Abstract
Aim: CNS infections due to parasites often prove fatal. In part, this is due to inefficacy of drugs to cross the blood–brain barrier. Methods: Here, we tested intranasal and intravenous route and compared adverse effects of Amphotericin B administration, through blood biochemistry, liver, kidney and brain histopathological evidence of toxicities in vivo post-administration. Results: It was observed that intranasal route limits the adverse side effects of Amphotericin B, in contrast to intravenous route. Conclusion: As parasites such as Naegleria fowleri exhibit unequivocal affinity toward the olfactory bulb and frontal lobe in the central nervous system, intranasal administration would directly reach amoebae bypassing the blood–brain barrier selectivity and achieve the minimum inhibitory concentration at the target site.
Plain language summary
Brain infections due to parasites are often fatal. One of the reasons is the inability of drugs to get to the brain. When given in large dose to reach the brain, the drug can cause serious side effects. Here, we tested the side effects of Amphotericin B (drug of choice against brain-eating amoebae), when given intranasally versus intravenous. Our findings clearly show that intranasal route limits the side effects of Amphotericin B. These are important findings and should serve as an important step in the development of effective therapy against parasitic infections affecting the brain.
Tweetable abstract
Targeting brain-eating amoebae: Amphotericin B-mediated host tissue toxicity can be limited when given intranasally.
Graphical abstract
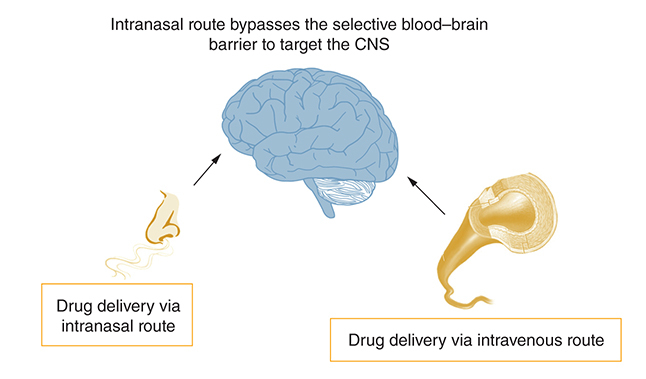
Primary amoebic meningoencephalitis (PAM) brought about by Naegleria fowleri is a severe infection that often leads to fatality within days, despite treatment [Citation1]. N. fowleri is a highly lethal opportunistic parasite with a mortality rate exceeding 90%, even with treatment. It enters the body through the nose, attaches to epithelial cells, and migrates along the olfactory neuroepithelial route to reach the brain by passing through the porous cribriform plate. Due to the nose being the entry point, N. fowleri is commonly found in the frontal lobe upon post-mortem examination. Despite perceived advancements in chemotherapy and supportive care, it is distressing to note that the mortality rate for PAM caused by N. fowleri remains over 90%. This infection was first described by Fowler and Carter in 1965 [Citation2]. In vitro, Amphotericin B, which targets ergosterol biosynthesis, is highly effective. However, its efficacy in targeting the parasite residing within the central nervous system (CNS) is limited. The limited efficacy of drugs in targeting the parasite within the CNS is partly attributed to their inability to effectively cross the blood–brain barrier and reach the target site. Additionally, the bioavailability of the drug within the cyst or abscess can also contribute to this limitation. To compensate for the limited efficacy of drugs in targeting the parasite within the CNS, large doses are administered intravenously to attain a minimum inhibitory concentration in the brain for parasite elimination. However, this approach leads to the distribution of the drug to unintended tissues, causing significant side effects such as hepatotoxicity, nephrotoxicity, and others [Citation1,Citation3,Citation4]. Liposomal formulations of drugs have shown promise in reducing drug-related toxicities, but the prognosis for the disease remains poor. Considering that the parasite enters through the nose and migrates along the olfactory neuroepithelial route, it is logical to administer drugs intranasally using solubilized Amphotericin B deoxycholate. Moreover, administering the drug through the intranasal route would help limit side effects such as hepatotoxicity, nephrotoxicity and others. Likewise, the current recommended treatment for COVID-19-associated cerebral mucormycosis entails intravenous administration of Amphotericin B, with each patient needing 60 to 100 injections (each comprising 50 mg of the drug). One of the main reasons for using a high dosage of the drug is that the intravenous route leads to the dilution of the compound in the plasma. As the blood–brain barrier is highly selective coupled with the limited ability of Amphotericin B to penetrate the CNS and target the fungi, it is necessary to administer greater doses of the drug in order to reach the minimum inhibitory concentration at the focal point of the infection. In such circumstances, it is reasonable to reconsider the treatment approach in order to minimize the side effects associated with the drug. Herein, both the intranasal and intravenous routes were examined and compared in terms of adverse effects of Amphotericin B administration. This was assessed via the analysis of blood biochemistry, as well as histopathological evidence of toxicities in the liver, kidneys, and brain following infection in vivo.
