Abstract
Renal Medullary Cancer (RMC) is a rare and aggressive type of renal cell cancer that presents predominantly in patients with sickle cell hemoglobinopathies, and is typically metastatic at the time of presentation. Although platinum based chemotherapeutic regimens have recently emerged as the best option for producing a clinically significant response as reported in various case series, the response is far from satisfactory, as most RMC patients still succumb to their disease within a year of diagnosis. There is currently no standard of care for treatment of this disease. We report, to our knowledge, the first case of RMC where in molecular characterization of the tumor was used to guide therapy. In our patient, molecular analysis identified a decreased expression of Ribonucleotide Reductase M1(RRM1) and phosphatase and tensin homolog (PTEN). Based on these results of PTEN deficiency, we started our patient on everolimus (an MTOR inhibitor) maintenance after treating him with an induction chemotherapy regimen of Paclitaxel-Cisplatin-Gemcitabine (PCG). His tumor responded to induction therapy and he went into complete remission and remained in remission for 7 months. He is now alive about 14 months from his diagnosis and is asymptomatic with minimal disease. The rarity of RMC makes it very difficult to do any meaningful clinical trials in this group of patients. The overall prognosis for RMC remains very poor and knowledge about driver mutations may help in guiding therapy to improve survival in this select group of patients, where there is dearth of available therapies.
Introduction
Renal Medullary Carcinoma (RMC) is a rare tumor found predominantly in young patients with Sickle Cell Trait (SCT) or Sickle Cell Disease (SCD).Citation1,2 The disease is highly aggressive, typically presenting with widespread metastases at the time of diagnosis.Citation3 The disease is predominant in males (M:F = 2:1) with a median age of 26 years and the tumors commonly occur in the right kidney.Citation4 The pathogenesis of this disease is unknown but in some cases inactivation of SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 (SMARCB1) protein has been observed suggesting its role in the development of this disease.Citation4,5 Historically, patients would succumb to this disease within weeks to months with a median of <12 months.Citation1,6 Because of the rarity of the tumor and its vicious nature, clinical trials are difficult to perform. There are some case reports and case seriesCitation1 that hint toward the benefit of using cisplatin-based chemotherapy regimen in RMC but almost all of them relapsed quickly after treatment. The overall prognosis of this disease remains poor with no cure.
We present here what is, to our knowledge, the first case in which molecular analysis of the tumor was used to direct maintenance therapeutic approach post cisplatin-based chemotherapy in a patient with metastatic RMC.
Case Report
A 27 year-old African-American male with SCT presented with hematuria and left flank pain in June 2013. Computed tomography (CT) of the abdomen and pelvis revealed a poorly defined mass at the midpole of the left kidney, 4.8 × 4.4 cm in size, with minimal hydronephrosis (). CT guided biopsy of the mass was performed at an outside institution. The pathology came back as RMC. The patient underwent left radical nephrectomy without any complications in an outside institution. The surgical specimen contained highly atypical cells arranged in nests, sheets, and adenoid cystic patterns, with intratumoral neutrophilic infiltration and peritumoral inflammation and fibrosis (). Staining was positive for thrombomodulin, EMA, CAM5.2, PAX-8, high molecular weight keratin, and E-Cadherin; and negative for PAX-2, uroplakin, p63, and Mucicarmine. The tumor was 6.5 cm in its longest dimension, and was pathologically staged as pT3a pN1 Mx. He had not had a staging CT chest prior to his surgery. Five weeks post his surgery, in July 2013; he was referred to our institution for further management. At that time, he had noticed recurrence of his flank pain. CT of the chest, abdomen, and pelvis revealed mediastinal, hilar, and gastrohepatic ligament lymphadenopathy with right pulmonary nodules suspicious for metastatic disease (). Bone scan had shown suspicious lesion in the left 8th rib, which was thought to be a metastatic site. He was unable to start chemotherapy with cisplatin based regimen because of renal insufficiency and was instead started on carboplatin (AUC 6) and Paclitaxel (175 mg/m2) Q3 weekly with denosumab (120 mg) Q6 weekly (per patients’ request to decrease frequent hospital visits) with plans to switch to cisplatin based regimen when renal function improves.
Figure 1. Pathological features of renal medullary carcinoma. Pathologic surgical specimen of left kidney, revealing highly atypical cells arranged in nests and sheets, with intratumoral neutrophilic infiltration and peritumoral inflammation and fibrosis.
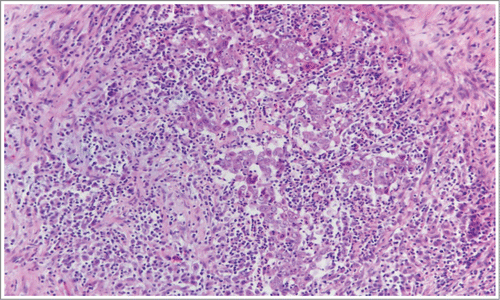
Figure 2. Sequential CT scans showing response to therapy. Metastatic Renal Medullary Carcinoma with Complete Remission of Disease post chemotherapy and it remained the same while on maintenance: CT scan prior to the initiation of chemotherapy revealed significant lymphadenopathy consistent with metastatic disease in the mediastinum (A), gastrohepatic ligament (B), and in the right lung (C). After Cycle 4 of chemotherapy, CT imaging revealed complete remission of mediastinal (D), gastrohepatic ligament (E), and pulmonary (F) nodules.
