Abstract
In the 24 years since first being marketed, the mesh nebulizer has been developed by five main manufacturers into a viable solution for the delivery of high-value nebulized drugs. Mesh nebulizers provide increased portability, convenience and energy efficiency along with similar lung deposition and increased ease of use compared with jet nebulizers. An analysis of EU and US clinical trial databases has shown that mesh nebulizers are now preferred over jet nebulizers for clinical trials sponsored by pharmaceutical companies. The results show a strong preference for the use of mesh nebulizers in trials involving high cost and niche therapy areas. Built-in capability to optimize the way patients use their mesh nebulizer and manage their disease will further increase uptake.
It is 24 years since the first ultrasonic mesh nebulizer was marketed by Omron in 1993 [Citation1]. During this period, five major manufacturers have developed a range of marketed products for both domiciliary and ventilator applications. In 1993, the market for aqueous drug delivery was dominated by one product - the jet nebulizer, a product which had been in continuous development since medicinal aerosol delivery started in the 19th century. Today the majority of jet nebulizers are inexpensive simple plastic devices operated from compressed gas, and when used in a domiciliary setting they require a grid connected air compressor. It is a significant challenge for a new technology to enter such a mature market, especially with the complexities of the different national regulatory and reimbursement systems that need to be dealt with for both the delivery device and the delivered drugs. Mesh nebulizers have been significantly more expensive than jet nebulizers because of the increased number of tolerance critical parts, components and assembly associated with both the electronic control circuits and the mesh. The mesh itself must be produced with consistent thickness, consistent physical properties and with thousands of consistent micron sized holes, which is a time consuming multistage production process. However, the increased costs are counterbalanced by significant advantages including: reduced drug waste, silent operation and portability. To date not all of these advantages are easy for the customer to benefit from when most of the existing drugs for nebulization are already packaged specifically for jet nebulizers.
Market uptake of mesh technology
Jet and ultrasonic nebulizers entered the market several decades before mesh devices. Therefore the mesh nebulizer has had to compete for a share of the pre-existing market since the launch of the first mesh nebulizer the Omron NE-U03 in 1993 [Citation1]. In an ever growing nebulizer market, perhaps as a consequence of decreasing prices, an aging population and increased occurrence of respiratory diseases, the jet nebulizer continues to dominate the market with approximately 73% of the world market, compared with ultrasonic and mesh nebulizers (measured as revenue in 2013) [Citation2]. Existing pharmaceuticals have all been developed and packaged for jet nebulizer use, and as a result mesh nebulizers were not an immediate replacement for jet nebulizers. It has taken time for new drug products which have been specifically developed for mesh nebulizers to be developed and approved. However, the mesh nebulizer has continued to establish a place for itself, leading to the domination of the specialist nebulizer market [Citation3] for higher value drugs jointly developed with pharmaceutical companies. As a result, the success of mesh nebulizers has been initially limited in the main market for jet nebulizers, namely treatment of asthma and chronic obstructive pulmonary disease (COPD), due to the lower cost of the drugs.
Recent market research reports indicate that the market for mesh nebulizers is expected to grow [Citation2,Citation3]. The world market has an expected growth of approximately a third higher than jet nebulizers from 2015 to 2018, in terms of unit shipments [Citation2]. In America, the estimates suggest there will be a market increase for mesh nebulizers of over double that of jet nebulizers from 2015 to 2018. In Europe, the Middle East and Africa, an increase at approximately the same rate is estimated for all nebulizer types. The Western European and the UK and Ireland market estimates, however, show that the mesh technology market is expected to grow at a rate of approximately two thirds faster than the jet nebulizer market from 2015 to 2018. This slower growth in the European market compared with the American market is based on Europe's continued recovery from the Eurozone crisis. Estimates indicate that there will be a decline in the average selling price of all nebulizer devices, with the greatest reductions expected for mesh nebulizers from 2015 to 2018 [Citation2,Citation3]. The reduction in the prices of mesh nebulizers is likely to be due to a combination of increased competition with the relatively less expensive prices of jet nebulizers and a reduction in manufacturing prices as a result of the less expensive technologies available for the production of mesh generators by various manufacturers. Despite this, the additional education required in order to ensure that the user can clean mesh devices adequately, combined with the further complexity of matching drug specifications to specific devices and the relatively high cost of mesh devices have created barriers to the use of mesh nebulizers within some markets. Overall, the mesh nebulizer market is expected to continue to grow. Key drivers include a reduction in device prices and growth of the domiciliary nebulizer market, where patients demand newer technology, higher quality, smaller, lighter, quieter and more reliable devices [Citation2], plus an expansion into new therapeutic areas.
Choice of the type and model of nebulizer that is used in a drug development program is becoming progressively more important as a result of increased restrictions by regulators. Drug approval is increasingly related to the specific nebulizer used in the later phases of a clinical trial program, with an advisory that the performance of other devices has not been evaluated. The aim of such regulation is presumably to increase the reproducibility of the administration of nebulized medications by removing the factor of device variation [Citation4–6]. Therefore it is important to make a carefully considered choice regarding the type and specifications of a nebulizer for use in a clinical trial within a drug development program. This ensures that it is best suited for the specific drug delivery profile and target patient group, and consequently to increase the chance of achieving commercial success [Citation6].
Aerosol generation in mesh nebulizers
The process of aerosol formation is similar in all ultrasonic mesh nebulizer designs; an alternating pressure behind the mesh forces liquid through the holes, a column of liquid is extruded from the front of the mesh and as the pressure cycle reverses, the end of the column separates as a liquid droplet. The size of the droplet is approximately twice the size of the mesh hole, as predicted in Rayleigh theory [Citation7]. However, the design of the aerosolization mechanism containing the mesh, and the mesh itself, can vary widely between different models. The mode of vibration, shape of the mesh, material that the mesh is made of and also the way that the mesh is created can all vary between mesh nebulizer brands, and have an effect upon the aerosolization performance of the device. In passive mesh nebulizers, the piezo element is mounted in close proximity to the mesh and the vibration is conducted through a thin fluid layer to the mesh. Active mesh nebulizers use the vibration of a piezo element to vibrate a mesh substrate that is in contact with a fluid reservoir so that the movement of the mesh pushes the liquid through the holes in the mesh. Most passive mesh nebulizer designs utilize a flat mesh geometry, whereas active mesh nebulizer designs utilize a domed mesh. The rigidity of the mesh structure is important to prevent oscillations of varying amplitude across the surface of the mesh, which could result in inconsistent aerosolization performance. In addition to the mechanical aspects of the vibration and how it is applied to the liquid/mesh, the material substrate from which the mesh is constructed and the way in which the holes are generated have important implications for the aerosolization of different drug formulations. These formulations may have widely different physicochemical properties, such as surface tension, viscosity and whether the formulation is a pure solution or a suspension of particles within a solution. Currently, the two main methods for mesh production are electroplating and laser cutting techniques, which are used to produce a tapered hole. A tapered hole is required to optimize mesh performance; it amplifies flow at the nozzle and reduces viscose losses. The electroplating method relies on the use of a lithographic plate and the eventual size of the mesh holes is determined by the duration of the electroplating process. The holes get smaller as the metal is deposited on the edge of the hole over time. Laser cutting involves the use of a laser beam to cut the mesh holes into a thin sheet of metal or polymer material. Laser cutting metal can result in molten material being deposited around the hole, which is then removed by polishing. Currently, most mesh nebulizer meshes are constructed from a metal alloy, to give the rigidity, mass, durability and inert chemical properties required for the aerosolization of different drug formulations. The metals used in mesh manufacture (i.e., platinum, palladium, nickel and stainless steel) along with the manufacturing techniques needed to create the consistent sizes of holes tend to be expensive. Future developments of mesh nebulizers will include the use of alternative materials, designs and production processes to produce lower cost meshes, which would allow their frequent replacement, and reduce the requirement for thorough cleaning.