Materials & methods
Study area
The study was conducted at the Monash Animal Research facility, located at Monash University in Malaysia. The research took place over a period from October 2017 to October 2018.
Experimental animals
The study utilized C57BL/6 mice as experimental animals, and their use was approved by the Research Ethics Committee following Sunway University's Code of Practice for the Ethical Conduct of Research and the Research Governance and Integrity Policy. Furthermore, the procedures performed on the mice were permitted by the Animal Ethics Committee of Monash University (Approval Code: MUM2017-06).
A total of 50 male adult C57BL/6 mice, aged between 22 and 26 weeks and weighing 20–38g, were housed under specific pathogen-free (SPF) settings during experimentation.
Study design
The animals were divided into three groups and assigned the following treatments: administration of saline alone intranasally as well as intravenous (iv.) route (n = 10), similar volumes to Group 3 mice; iv. administration of Amphotericin B deoxycholate at a concentration of 4 mg/kg (n = 20) [Citation5]; and combined intranasal (IN) administration of Amphotericin B deoxycholate at a dose of 2 mg/kg and iv. administration of Amphotericin B deoxycholate at a dose of 2 mg/kg to achieve a total effective concentration of 4 mg/kg (n = 20). To simplify, group 2 is denoted to as the iv. group, and group 3 is denoted to as the IN group. Notably, several formulations of Amphotericin B are available clinically with varying levels of safety with AmBisome exhibiting higher safety. However, Amphotericin B deoxycholate was selected in this study over Amphotericin B-AmBisome due to its availability. The animals in both groups were anesthetized via intraperitoneal injection of 0.9 ml/kg (4.5 mg/kg) of ketamine–xylazine–zoletil. 10 mM Amphotericin B deoxycholate (Sigma Aldrich) was solubilized in distilled water adjusted to pH11. Subsequently, Amphotericin B deoxycholate was administered intravenously to the animals in the iv. group via the tail vein at a dose of 4 mg/kg daily (equivalent to 170 μl of maximum dosage once daily). In Group 3, mice received daily administration of Amphotericin B deoxycholate IN (2 mg/kg) and iv. (2 mg/kg). Daily observations were conducted to assess the mice's alertness, activity, breathing, body condition and weight, coat condition, food and water consumption, signs of dehydration, feces appearance, movement and gait, eye condition, nasal condition, urine output, vocalization, motor coordination or ataxia, lethargy and signs of pain. Mice were euthanized, and their livers, brains, and kidneys were saved and fixed in 4% paraformaldehyde (PFA) on. Day 14. The tissues were then transferred to a solution of 30% sucrose in 0.1 M phosphate-buffered saline (PBS) and stored at -80°C. Sections of 5–15 μm thickness were prepared in sagittal planes, rehydrated and subjected to hematoxylin and eosin staining. Images of the stained sections were captured using an inverted microscope [Citation6]. The specific areas of interest for examination included the anterior olfactory nucleus, olfactory bulb, thalamus, hippocampus, midbrain, cerebellum, cerebral cortex, pons and medulla of the brain. In addition, the liver was examined for any abnormalities in its architecture, and the kidneys were assessed for medullary tubular necrosis and glomerular ischemia.
Statistical analyses
Statistical analysis was performed using a two-sample t-test with a two-tailed distribution, where applicable.
Results
During the course of experimentation, one mice in the control group, five in iv. group and three in IN group died. These mice were excluded and the remaining mice were used for data presentation. Changes in body mass and motor incoordination were not observed in the control group and IN group and they maintained an average weight of 28.8 ±0.4 g. However, the iv. group exhibited a slight decline in weight to approximately 26.4 ±0.5 g. The estimated glomerular filtration rate (eGFR) was used to assess renal function as well as urea, uric acid, creatinine, whereas enzymes of the liver, including aspartate transferase, alanine transferase and alkaline phosphatase, were evaluated to assess liver function.
Liver enzymes showed a more significant elevation in the iv. group compared with the IN group, suggesting a greater severity of liver toxicity with intravenous treatment of Amphotericin B compared with intranasal treatment. This finding is consistent with other recent studies that have reported similar results [Citation7–11].
