
- Rudan I , Boschi-PintoC, BiloglavZ, MulhollandK, CampbellH. Epidemiology and etiology of childhood pneumonia.Bull. World Health Organ.86(5), 408–416 (2008).
- Simoes EAF . Respiratory syncytial virus infection.Lancet354(9181), 847–852 (1999).
- Sigurs N , GustafssonPM, BjarnasonRet al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Cri. Care Med. 171(2), 137–141 (2005).
- Simoes EA , GroothuisJR, Carbonell-EstranyXet al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J. Pediatr. 151(1), 34–42 (2007).
- Hall CB , WeinbergGA, IwaneMKet al. The burden of respiratory syncytial virus in young children. N. Engl. J. Med. 360(6), 588–598 (2009).
- Madhi SA , LevineOS, HajjehR, MansoorOD, CherianT. Vaccines to prevent pneumonia and improve child survival.Bull. World Health Organ.86(5), 365–372 (2008).
- Murata Y , FalseyAR. Respiratory syncytial virus infection in adults.Antivir. Ther.12(4), 659–670 (2007).
- Falsey AR , HennesseyPA, FormicaMA, CoxC, WalshEE. Respiratory syncytial virus infection in elderly and high-risk adults.N. Engl. J. Med.352(17), 1749–1759 (2005).
- Thompson WW , ShayDK, WeintraubEet al. Mortality associated with influenza and respiratory syncytial virus in the United States. J. Am. Med. Assoc. 289(2), 179–186 (2003).
- Morris SK , DzolganovskiB, BeyeneJ, SungL. A meta-analysis of the effect of antibody therapy for the prevention of severe respiratory syncytial virus infection.BMC Infect. Dis.5(9), 106 (2009).
- Carbonell-Estrany X , SimõesEA, DaganRet al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics 125(1), e35–51 (2010).
- Young D . FDA: MedImmune’s Rezield has 3x allergic reactions as Synagis.BioWorld Today21(104), 1–5 (2010).
- Murata Y . Respiratory syncytial virus vaccine development.Clin. Lab. Med.29(4), 725–739 (2009).
- Haas MJ . Besieging RSV.SciBX2(1), 1–3 (2009).
- Nokes JD , CanePA. New strategies for control of respiratory syncytial virus infection.Curr. Opin. Infect. Dis.21(6), 639–643 (2008).
- Kapikian AZ , MitchellRH, ChanockRM, ShvedoffRA, StewartCE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine.Am. J. Epidemiol.89(4), 405–421 (1969).
- Kim HW , CancholaJG, BrandtCDet al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89(4), 422–434 (1969).
- Neilson KA , YunisEJ. Demonstration of respiratory syncytial virus in an autopsy series.Pediatr. Pathol.10(4), 491–502 (1990).
- Delgado MF , CovielloS, MonsalvoACet al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 15(1), 34–41 (2009).
- Bueno SM , GonzálezPA, PachecoRet al. Host immunity during RSV pathogenesis. Int. Immunopharmacol. 8(10), 1320–1329 (2008).
- Welliver TP , GarofaloRP, HosakoteYet al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 195(8), 1126–1136 (2007).
- Devincenzo J , Lambkin-WilliamsR, WilkinsonTet al. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc. Natl Acad. Sci. USA 107(19), 8800–8805 (2010).
- Bonfanti JF , RoymansD. Prospects for the development of fusion inhibitors to treat human respiratory syncytial virus infection.Curr. Opin. Drug Dev.12(4), 479–487 (2009).
- Olszewska W , OpenshawP. Emerging drugs for respiratory syncytial virus infection.Expert Opin. Emerg. Drugs14(2), 207–217 (2009).
- Chapman J , AbbottE, AlberDGet al. RSV604, a novel inhibitor of respiratory syncytial virus replication. Antimicrob. Agents Chemother. 51(9), 3346–3353 (2007).
- Lamb RA , JardetzkyTS. Structural basis of viral invasion: lessons from paramyxovirus F.Curr. Opin. Struct. Biol.17(4), 427–436 (2007).
- Roymans D , De BondtHL, ArnoultEet al. Binding of a potent small-molecule inhibitor of six-helix bundle formation requires interactions with both heptad-repeats of the RSV fusion protein. Proc. Natl Acad. Sci. USA107(1), 308–313 (2010).
- Cianci C , LangleyDR, DischinoDDet al. Targeting a binding pocket within the trimer-of-hairpins: small-molecule inhibition of viral fusion. Proc. Natl Acad. Sci. USA 101(42), 15046–15051 (2004).
- Legg JP , HussainIR, WarnerJA, JohnstonSL, WarnerJO. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis.Am. J. Respir. Crit. Care Med.168(6), 633–639 (2003).
- Aung S , RutiglianoJA, GrahamBS. Alternative mechanisms of respiratory syncytial virus clearance in perforin knockout mice lead to enhanced disease.J. Virol.75(20), 9918–9924 (2001).
- Welliver RC . The immune response to respiratory syncytial virus infection: friend or foe?.Clinic. Rev. Allerg. Immunol.34(2), 163–173 (2008).
- DeVincenzo JP . A new direction in understanding the pathogenesis of respiratory syncytial virus bronchiolitis: how real infants suffer.J. Infect. Dis.195(8), 1084–1086 (2007).
- Buckingham SC , JafriHS, BushAJet al. A randomized, double-blind, placebo-controlled trial of dexamethasone in severe respiratory syncytial virus (RSV) infection: effects on RSV quantity and clinical outcome. J. Infect. Dis. 185(9), 1222–1228 (2002).
- Ermers MJJ , RoversMM, van Woensel JB et al. The effect of high dose inhaled corticosteroids on wheeze in infants after respiratory syncytial virus infection: randomized double blind placebo controlled trial. BMJ338, b897 (2009).
- DeVincenzo JP , El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J. Infect. Dis.191(11), 1861–1868 (2005).
- Buckingham SC , BushA, DeVincenzoJP. Nasal quantity of respiratory syncytial virus correlates with disease severity in hospitalized infants.Pediatr. Infect. Dis. J.19(2), 113–117 (2000).
- Hall CB . Respiratory syncytial virus in young children.Lancet375(9725), 1500–1502 (2010).
Websites
- Keeping the promise: a forward-looking review to promote an agreed action agenda to achieve the Millennium Development Goals by 2015. United Nations, New York, NY, USA (2010). www.un.org/millenniumgoals
- Global action plan for prevention and control of pneumonia (GAPP). WHO-Unicef, Geneva, Switzerland (2009). http://whqlibdoc.who.int/hq/2009/WHO_FCH_CAH_NCH_09.04_eng.pdf
- Clinical Trials. http://clinicaltrials.gov