Mesh nebulizer manufacturers
In the 24 years since first being marketed, the mesh nebulizer has been developed by five main manufacturers, but generic devices are now coming to market, photographs and details are widely available on manufacturer and other websites [Citation8–14].
Omron: Omron launched the NE-UO3 in Japan in 1993. This nebulizer was a huge step forward for portable nebulizers, with the benefits of being small, lightweight, silent and efficient, and it could be powered by four AA batteries for up to 5 h [Citation15,Citation16]. Nonetheless, there were a number of drawbacks associated with this nebulizer, including the ceramic mesh, a relatively slow output rate and large mass median aerodynamic diameter [Citation16]. After continued developments to their mesh technology, the MicroAir NE-U22 passive mesh nebulizer model was launched in 2002. The design comprised a flat electroformed mesh with a gravity liquid feed and a removable nebulizer head [Citation17], with a notable improvement in the robustness, ease of cleaning and performance compared with the earlier model.
Philips: In 2002, the Omron Healthcare Ltd aerosol generation technology was licensed by Profile Therapeutics Ltd (which later became Respironics Respiratory Drug Delivery [UK] Ltd) and integrated into their third-generation Adaptive Aerosol Delivery (AAD) device, the I-neb AAD System nebulizer [Citation4], which was launched in 2004 [Citation18]. This device incorporated the superior efficiency of mesh technology with the ‘intelligent’ AAD technology. It released aerosol only into the patient's inhalation, based on the inhalation length predicted by the AAD algorithm from previous inhalation data [Citation18,Citation19]. The advanced technology provided patients with feedback and also allowed the delivery of precise doses with reduced wastage, thus reducing the amount of drug required to be loaded into the device in order to deliver an effective dose to the patient. The addition of a metering chamber with an overflow portion allowed the metering of smaller and more precise doses [Citation20]. This allowed the I-neb AAD System to be used with existing drugs packaged for jet nebulizers, and also made the I-neb AAD System nebulizer suitable for the delivery of expensive medications or medications with a narrow therapeutic window [Citation19]. In 2014, the Aeroneb Go nebulizer (launched in 2004) [Citation21] along with the rest of the Aerogen Ltd domiciliary nebulizer range was acquired by Philips via a technology license.This allowed Aerogen Ltd to focus their mesh nebulizer business on hospital use [Citation22]. In 2016, the InnoSpire Go was launched, which was designed with a two-piece construction to provide improved ease of use compared with older designs of mesh nebulizer [Citation23].
Pari: The eFlow mesh nebulizer was approved for sale in 2004 [Citation24]. The original eFlow nebulizer, designed by Pari GmbH using The Technology Partnership plc mesh technology, had an active mesh with a gravity liquid feed, aerosol reservoir chamber and inhalation and exhalation valves [Citation25]. The storage of aerosol generated during exhalation, and subsequent release during inhalation, proved to be a significant advancement for mesh nebulizer performance. In addition to the continued development of the eFlow nebulizer platform, a flexible manufacturing process was developed in conjunction with The Technology Partnership plc in 2002 that could produce laser cut and polished meshes with different sized holes for different clinical applications [Citation4,Citation24,Citation26]. This feature was a significant advantage of the eFlow nebulizer platform when considered for the delivery of different drugs. Pari GmbH launched the Velox mesh nebulizer in 2015, based on the proven eFlow technology platform incorporated into an improved ergonomic design.
Vectura: In 2014 the Vectura Group plc acquired Activaero GmbH [Citation27], the developers of FAVORITE smart nebulizer systems technology, which will be used to deliver both Vectura's own and partners’ pharmaceutical products. The Akita system delivers a regulated flow of air to the patient during inhalation, which results in slow deep inhalation by the patient. Aerosol delivery is controlled by a programmable smart card allowing an aerosol bolus to be delivered at a precise point during inhalation. The timing of the aerosol bolus can be used to target the aerosol deposition in different regions of the lung from central to peripheral [Citation28]. Akita systems are available with either a jet nebulizer or a mesh nebulizer developed by Pari, and the smart card also allows patient adherence data to be collected. The FOX nebulizer is a hand held device which uses a mechanical flow regulator to control the patient's inhalation flow. This allows the patient to take a slow deep inhalation maneuver during which the aerosol is delivered. The first FOX system was launched by Bayer AG as the Breelib for the delivery of Ventavis in 2017 [Citation29].
Aerogen: The Aeroneb Pro nebulizer, launched in 2002 [Citation30], and the subsequent Aeroneb Solo nebulizer model (Aerogen Ltd) [Citation31] were rebranded by Aerogen Ltd in 2015 and marketed subsequently as Aerogen Pro and Aerogen Solo. The introduction of these nebulizers resulted in a new use for mesh nebulizers within the area of aerosol delivery to patients during mechanical ventilation. This was due to the increased doses delivered to the patient when mesh nebulizers were used in combination with a ventilator, and the reduced risk of side effects, such as barotrauma and volutrauma, which could result from use of a jet nebulizer. Aerogen Ltd also developed the Pulmonary Drug Delivery System, which allowed the nebulizers to be breath-activated when used with a ventilator [Citation32]. Consequently, the Aerogen Pro and Aerogen Solo mesh nebulizers have become the delivery systems of choice for use with both invasive and noninvasive ventilators. Controls for the nebulizer have been integrated into the ventilator by several market leading ventilator manufacturers, including GE Healthcare. Another Aerogen device that is popular in noninvasive ventilator aerosol delivery is the Nivo mesh nebulizer (Aerogen Ltd) [Citation33], which is used with the Respironics AF531 oronasal mask (Respironics, Inc., a Philips Healthcare Company, PA, USA). This was the first mesh nebulizer developed specifically for noninvasive ventilation.
Generic devices: Since 2004, the mesh nebulizer market has been dominated by these five manufacturers. However, the original mesh nebulizer patents are beginning to expire, which has permitted other manufacturers to enter the market; by 2015, over ten new nebulizer manufacturers had been identified [Citation34]. Most of these suppliers are making products using the expired patent designs and have not filed any patents of their own. The products from these manufacturers are sold under a number of different brands as various models of mesh nebulizers. These models are usually not supported by publications or the extensive test and characterization results that are normally published by established companies in the field. As has been seen with jet nebulizers, it is expected that there will be significant differences in performance between these brands [Citation35,Citation36], which may affect the delivery of aerosol to the lungs. The consistency of aerosol particle size performance, especially after replacement of meshes, as well as the overall performance and reliability of these models will be open to question until their operational performance has been thoroughly tested and the results published for digest by the clinical fraternity, as typically occurs for the main developers [Citation19,Citation37–40]. This is not to say that all the new entrants into the field only sell copies; the Industrial Technology Research Institute of Taiwan (Hsinchu, Taiwan) and the Microbase Technology Corporation (Bade City, Taiwan) are actively developing atomizer technology. These two companies have filed over 45 patent applications, and have 13 granted EU/US patents. Rossmax International Ltd (Taipei, Taiwan), using technology from the Industrial Technology Research Institute of Taiwan patented in 2006 [Citation41], has developed both branded and original equipment manufacturer mesh nebulizers. Some designs include novel features, such as a laser cut polymer mesh, which could have the potential to induce a reduction in the future price of mesh technology and thus open up the mesh nebulizer to wider use. However, if these new developments do lead to improved nebulizer performance, these companies will also need to ensure that they publish data to support their claims, and such data would be considered a prerequisite for including these new devices in pharmaceutical developments.