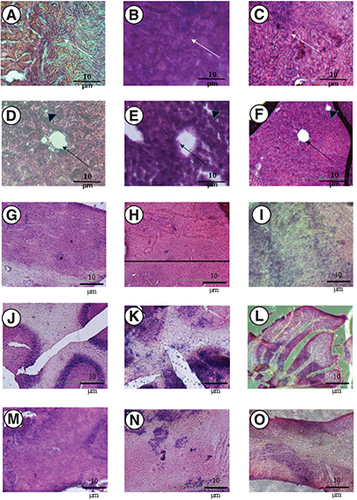
The level of alkaline phosphatase (U/l) in the IN group was 60 ± 6.6, while it increased to 70 ± 3.3 in the iv. group (control group was 55 ± 2.6). As for aspartate transferase (U/l), it was 188 ± 13 in the IN group and increased to 350 ± 21 in the iv. group (control group was 95 ± 6). In the case of alanine transferase (U/L), the level was 37 in the IN group, but increased to 65 ± 5.5 in the iv. group (control group was 45 ± 2.1). In contrast, the estimated glomerular filtration rate (eGFR) in the IN group was 68 ± 4.6 ml/min/1.73 m2, while it was 71 ± 4 ml/min/1.73 m2 in the iv. group (control group was 55 ± 1). Similar trend was observed with urea (8 ± 0.3 mmol/l in the IN group and increased to 10.5 ± 0.2 in the iv. group, while the control group was 7 ± 0.4), uric acid (98 ± 7 μmol/l in the IN group and increased to 135 ± 21 in the iv. group, while the control group was 110 ± 4), and creatinine (50 ± 3 in the IN group and increased to 65 ± 5 in the iv. group, while the control group was 55 ± 4.7). Mice in the IN group, iv. group and control group displayed intact renal tubules, particularly in the proximal tubule area, without any signs of atrophy or glomerular ischemia, which are characteristic features of Amphotericin-induced nephrotoxicity. The architecture of the liver in both the IN and iv. groups did not show any signs of multifocal hepatocellular necrosish, hepatic centrilobular degeneration or macrophage vacuolation, that are typically observed in cases of Amphotericin B-induced hepatotoxicity. In the iv. group, the liver tissues exhibited hepatocyte necrosis accompanied by fragmentation toward a central vein. Additionally, there was distortion and malalignment of the portal triad, which includes bile ducts, portal venules and hepatic arteries, as a consequence of hepatocyte necrosis (A–O). The laboratory blood biochemistry results pertaining to liver functions aligned with the histopathological findings of nephrotoxicity and hepatotoxicity. Furthermore, the investigation of the brain encompassed the olfactory bulb, medulla–midbrain borders, olfactory cortex and cerebellum for all groups. The examination of the brain revealed mild neutrophilic inflammation in the perivascular area (Virchow-Robin space) for the intravenous group. No observable hemorrhage was detected in the examined brain regions. Additionally, necrosis and gangliosis were not observed in the intranasal group. Conversley, the intravenous group showed prevalent neutrophilic infiltration in the borders of the medulla and midbrain, as well as in the olfactory cortices. The presence of foci of neutrophilic infiltrations indicated inflammation (). Images are representative of each group. The findings observed were consistent in 50% of the mice in each group.
(A–O) Three groups of mice were used including, a negative control group; an intravenous group (injected with 4 mg/kg of Amphotericin B deoxycholate, referred to as iv.); and an intranasal group (2 mg/kg) + iv. group (2 mg/kg, referred to as IN group). Note that A (negative control), and C (IN group) show that glomeruli are devoid of ischemia and integrity of renal tubules remain unscathed with no signs of necrosis, while group B (iv.) exhibited tubular necrosis (white arrow). The sagittal sections of a liver lobular unit from caudate lobes are shown for (D) negative control; (E) iv. group; (F) IN group. Note that the architecture of the liver lobular unit is normal for D (negative control), and F (IN group), whereas the hepatocyte shredded into the hepatic central vein in group E (iv. group) mice (black arrow) and the intrahepatic duct was distorted with malalignment of hepatic acinus (black arrowhead). The tissues were stained in haematoxylin-eosin and were visualized under an inverted microscope (x400). (G–O) Shows different regions of the brain. For the olfactory bulb, (G) negative control; (H) iv. group; and (I) IN group. For the cerebellum, (J) negative control; (K) iv. group (L) IN group. For regions of pons – midbrain (M) negative control; (N) iv. group; (O) IN group.
Discussion
N. fowleri causes an uncommon but deadly disease called PAM, and there has been an increase of reported PAM cases, particularly since the year 2000 [Citation11]. Recently, it has been suggested that changes and rapid alterations in iron availability have been witnessed in various regions, and may be linked to climate change [Citation12]. Furthermore, it has been suggested that there is a need to comprehend the availability and the role of iron in the pathogenicity and distribution of N. fowleri, with an increase in number of cases in future likely, hence the importance of developing new therapeutic options to treat this devastating infection [Citation12]. The promising findings from our study, suggest that the intranasal route of drug administration can potentially reduce side effects. The involvement of the paravascular system in the olfactory and trigeminal routes, utilized by the intranasal route, highlights its potential as a therapeutic strategy for delivering drugs to the CNS. A recent study has provided validation by demonstrating the swift delivery of molecules from the imine group to the brain through the glymphatic system, which is an important and efficient route [Citation13]. The intranasal route of drug delivery is advantageous due to its non-invasiveness and tolerability for frequent application. It enables enhanced drug delivery to the brain and achieving the desired concentration at the infection site with lower doses. The efficacy of intranasal delivery of Amphotericin B has been demonstrated in the treatment of invasive mucormycosis, and it has been suggested as a potential treatment approach for this infection in relevant studies [Citation14,Citation15]. A recent study demonstrated that vaporized drugs are more effective than their liquid forms, as observed with the antifungal properties of lemongrass. Lemongrass in vapor form exhibited higher efficacy against Candida albicans compared with its liquid phase. The elevated antifungal attributes in vapor form indicate increased cellular damage, possibly due to enhanced drug absorption within the membrane. However, the specific molecular mechanisms underlying this phenomenon have not been thoroughly investigated [Citation16].
Conclusion
In summary, the intranasal route of drug delivery offers numerous advantages compared with the intravenous route. These include bypassing the blood–brain barrier, avoiding venous drainage, direct delivery to the central nervous system, and the potential for delivery in the form of gas phase through the porous cribriform plate, which opens up the possibility of using nasal inhalers. However, further extensive clinical research is required to validate and establish the efficacy of this approach. Furthermore, the use of liposomal formulation of Amphotericin B, as well as other novel compounds with anti-amoebic activities, such as novel azoles, and other recently mined compounds [Citation17–22] should be assessed intranasally, as well as versus other free-living amoebae. Future work comparing both the intransal route and intravenous route in male and female mice should also be carried out, to mitigate any effects of gender. In summary, the results strongly support the notion that utilizing the intranasal route for Amphotericin B administration effectively reduces the occurrence of adverse side effects. Given N. fowleri's preference for the frontal lobe and olfactory bulb in the CNS [Citation23], and its entry through the nose, intranasal administration may directly target the amoebae without being hindered by the blood–brain barrier, ensuring effective delivery of the drug to the desired site and achieving the necessary concentration for inhibiting the parasite. The effectiveness of intranasal drug delivery for PAM caused by N. fowleri still needs to be assessed in future studies. Importantly, the ability for patients to self-administer intranasal treatment could be advantageous, especially in rural areas of developing countries where access to tertiary hospitals may be limited.
Adverse effects of Amphotericin B administration were investigated, in vivo.
No major changes were observed in body mass and motor incoordination.
Liver enzymes showed elevation in the intravenous group compared with the intranasal group.
Liver tissues exhibited hepatocyte necrosis with distortion and malalignment of the portal triad.
Glomerular filtration rate, urea, uric acid and creatinine showed elevation in the intravenous group compared with intranasal group.
Brain revealed neutrophilic inflammation in the perivascular area in the intravenous group.
Intravenous group showed neutrophilic infiltration in the borders of the medulla and midbrain, as well as in the olfactory cortices.
Overall, intranasal route for Amphotericin B administration effectively reduces the occurrence of side effects.
Author contributions
R Siddiqui and NA Khan conceptualized the study. R Siddiqui and NA Khan received funding to carry out the research. R Siddiqui, SK Maciver, T Yu Yee Ong and NA Khan designed the study, carried out experiments and analysed the data amid critical discussions. T Yu Yee Ong and R Siddiqui prepared the first draft of the manuscript and SK Maciver and NA Khan corrected the manuscript. All authors reviewed and approved the final manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
This work was supported by Sunway University internal grant (2017–04). This work was supported by Sunway University internal grant (2017–04). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Visvesvara GS , MouraH, SchusterFL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol.50, 1–26 (2007).
- Fowler M , CarterRF. Acute pyogenic meningitis probably due to Acanthamoeba sp.: a preliminary report. Br. Med. J.2, 740–742 (1965).