Mesh nebulizer performance
Advantages of mesh nebulizer design
The main advantage of mesh nebulizers is the additional portability and convenience they provide to the user, which is a result of a number of factors detailed in .
Table 1. A comparison of relative features, advantages and disadvantages of jet and mesh nebulizers.
Energy efficiency lies at the heart of the increased portability and convenience that mesh nebulizers offer compared with jet nebulizers. The lack of compressor and low energy requirement for the single pass aerosol generation process result in a handheld aerosol generator that is small in size, weight and virtually silent in operation. The single pass aerosol generation with no need for baffles has also resulted in reduced residual volume, fast nebulization times and long battery life. The minimal residual volume of around 100 μl [Citation6,Citation18,Citation42] compared with the large volume of liquid that remains within a jet [Citation4,Citation6,Citation15,Citation43] or ultrasonic nebulizer cup [Citation6], has been a key advantage in the successful commercialization of many of the mesh nebulizer brands. This has allowed a reduction in fill volumes in comparison with previous nebulizers. For example, a fill volume of 2000 μl was required for a conventional jet nebulizer (LC Plus; Pari GmbH) to achieve a predicted lung dose of 404 μg, whereas an almost five-times lower fill volume of 420 μl was required for an I-neb AAD System breath-activated mesh nebulizer to achieve an equivalent predicted lung dose of 420 μg [Citation19]. An in vivo example is provided by a study that used γ-scintigraphy to test lung deposition in patients. Nebulization of 500 μl of solution via the MicroAir NE-U22 mesh nebulizer was compared with 2500 μl solution via the Pari LC Plus jet nebulizer. The lung deposition achieved with the MicroAir NE-U22 nebulizer was nearly triple that of the lung deposition achieved with the LC Plus nebulizer (18.1 and 6.4% mean lung deposition as a percent of volume fill, respectively) [Citation44]. This enhanced efficiency of mesh devices over previous nebulizer designs makes them ideal delivery systems for medications with a high cost [Citation6]. This has led to the launch of drugs with smaller unit dose vial sizes of around 1 ml [Citation45], compared with traditional unit dose vial sizes of around 2.5 ml. Moreover, unlike ultrasonic nebulizers, mesh devices are suitable for the nebulization of a large variety of medications [Citation6], with some new drugs developed specifically for nebulization by mesh nebulizers. For example, the I-neb AAD System nebulizer was the first mesh device developed for specific drug applications, such as Colistimethate Sodium (Profile Pharma Ltd, Chichester, UK) and Iloprost (Bayer plc, Newbury, UK), with the drug formulations optimized to the high device efficiency. In contrast, the continued use of existing nebulized drugs has caused some mesh nebulizer manufacturers to deliberately reduce the efficiency of mesh devices in order to ensure that an equivalent dose is delivered to the patient as those by jet and ultrasonic nebulizers [Citation4]. One of the main drawbacks of mesh nebulizers, however, is their cost [Citation43]. Mesh nebulizer technology has improved rapidly, providing a solution to the demand for small, efficient and portable devices for the delivery of inhaled medication.
Mesh nebulizers confer particular advantages when used for the delivery of aerosols to ventilated patients, as the generation and introduction of aerosol into the ventilation system does not result in additional flows and pressures that can arise with the use of jet nebulizers. Even greater advantage is gained when the mesh nebulizer is combined with breath-activated systems such as in the Pulmonary Drug Delivery System, which allows aerosol generation to be synchronized with the ventilator for maximum delivery efficiency. These features allow the technology to be easily adapted to a wide variety of ventilator support circuits, enabling aerosol delivery including noninvasive ventilation, continuous positive airway pressure, high-flow nasal therapy [Citation46] and neonatal applications. The mesh nebulizer is normally inserted into the inhalation arm of the ventilator circuit for volume ventilators, whereas in continuous positive airway pressure or noninvasive ventilation circuits it should be positioned after the patient leak port. The ability to alter the position of the mesh nebulizer in the circuit allows significant improvements in delivered dose for both adult and neonatal patients [Citation47,Citation48]. Compared with pressurized metered dose inhalers, nebulizers can be used to deliver a wider range of drugs to ventilated patients including antibiotics, surfactant, mucolytics and prostaglandins [Citation49]. As the Aerogen Ltd system has become the industry standard for ventilator drug delivery, the potential for delivering new drugs is being investigated, such as Amikacin (Bayer HealthCare Pharmaceuticals, Inc., NJ, USA) for ventilator-associated pneumonia. As a consequence of Aerogen's successful aerosol delivery via mesh nebulizer in mechanical ventilation, other manufacturers are investigating the use of mesh nebulizers for ventilator drug delivery.
Mesh nebulizers versus jet nebulizers in vivo
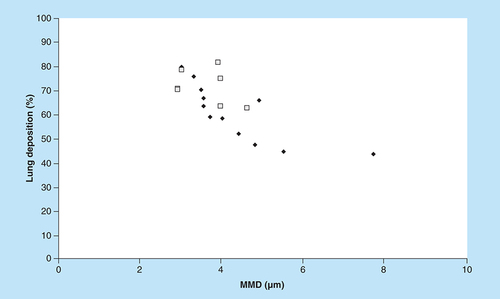
Scintigraphic aerosol deposition studies are used to give in vivo data concerning the fate of inhaled aerosols. Mesh nebulizers were compared with conventional jet nebulizers with mains-powered compressors in . The results of the in vivo studies in [Citation44,Citation50–57] suggest that lung deposition is also similar for both types of nebulizers. For instance, when an aerosol with a mass median diameter (MMD) of 3 μm was produced by both a mesh nebulizer and a jet nebulizer, the lung deposition was 80% of the delivered dose. Therefore, although the jet nebulizer can produce a more polydispersed aerosol, the performance of jet nebulizers and mesh nebulizers were similar in vivo. This suggests that the mesh nebulizer is as effective as a jet nebulizer in producing aerosol particles suitable for inhalation, with high deposition in the lungs.
Ease of use & cleaning
Mesh nebulizers have been designed to be small, quiet and portable, with the intention of improving convenience and appeal to the user and to allow for the maintenance of an active lifestyle. The appeal of an inhalation device is an important factor for patient adherence, as patients tend to use devices more regularly when they like them [Citation58,Citation59], and therefore can result in improved clinical outcomes [Citation60]. In order to determine if the improvements in the design of mesh nebulizers have been positively perceived by the user, comparisons were made of patients’ opinions of their old nebulizers and new mesh nebulizers. Goodman et al. assessed the preferences of patients with COPD after the use of the I-neb AAD System nebulizer for 3 months, in comparison with their previous jet nebulizer (96%) or ultrasonic nebulizer (4%) [Citation61]. The authors found that patients had a statistically significant (p ≤ 0.001) preference for the I-neb AAD System nebulizer with almost every question, including ease of cleaning, assembly/disassembly, device size, shape and weight, ease of operation and overall desirability. Furthermore, through the completion of the validated Chronic Respiratory Questionnaire at the start and end of the study, patients reported a statistically significant improvement in the Chronic Respiratory Questionnaire dimensions of dyspnea and fatigue with the I-neb AAD System nebulizer over their previous nebulizer, indicating an improvement in quality of life. In a study by Naehrig et al., cystic fibrosis patients’ use of an eFlow Rapid mesh nebulizer (Pari GmbH), for a year, in place of their conventional nebulizer, was evaluated. The patients reported a reduction in the total treatment time per day from 31 min with a jet nebulizer to 10 min with the eFlow Rapid mesh nebulizer. As a consequence, the inhalation time (p < 0.01) and the tolerability of the eFlow Rapid nebulizers (p = 0.021) were preferred by the patients to previous devices. Patient satisfaction with cleaning the device (p = 0.93) and its integration into daily physiotherapy (p = 0.95) was equally high for the eFlow Rapid nebulizer and the patients’ previous devices [Citation62]. In the early days of mesh nebulizer adoption in the clinical area, there were concerns over the difficulty of cleaning mesh nebulizers and the potential for clogging of the mesh holes [Citation4,Citation43], which could potentially increase nebulization times and reduce the performance of mesh devices. In spite of these initial concerns, in both studies detailed above, the ease and satisfaction of cleaning the device were found to be high for the I-neb AAD System and eFlow Rapid mesh nebulizers [Citation61,Citation62]. Rottier et al. assessed performance changes of eFlow Rapid mesh nebulizers and Pari LC Plus jet nebulizers with a Pari TurboBoy N compressor after 6 months’ use by cystic fibrosis patients. Regardless of cleaning instructions at the start of the study, most eFlow Rapid devices were found to be polluted after 6 months of use [Citation63]. Slight increases in MMD were observed after 6 months’ nebulizer use, with an increase of 5.1% for the eFlow Rapid nebulizer, in comparison with a three-times greater increase in MMD of 15.5% for the LC Plus nebulizer. Nebulization times also increased by a mean of 2.3 min for the eFlow Rapid (nebulizer automatically turned off after 10 min, and 51% of the devices tested turned off automatically) and 6.1 min for the LC Plus nebulizers [Citation63]. Bakuridze et al. published contrasting results to Rottier et al. following an assessment of eFlow Rapid mesh nebulizers throughout 2 months’ simulated use [Citation64]. Although a shorter time frame was used to collect the results from the study by Bakuridze et al., it has been suggested that the results from the study by Rottier et al. could have been due to a lack of patient compliance with disinfection of the nebulizer, as well as the use of the nebulizer with other medications [Citation65]. Together, these results highlight the importance of patient compliance with mesh nebulizer cleaning and disinfection procedures recommended by the manufacturer for the prevention of pollution of the mesh and maintenance of nebulizer performance. From the studies discussed above, it can be concluded that the ease of use of mesh nebulizers is high, with the studies by Naehrig et al. and Goodman et al. demonstrating a user preference for mesh nebulizers over the users’ previous devices. Furthermore, Bakuridze et al. suggested that when care instructions are followed, mesh nebulizer performance is unaffected by repeated use; however, low patient compliance with mesh nebulizer care can potentially compromise nebulizer performance, as it can with jet nebulizers.
Mesh nebulizers in clinical trials
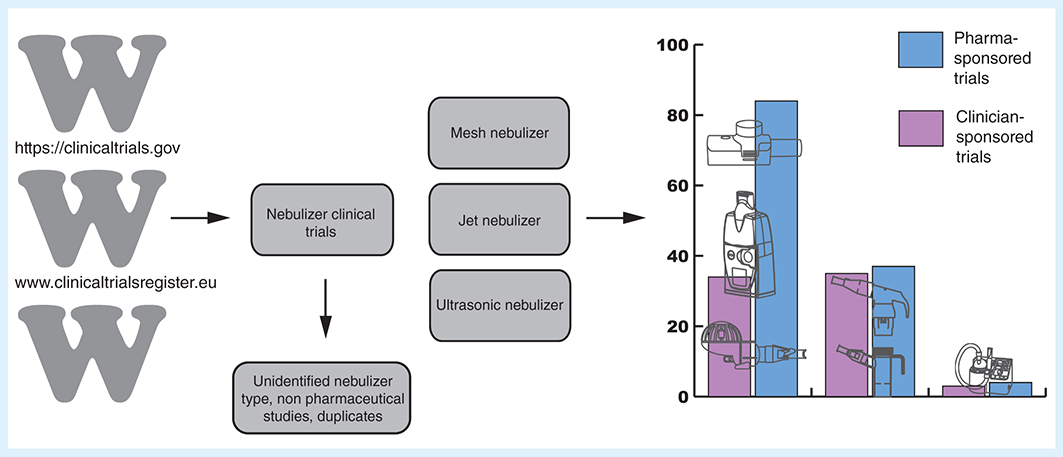
To evaluate how successfully mesh nebulizers are progressing in the new pharmaceutical development market, we have analysed the US and EU clinical trial databases to see how many pharmaceutical drug developments are now using mesh compared with jet nebulizers. This could be an indication of how well mesh technology has been accepted. The adoption of clinical trial registers has provided a useful resource to examine the penetration of mesh nebulizers into clinical trials of new and existing drugs. The use of clinical trial registers have been a recent development in the US and EU, with data collected since 2000 and 2004, respectively.
Clinical trials register review method
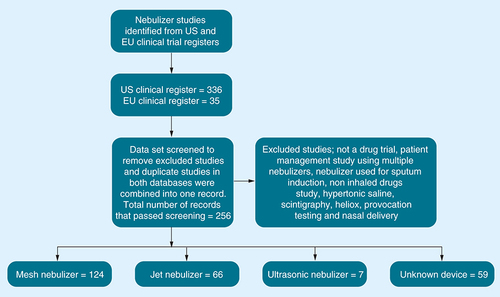
A search of the US and EU clinical trial registers was undertaken in January 2017 for this review, using the following databases: EU clinical trials register and the US National Institute of Health clinical trials registry. A total of 256 clinical trials involving nebulizers were found, clinical trials that were registered in both databases were combined as one dataset. Clinical trials were excluded from the analysis due to the nebulizer not being specified, the study being withdrawn, the study not being a drug trial or for noninhaled drugs, or the trial investigating hypertonic saline or heliox, scintigraphy, provocation tests, using spinning top aerosol generators or nasal delivery. A total of 197 studies were analysed by nebulizer type, sponsor, therapy area, clinical trial phase and year ().
Results of the review of clinical trial registers
The combined search results from both the US and EU clinical trial databases found 256 nebulizer studies; in 59 (23%) studies, the type of nebulizer device could not be identified (). The results in show that there was a rise in the number of registered clinical trials from 2006 for both jet and mesh nebulizers, although this may be partly representative of an increased compliance with the use of registries rather than an increase in the number of clinical trials undertaken. Between 2006 and 2016, the number of registered clinical trials that used mesh nebulizers was higher than the number of those that used jet nebulizers. The inclusion of mesh nebulizers in clinical trial registries came after both the establishment of clinical trial registries in the EU and the launch of three of the most popular models of mesh nebulizer, the I-neb AAD System, Aeroneb Go and eFlow, in 2004 [Citation18,Citation21,Citation24].