- Tonomura Y , YamamotoE, KondoCet al. Amphotericin B-induced nephrotoxicity: characterization of blood and urinary biochemistry and renal morphology in mice. Human Exp. Toxicol.28, 293–300 (2009).
- Siddiqui R , AliIKM, CopeJ, KhanNA. Biology and pathogenesis of Naegleria fowleri. Acta Trop.164, 375–394 (2016).
- Goswick SM , BrennerGM. Activities of Azithromycin and Amphotericin B against Naegleria fowleri In Vitro and in a Mouse Model of Primary Amebic Meningoencephalitis. Antimicrob. Agents Chemother.47, 524–528 (2003).
- Fischer AH , JacobsonKA, RoseJ, ZellerR. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols doi: 10.1101/pdb/prot4986 (2008). ( pdb-prot4986).
- Sandbhor P , GodaJ, MohantyBet al. Targeted nano-delivery of chemotherapy via intranasal route suppresses in vivo glioblastoma growth and prolongs survival in the intracranial mouse model. Drug. Deliv. Transl. Res.16, 1–9 (2022).
- Wang X , MohammadIS, FanLet al. Delivery strategies of amphotericin B for invasive fungal infections. Acta Pharmaceut. Sin. B.11(8), 2585–2604 (2021).
- Appu AP , ArunP, KrishnanJK, MoffettJR, NamboodiriAM. Rapid intranasal delivery of chloramphenicol acetyltransferase in the active form to different brain regions as a model for enzyme therapy in the CNS. J. Neurosci. Method.259, 129–134 (2016).
- Krishnan JK , ArunP, ChembukaveBet al. Effect of administration method, animal weight and age on the intranasal delivery of drugs to the brain. J. Neurosci. Method.286, 16–21 (2017).
- Maciver SK , PiñeroJE, Lorenzo-MoralesJ. Is Naegleria fowleri an emerging parasite?Trend. Parasitol.36(1), 19–28 (2020).
- Maciver SK , McLaughlinPJ, AppsDK, PiñeroJE, Lorenzo-MoralesJ. Opinion: iron, climate change and the ‘Brain Eating Amoeba’ Naegleria fowleri. Protist.172(1), 125791 (2021).
- Krishnan JKS , ArunP, AppuAPet al. Intranasal delivery of obidoxime to the brain prevents mortality and CNS damage from organophosphate poisoning. Neurotoxicol.53, 64–73 (2016).
- Khafagy R , GuptaS, CampisiP, WatersV. Treatment of localized mucormycosis using nasal amphotericin B irrigation in pediatric oncology. Pediatr. Blood Cancer.67(4), e28175 (2020).
- Siddiqui R , AbouleishMY, KhamisM, IbrahimT, KhanNA. Cerebral mucormycosis: intranasal route to deliver amphotericin B for effective management?. Curr. Med. Res. Opin.38(2), 299–301 (2022).
- Tyagi AK , MalikA. Liquid and vapour-phase antifungal activities of selected essential oils against candida albicans: microscopic observations and chemical characterization of Cymbopogon citratus. BMC Complement. Altern. Med.10, 65 (2010).
- Debnath A . Drug discovery for primary amebic meningoencephalitis: from screen to identification of leads. Exp. Rev. Anti-infect. Ther.19(9), 1099–1106 (2021).
- Rajendran K , AnwarA, KhanNA, SiddiquiR. Brain-eating amoebae: silver nanoparticle conjugation enhanced efficacy of anti-amoebic drugs against Naegleria fowleri. ACS Chem. Neurosci.8(12), 2626–2630 (2017).
- Rice CA , ColonBL, AlpM, GökerH, BoykinDW, KyleDE. Bis-benzimidazole hits against Naegleria fowleri discovered with new high-throughput screens. Antimicrob. Agent. Chemother.59(4), 2037–2044 (2015).
- Rice CA , TrothEV, RussellAC, KyleDE. Discovery of anti-amoebic inhibitors from screening the MMV pandemic response box on Balamuthia mandrillaris, Naegleria fowleri, and Acanthamoeba castellanii. Pathogen.9(6), 476 (2020).
- Anwar A , MungrooMR, KhanSet al. Novel azoles as antiparasitic remedies against brain-eating amoebae. Antibiotics.9(4), 188 (2020).
- Mungroo MR , KhanNA, MaciverS, SiddiquiR. Opportunistic free-living amoebal pathogens. Pathogen. Glob. Health.116(2), 70–84 (2022).
- Ong TYY , KhanNA, SiddiquiR. Brain-eating amoebae: predilection sites in the brain and disease outcome. J. Clin. Microbiol.55(7), 1989–1997 (2017).