Mesh nebulizers have been used in more clinical trials than jet or ultrasonic nebulizers with 60% of the clinical trials having used a mesh nebulizer in comparison with the 36% that included a jet nebulizer and 4% that included an ultrasonic nebulizer. This suggests that the mesh nebulizer has been well accepted since it was first introduced to the market in 1993 [Citation1].
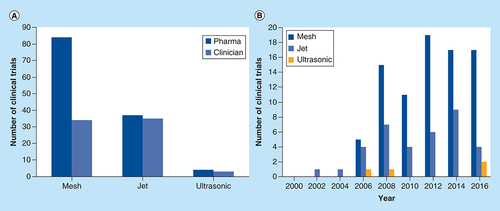
The majority of clinical trials on the registry that used mesh nebulizers were sponsored by pharmaceutical companies (71%), whereas only 29% of the mesh nebulizer clinical trials were sponsored by clinicians (). Ultrasonic nebulizers were used in the lowest number of clinical trials, with inclusion being equally low for both the pharmaceutical company and clinician sponsored clinical trials. Jet nebulizers were also used equally by clinicians and pharmaceutical companies, although the overall levels of inclusion were substantially higher than those of ultrasonic nebulizers. The low inclusion rates of the ultrasonic nebulizer in both pharmaceutical company and clinician sponsored clinical trials were possibly as a result of the high expense and the restriction of the types of drugs that can be nebulized by an ultrasonic nebulizer, limiting its appeal.
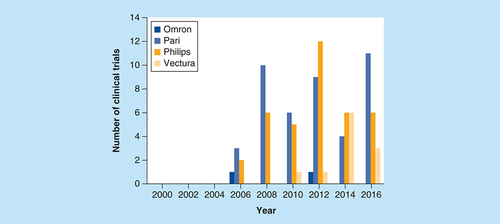
There was a general increase in the number of mesh nebulizer clinical trials sponsored by pharmaceutical companies between 2006 and 2012, with a consistently higher mesh nebulizer inclusion compared with that of jet nebulizers from 2006 .
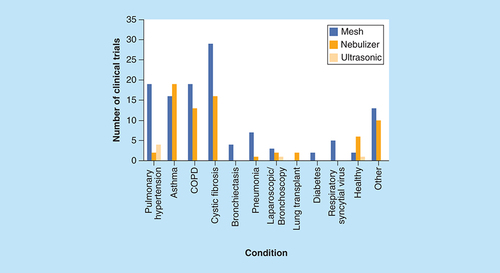
An analysis of the types of nebulizers used in clinical trials for different therapy areas showed that there was a preference for mesh nebulizers in clinical trials of high cost and niche therapy areas, such as cystic fibrosis and pulmonary hypertension (). This is likely to be a result of increased investment into treatment areas where a disease has a high potential to be life threatening and where there is a higher drug expense. For example, iloprost, for the treatment of pulmonary hypertension, costs approximately $70,000 per annum per patient [Citation6], and thus demands a delivery device with high efficiency and minimal wastage. In more common disease areas such as asthma, jet nebulizers were favoured over mesh nebulizers, possibly due to the wider demand for therapy, making lower cost jet nebulizers a more viable option for widespread treatment as well as the lower cost of medication reducing the necessity to prioritize high efficiency over the cost of the device.
Between 2006 and 2016, Pari GmbH mesh nebulizers were the most preferred make of nebulizer used in domiciliary clinical trials (44%), compared with Philips (38%) and Vectura (11%) (); Omron was the least preferred make. However a Vectura mesh nebulizer has only been available since 2007 and the data show that it is gaining popularity.
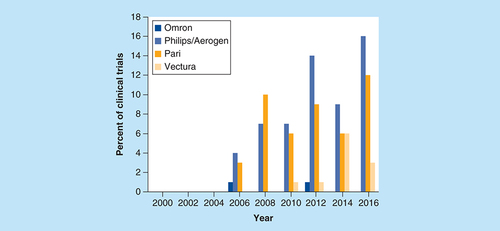
Since Philips’ acquisition of Aerogen Ltd domiciliary devices in 2014 [Citation22] left Aerogen to focus on the hospital sector, as a result of this, the combined Aerogen Ltd and Philips domiciliary mesh devices were used in 49% of all clinical trials, followed by Pari GmbH with 40% and Vectura with 9%. Omron Healthcare Ltd devices, however, were included in just 2% of clinical trials ().
Aerogen Ltd mesh nebulizers dominate the field of aerosol delivery during mechanical ventilation, with 76% of ventilator clinical trials having used Aerogen Ltd devices between 2000 and 2016, and 14% using Pari devices. In comparison, only 10% of clinical trials involving ventilators used a jet nebulizer. This may indicate that the increased drug doses delivered by mesh nebulizers compared with jet nebulizers when a ventilator is used have caused mesh nebulizers to take over as the respiratory device of choice in the field of aerosol delivery during mechanical ventilation.
Based on the description of the clinical trial in the registers, an assessment was also made of the phase of drug development. Surprisingly, only 15% appeared to be Phase I studies, with 37, 21 and 27% in Phases II, III and IV, respectively. Assuming the classifications were broadly correct, this is in stark contrast to the normal pattern of drug development, where Phase I studies are the highest proportion due to attrition at later stages. While there was some bias toward rejecting early phase studies because the nebulizer type could not be identified (see ), this was insufficient to account for this finding. Therefore, either companies are using an inhaler for Phase I, which would seem unlikely given the speed and cost advantages of using a nebulized formulation in early phase development [Citation66], or there is an under-reporting of early phase trials in the registers. Registration of clinical trials is voluntary, but is required if the results of the study are to be published in mainstream medical journals. As the results of Phase I studies are rarely published, it may be that many companies choose not to register early phase trials. Only three of the large pharmaceutical companies were represented in the Phase I studies that were reported. Mesh nebulizers were the most common type studied across all the phases, although in Phase IV studies, jet nebulizers were used almost as frequently.
With the choice of the specific nebulizer used in a clinical trial becoming more important, it is conceivable that the increased inclusion of mesh nebulizers in registered clinical trials could lead to an increase in the mesh nebulizer market share if more drugs are approved specifically for use with a mesh nebulizer. For instance, iloprost was the first inhaled drug to be approved in the USA for use with a specific mesh nebulizer, the I-neb AAD System nebulizer in 2005. Colistimethate sodium was approved for nebulization by the I-neb AAD System nebulizer in 2006; eFlow mesh nebulizers were approved for the nebulization of both aztreonam (Gilead Sciences, Inc., CA, USA) and colistimethate sodium (Pari GmbH) in 2010, and the Tolero nebulizer (Pari GmbH) was approved for the nebulization of tobramycin (Pari GmbH) in 2015.
There were 19 new inhaled drugs being tested in clinical trials using mesh nebulizers between 2012 and 2016, distributed between 13 different pharmaceutical companies. 14 of the studies were in Phase II, indicating that the combination of mesh nebulizer and new pharmaceutical is becoming a mature development strategy where the benefits outweigh the risk compared with choosing a jet nebulizer. Mesh nebulizers have become firmly established within the nebulizer market and their high inclusion rates in clinical trials suggest that these devices have succeeded in meeting both pharmaceutical company and patient needs. The cost of the mesh nebulizer, however, may be a barrier to its use in lower cost therapy markets such as asthma, and may also restrict its use in clinician sponsored clinical trials. However in 2016, Sunovion filed the first application for the approval of a mesh nebulizer in combination with a drug for COPD (SUN-101) [Citation67]. The approval of SUN-101 will be a significant step forward for the use of mesh nebulizers in this important market segment, and other companies such as Vectura also have products in late stage development, such as VR475 in Phase III trials for severe asthma [Citation68].
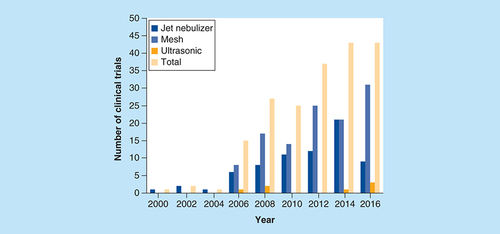
(A) Total number of clinical trials between 2000 and 2016 that used the different types of nebulizers, sponsored by either pharmaceutical companies or clinicians. (B) Clinical trials sponsored by pharmaceutical companies registered by year 2000–2016 that use mesh, jet or ultrasonic nebulizers.
Conclusion & future perspective
The nebulizer market is forecast to grow (by volume) at a rate of over 5% in the next 10 years, with the highest growth rate found in the Asia region. This Asian growth is driven by the continued growth of generic nebulized drugs in China, and these nebulized drugs have the largest share of the asthma and COPD market. As new drugs are developed by pharmaceutical companies, the regulatory authorities will expect the drug to be registered with the nebulizer used in the pivotal trials. This can result in a specific drug–device combination being registered. When the drug is registered with only one device, this allows the drug packaging to be optimized for delivery with that specific device. Typically with a high-efficiency mesh nebulizer, the drug can be packaged in a 0.5–1 ml vial to suit the high efficiency of a mesh nebulizer compared with 2–5 ml required for a jet nebulizer. This can result in very significant cost of goods savings for the pharmaceutical company for drugs that are expensive to produce and also significant reductions in treatment time for the patient.
The adoption of mesh nebulizers as the delivery system of choice for pharmaceutical partners will continue to transform the nebulizer market as these new drugs gain marketing approval. The increased cost of the devices and the associated payment of royalties to the device manufacturers do add some additional costs to the pharmaceutical development budget, however the advantages gained for both the pharmaceutical company and the patient can bring significant overall benefits.
Mesh nebulizers are now an important delivery system for the treatment of high-value drugs in diseases such as cystic fibrosis and pulmonary hypertension where alternate delivery systems such as metered dose inhalers (MDIs) and dry powder inhalers (DPIs) have not been popular due to the higher cost of developing these devices and the limited mass of drug that can be delivered in a single inhalation. However, in the asthma and COPD markets where MDIs and DPIs are often the delivery system of choice, nebulizer use has been limited to patient groups such as the young and elderly who may be unable to use an MDI or DPI [Citation69]. This has limited the penetration of mesh nebulizers in these established markets where the current costs of delivering generic drugs is low, and developers of new formulations such as long-acting bronchodilators have initially focussed on the main MDI and DPI markets. There are indications that this trend is now changing with specialist nebulizer formations being developed for mesh nebulizers, as pharmaceutical manufacturers become increasingly confident of the commercial benefits of using a mesh nebulizer in the development program [Citation69]. The use of a mesh nebulizer also allows pharmaceutical manufacturers to differentiate their new nebulized product from a product associated with pneumatic nebulizers, in terms of both patient benefits such as fast and silent treatments, new clinical benefits such as adherence monitoring and the ability to differentially price the product in the market.
One reason for the popularity of Pari, Philips and Vectura brands of mesh nebulizer with pharmaceutical companies compared with Omron is not just based on the performance or cost of the device, but on the support that these manufacturers provide for the pharmaceutical companies to assist in the correct specification, testing and regulatory approval of the mesh nebulizer. Developing a combination product requires the long-term support of the device manufacturer, and these manufacturers can provide that support both through the development program and through regulatory approval, and most importantly they can provide global full life marketing support. Though the mesh nebulizer market has seen the recent introduction of a number of generic manufacturers, these suppliers appear to be focused on the existing general purpose nebulizer device market, and have not invested so far in the support infrastructure required to support major pharmaceutical developments.
In the 20+ years since the first mesh nebulizer was marketed, mesh nebulizers have changed dramatically. Challenges facing the commercialization of these devices have largely been overcome, and mesh nebulizers are now the devices of choice for alternative, high-cost and niche inhaled therapy areas. Mesh nebulizers are now widely used in clinical trials and are especially common in early-stage clinical trials sponsored by pharmaceutical companies and high cost or niche therapy areas. In fact, trials in which mesh nebulizers are used consistently outnumber trials using jet nebulizers, suggesting that mesh nebulizers have been well accepted. Mesh nebulizers are now being used in the development of formulations for asthma and COPD with the first drugs awaiting US FDA approval.
Mesh nebulizers have also been shown to be well accepted by patients due to the increased portability and convenience, and decreased nebulization times when compared with jet nebulizers. The commercial acceptance of mesh nebulizers is largely due to their low residual volumes and accurate lung delivery, making them ideal devices for the delivery of a wide variety of medications, including high-cost drugs. Overall, it can be seen that mesh nebulizers have now been successfully integrated into inhaled treatment therapy, with the potential improvements in technology and wider use in clinical trials, mesh nebulizers are likely to be a popular choice for the delivery of inhaled medications in the years to come.
Market uptake of mesh technology
Mesh technology entered the market for nebulized therapy in 1993.
Existing pharmaceuticals had been developed and packaged for jet nebulizer use, and as a result, mesh nebulizers with high nebulization efficiency and low residual volumes were not an immediate replacement for jet nebulizers.
It has taken time for new drug products which have been specifically developed for mesh nebulizers to be developed and approved. Five drugs have now been approved for use with a specific mesh nebulizer, and there are a further 19 in clinical development with 13 pharmaceutical companies.
Aerosol generation in mesh nebulizers
The process of aerosol formation is similar for all mesh nebulizer designs, but the design of the aerosolization mechanism containing the mesh can vary widely.
Passive and active flat meshes and domed active mesh designs with meshes constructed from metal alloys are prevalent but expensive to manufacture, however it is expected that the use of alternative materials and production processes will reduce the cost in the future.
Mesh nebulizer manufacturers
The market has been dominated by five main mesh nebulizer manufacturers, but as the original mesh nebulizer patents have begun to expire, generic manufacturers have entered the market.
Mesh nebulizer performance
Mesh nebulizers offer increased portability due to the small size, weight and energy efficiency arising from use of the piezo element to generate the vibration of the mesh.
Nebulization is fast due to the number of holes creating aerosol droplets.
The high nebulization efficiency makes them especially suitable for high-cost drug formulations.
Lack of gas flow required for aerosol generation is advantageous when delivering aerosols to ventilated patients.
Lung deposition studies have shown that mesh nebulizers are as effective as jet nebulizers in producing aerosol for deposition in the lungs.
Studies have shown patient preference for mesh nebulizers over (predominantly) jet nebulizers and high perceptions of ease of use, though compliance with manufacturers’ cleaning recommendations has been shown to be important.
Mesh nebulizers in clinical trials
Analysis of data from clinical trial databases could provide an indication of how well mesh technology has been accepted.
The use of mesh nebulizers in clinical trials has been steadily increasing since 2006.
Pharmaceutical companies sponsored 71% of the trials of mesh nebulizers.
Mesh nebulizers were strongly preferred over jet nebulizers for trials involving high cost and niche therapy areas.
The high inclusion rates of mesh nebulizers in clinical trials suggest that these devices have succeeded in meeting both pharmaceutical company and patient needs and may increase in the future as new drug developments are approved.
Future perspective
Challenges in the commercialization of mesh nebulizers have largely been overcome and they are now the device of choice for high cost and niche therapy areas.
Increasing registration of new drug–device mesh nebulizer combinations will allow cost of goods savings for manufacturers of drugs that are expensive to produce.
Mesh nebulizer drug–device combinations also allow product differentiation in terms of patient benefits such as fast and silent treatment, as well as clinical benefits such as integrated adherence monitoring.
Although the use of mesh nebulizers for delivery of asthma and chronic obstructive pulmonary disease mediations has been limited by the packaging of existing drugs, there are now specialist formulations being developed for delivery via mesh nebulizers for the treatment of these diseases.
The main mesh nebulizer manufacturers will continue to dominate the field of new drug–device combinations until generic mesh nebulizer manufacturers invest in the support infrastructure required to support new pharmaceutical developments.
The popularity of mesh nebulizers can be expected to grow further, fuelled by patient acceptance based on portability, convenience and speed of treatment as well as by commercial acceptance due to their low residual volumes and accurate lung delivery.
Acknowledgements
The authors acknowledge N Smith of PS5 Consultants Ltd for editorial assistance.
Financial & competing interests disclosure
J Pritchard and R Hatley are employees of Respironics Respiratory Drug Delivery (UK) Ltd who develop, manufacture and sell some of the products referenced in the paper. J Denyer is an employee of PS5 Consultants Ltd who were contracted to provide writing assistance for this article. D von Hollen is an employee of Respironics, Inc., a Philips Healthcare company, Murrysville, PA, USA. J Pritchard, R Hatley and D von Hollen are all full-time employees of Philips, who allowed the time required to author this paper during the normal course of employment.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Omron NE-U22 MicroAir product brochure. Vernon Hills, IL. Omron Healthcare Inc. www.quickmedical.com/downloads/omron-respiratory-neu22v_brochure.pdf.
- Ingram H: Nebulisers–World–2014 [Internet]. IHS Technology (2014). https://technology.ihs.com/435839/nebulizers-2014.
- Executive summary: European anesthesia, respiratory and sleep management device markets. iData research Inc., Burnaby, BC, Canada (2011).
- Kesser KC Geller DE . New aerosol delivery devices for cystic fibrosis. Respir Care.54 (6), 754–767 (2009).
- Hess DR . Aerosol delivery devices in the treatment of asthma. Respir Care.53 (6), 699–723 (2008).
- Elphick M von Hollen D Pritchard JN Nikander K Hardaker LEA Hatley RHM . Factors to consider when selecting a nebulizer for a new inhaled drug product development program. Expert Opin. Drug Deliv.12 (8), 1375–1387 (2015).
- Rayleigh JW . On the instability of jets. Proc. London Math Soc.10 (1), 4–13 (1878).
- MicroAIR U22 (2017). www.omron-healthcare.com/en/products/respiratorytherapy#.
- I-neb AAD System (2017). www.philips.co.uk/healthcare/solutions/sleep-and-respiratory-care/respiratory-drug-delivery.
- InnoSpire Go (2017). www.philips.co.uk/c-p/HH1342_00/innospire-go-portable-mesh-nebuliser.
- eFlow rapid nebuliser system (2017). www.pari.com/de-en/products/lower-airways/eflow-rapid-nebuliser-system/.
- Fox (2017). www.vectura.com/technologies/device-technologies/nebuliser/.
- Aerogen Solo (2017). www.aerogen.com/aerogen-solo-3/.
- Medical Expo Vibrating mesh nebulizers (2017). www.medicalexpo.com/medical-manufacturer/vibrating-mesh-nebulizer-30663.html.
- Dhand R . Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respir Care.47 (12), 1406–1416 (2002).
- Omron MicroAir portable nebulizer model NE-U03V instruction manual. Omron Healthcare Inc., Vernon Hills, IL, USA.
- OMRON HEALTHCARE CO LTD: EP-1327480 (2003).
- Denyer J Dyche T . The adaptive aerosol delivery (AAD) technology: past, present and future. J. Aerosol. Med. Pulm. Drug Deliv.23 (Suppl. 1), S1–S10 (2010).
- Hardaker LEA Hatley RHM . In vitro characterization of the I-neb adaptive aerosol delivery (AAD) system. J. Aerosol. Med. Pulm. Drug Deliv.23 (Suppl. 1), S11–S20 (2010).
- RESPIRONICS RESPIRATORY DRUG DELIVERY (UK) LTD: EP-1465692 (2004).
- Evaluate Ltd . Aerogen and medical industries America announce USA introduction of Aeroneb Go nebulizer (2004). www.evaluategroup.com/Universal/View.aspx?type=Story&id=71104.
- Koninklijke Philips N.V . Philips strengthens home healthcare solutions business through agreement with Aerogen (2014). www.newscenter.philips.com/gb_en/standard/news/press/2014/20140107-philips-strengthens-home-healthcare-solutions-business-through-agreement-with-aerogen.wpd#.VNoJ8y57LIU.
- Hatley R Rowe L Rabbetts I Quadrelli F . Optimizing patient experience of nebulizer treatments. Eur. Respir. J.48 (Suppl. 60), PA4082 (2016).
- Lass JS Sant A Knoch M . New advances in aerosolized drug delivery: vibrating membrane nebuliser technology. Expert Opin Drug Deliv.3 (5), 693–702 (2006).
- PARI GMBH: US6962151 (2005).
- THE TECHNOLOGY PARTNERSHIP: US7316067 (2008).
- Acquisition of Activaero (2014). www.vectura.com/news/acquisition-activaero/.
- Bennett WD . Controlled inhalation of aerosolised therapeutics. Expert Opin. Drug Deliv.2 (4), 763–767 (2005).
- First launch of a product incorporating Vectura's FOX smart nebuliser technology (2017). www.vectura.com/news/first-launch-product-incorporating-vecturas-fox-smart-nebuliser-technology/.
- Evaluate Ltd . Aerogen introduces the Aeroneb Pro for respiratory therapy in the hospital (2002). www.evaluategroup.com/Universal/View.aspx?type=Story&id=71142.
- STAMFORD DEVICES LTD: EP-2624967 (2013).
- NOVARTIS AG: US7971588 (2011).
- KONINKLIJKE PHILIPS ELECTRONICS N.V.: USD683012S (2013).
- Medical Expo: vibrating mesh nebulizers. www.medicalexpo.com/medical-manufacturer/vibrating-mesh-nebulizer-30663.html.
- Berg EB Picard RJ . In vitro delivery of budesonide from 30 jet nebulizer/compressor combinations using infant and child breathing patterns. Respir. Care.54 (12), 1671–1678 (2009).
- Loffert DT Ikle D Nelson HS . A comparison of commercial jet nebulizers. Chest.106 (6), 1788–1792 (1994).
- Hatley RHM Hardaker LEA Zarins-Tutt J et al. Investigation of optical density for the characterization of nebulizer meshes. In : Respiratory Drug Delivery 2016. DalbyRNByronPRPeartJSumanJDYoungPMTrainiD ( Eds). Proceedings of Respiratory Drug Delivery 2016. Scottsdale, AZ, USA, 17–21 April 2016. Virginia Commonwealth University, Richmond, VA, USA, 489–492 (2016).
- Keller M Tservistas M Bitterle E Bauer S . Aerosol characterization of Alpha-1 antitrypsin after nebulization with the eFlow: a novel vibrating perforated membrane nebulizer. In : Respiratory Drug Delivery 2006. DalbyRNByronPRPeartJSumanJDFarrSJ ( Eds). Proceedings of Respiratory Drug Delivery 2006. Boca Raton, FL, USA, 23–27 April 2006. Virginia Commonwealth University, Richmond, VA, USA, 733–736 (2006).
- Fink JB Simmons R . Nebulization of steroid suspension: an in vitro evaluation of the aeroneb go and the pari LC plus nebulizers. Chest126 (4), 816S–817S (2004).
- Hibbitts A Sivadas N Tewes F et al. Investigation of fluid physicochemical properties on output in vibrating mesh nebulisers. J. Aerosol. Med. Pulm. Drug Deliv.24 (3), A48–A49 (2011).
- INDUSTRIAL TECHNOLOGY RESEARCH INSTITUTE: US7669782 (2010).
- Newman S Pitcairn G Pickford M Gee-Turner A Asai K . The MicroAir electronic-mesh nebuliser deposits aerosol in the lungs more efficiently than a conventional jet nebuliser. J. Aerosol. Med. Pulm. Drug Deliv.18 (2), 249 (2005).
- Ari A . Jet, ultrasonic and mesh nebulizers: an evaluation of nebulizers for better clinical outcomes. Eurasian J. Pulmonol.16, 1–7 (2014).
- Coates AL Green M Leung K et al. A comparison of amount and speed of deposition between the PARI LC STAR jet nebulizer and an investigational eFlow nebulizer. J. Aerosol. Med. Pulm. Drug Deliv.24 (3), 157–163 (2011).
- Highlights of prescribing information . Cayston (aztreonam for inhalation solution). Gilead Sciences, Inc. ; Foster City, CA, USA (2012).
- Réminiac F Vecellio L Mac Loughlin R et al. Nasal high flow nebulization in infants and toddlers: an in vitro and in vivo scintigraphic study. Pediatr Pulmonol.52 (3), 337–344 (2017).
- Ari A Atalay OT Harwood R Sheard MM Aljamhan EA Fink JB . Influence of nebulizer type, position and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care.55 (7), 845–851 (2010).
- Berlinski A Willis JR . Albuterol delivery by four different nebulizers placed in four different positions in a pediatric ventilator in vitro model. Respir Care.58 (7), 1124–1133 (2013).
- Ari A . Aerosol therapy in pulmonary critical care. Respir Care.60 (6), 858–879 (2015).
- Newman SP Pitcairn GR Hooper G Knoch M . Efficient drug delivery to the lungs from a continuously operated open-vent nebulizer and low pressure compressor system. Eur. Respir. J.7 (6), 1177–1181 (1994).
- Johnson MA Newman SP Bloom R Talaee N Clarke SW . Delivery of albuterol and ipratropium bromide from two nebulizer systems in chronic stable asthma: efficacy and pulmonary deposition. Chest96 (1), 6–10 (1989).
- Brand P Beckmann H Maas Enriquez M et al. Peripheral deposition of α1-protease inhibitor using commercial inhalation devices. Eur. Respir. J.22 (2), 263–267 (2003).
- Coates AL Green M Leung K et al. Rapid pulmonary delivery of inhaled tobramycin for pseudomonas infection in cystic fibrosis: a pilot project. Pediatr. Pulmonol.43 (8), 753–759 (2008).
- Behr J Zimmermann G Baumgartner R et al. Lung deposition of a liposomal cyclosporine a inhalation solution in patients after lung transplantation. J. Aerosol. Med. Pulm. Drug Deliv.22 (2), 121–130 (2009).
- Zeman KL Wu J Bennett WD . Targeting aerosolized drugs to the conducting airways using very large particles and extremely slow inhalations. J. Aerosol. Med. Pulm. Drug Deliv.23 (6), 363–369 (2010).
- Lenney W Edenborough F Kho P Kovarik JM . Lung deposition of inhaled tobramycin with eFlow rapid/LC Plus jet nebuliser in healthy and cystic fibrosis subjects. J. Cyst. Fibros.10 (1), 9–14 (2011).
- Nikander K Prince I Coughlin S Warren S Taylor G . Mode of breathing–tidal or slow and deep–through the I-neb adaptive aerosol delivery (AAD) system affects lung deposition of 99mTc-DTPA. J. Aerosol. Med. Pulm. Drug Deliv.23 (Suppl. 1), S37–S43 (2010).
- Elliott D Dunne P . Guide to aerosol delivery devices for physicians, nurses, pharmacists and other healthcare professionals. Am. Assoc. Resp. Care (2011). www.aarc.org/wp-content/uploads/2014/08/aerosol_guide_pro.pdf.
- Anderson P . Patient preference for and satisfaction with inhaler devices. Eur Respir Rev.14 (96), 109–116 (2005).
- Braido F Chrystyn H Baiardini I et al. ‘Trying, But Failing’–the role of inhaler technique and mode of delivery in respiratory medication adherence. J. Allergy Clin. Immunol. Pract.4, 823–832 (2016).
- Goodman N Morgan M Nikander K Hinch S Coughlin S . Evaluation of patient-reported outcomes and quality of life with the I-neb AAD System in patients with chronic obstructive pulmonary disease. J. Aerosol. Med. Pulm. Drug Deliv.23 (Suppl. 1), S61–S70 (2010).
- Naehrig S Lang S Schiffl H Huber RM Fischer R . Lung function in adult patients with cystic fibrosis after using the eFlow Rapid for 1 year. Eur. J. Med. Res.16, 63–66 (2011).
- Rottier BL van Erp CJP Sluyter TS Heijerman HGM Frijlink HW de Boer AH . Changes in performance of the Pari eFlow Rapid and Pari LC Plus during 6 months use by CF patients. J. Aerosol. Med. Pulm. Drug Deliv.22 (3), 263–269 (2009).
- Bakuridze L Andrieu V Dupont C Dubus JC . Does repeated disinfection of the e-Flow Rapid nebulizer affect in vitro performance?J. Cyst. Fibros.6 (4), 309–310 (2007).
- Correspondence . Performance of PARI eFlow. J. Aerosol. Med. Pulm. Drug Deliv.23 (2), 113–118 (2010).
- Pritchard JN . Rethinking the paradigm for the development of inhaled drugs. Int. J. Pharm.496 (2), 1069–1072 (2015).
- Sunovion submits new drug application for SUN-1–1/eFlow to the FDA for the treatment of patients with chronic obstructive pulmonary disease (COPD) (2016). www.sunovion.us/featured-news/press-releases/.
- Vectura Group plc Corporate presentation (2017). www.vectura.com/index.php/download_file/view/548/493/.
- Pritchard JN . Nebulized drug delivery in respiratory medicine: what does the future hold?Ther. Deliv.8 (6), 391–399 (2017).