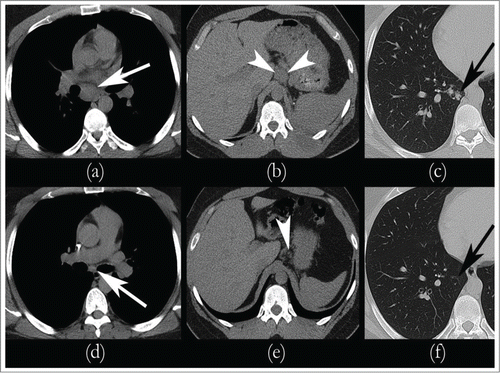
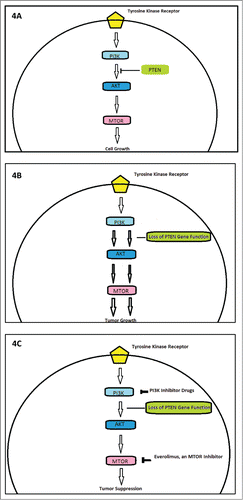
We switched him to PCG (paclitaxel 80 mg/m2, cisplatin 35mg/m2 and gemcitabine 1000 mg/m2 on days 1 and 8 every 21 d), when his renal function stabilized with GFR >50. He tolerated the chemotherapy well except for myelosuppression needing dose reduction and addition of neulasta from his C#3 onwards. He got a total of 6 cycles with platinum based chemotherapy, out of which 5 were PCG. CT of chest and abdomen after cycle 2 of PCG revealed marked decrease mediastinal, hilar, and gastrohepatic ligament lymphadenopathy with complete resolution (CR) of lung nodules and lymph nodes post completion of cycle 4 of PCG chemotherapy (). Given the rarity of the tumor and no standard of care for treatment, his surgical specimen was sent to Caris Life Sciences for molecular profiling and next generation sequencing in the hopes of finding a targetable mutation. Tumor immunohistochemistry revealed below threshold expression of Ribonucleotide Reductase M1 (RRM1) expression (), near complete loss of phosphatase and tensin homolog (PTEN) expression(Fig3 B) and 50% c-MET expression (positive but CISH was negative for cMET amplification); as shown in , loss of PTEN gene function removes the negative feedback mechanism exerted on PI3- kinanse, thereby increasing downstream signaling and increased un-inhibited cell growth. The tumor biomarker profile indicated potential benefit of gemcitabine, nab-paclitaxel, and doxorubicin. Since most of the literature showed that patients had early relapse of their disease post chemotherapy, we wanted to explore maintenance therapy for his disease to prevent early relapse.Citation1,7 His molecular analyses had shown low level of PTEN expression by IHC(5%), frequently correlated with PTEN gene deletion and functional loss. We opted to give him maintenance everolimus (mTOR-mammalian target of rapamycin, inhibitor) 10 mg/daily therapy, starting beginning of December 2013. demonstrates potential targets for therapy in patients with PTEN gene loss of function, including an MTOR inhibitor. His follow up bone scans were stable and upon repeat review, the rib lesion was thought to be a bony island of uncertain significance.
Figure 3. Immunohistochemical Identification of Molecular Targets for Therapy. (A) Negative expression of RRM1 (defined as less than 2+/50%) was detected in the patient sample (1+/90%). (B) Negative expression of PTEN (defined as ≤1+/50%) was detected in the patient sample (1+/5%, a near complete loss of expression).
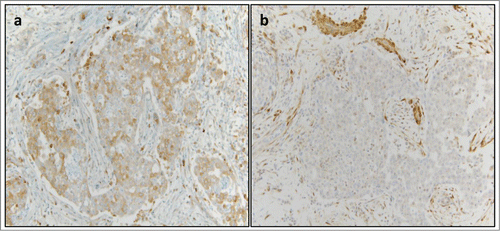
Figure 4. Outline of the role of PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homolog; mTOR, mammalian target of rapamycin. (A) Exhibits role of tumor suppressor gene PTEN in regulating PI3K mediated cell cycle signaling. (B) Demonstrates role of loss of PTEN inhibition in tumor genesis. (C) Highlights potential targets of cell cycle inhibitor, including Everolimus which was used in our patient.
He tolerated everolimus well, follow up CT chest and abdomen showed maintenance of CR until the end of June 2014, when his imaging showed a significant increase in his mediastinal and hilar and aortocaval lymph nodes. However, all his nodes were less than 2 cm and he was clinically asymptomatic. The repeat biopsy from his aortocaval lymph node was positive for recurrence of his disease. He was switched to gemcitabine single agent therapy in July 2014 and continues to tolerate it well. He is presently alive and almost 14 months from his diagnosis. We had wanted a repeat biopsy for next generation sequencing but due to unavoidable circumstances this was not possible in spite of disease progression.
Discussion
The patient in this study presented with RMC that was widely metastatic shortly after the time of presentation. We had done molecular profiling to determine possible targets with intent to treat. He was found to have near absence of PTEN expression by immunohistochemistry and we used this information to guide his maintenance therapy (). He tolerated this therapy well with no significant side-effect and was in clinical and radiological remission for 7 months since stopping of his induction chemotherapy and is now alive about 14 months since his diagnosis. Our case is one of the very few cases with advanced RMC who achieved CR from his initial chemotherapy. To the best of our knowledge, this is the first case to show that profiling and subsequent targeted therapy had therapeutic benefit in RMC and should be considered in these patients. Our patient is alive; about 14 months post his diagnosis.
RMC was first described in 1995 by Davis et al as “the seventh sickle cell nephropathy.” Since that initial publication, the literature has become populated with case reports and periodic reviews of the literature, which form the principle body of knowledge available on this tumor. The disease is usually widely metastatic at the time of diagnosis. The tumor microscopically presents with islands of reticular growth, with adenoid cystic-like spaces, solid sheets, and anastomosing tubules.Citation8 The cancer cells are highly atypical, with a rhabdoid or plasmacytoid shape as well as dark cytoplasm, clear nuclei, and prominent nucleoli.Citation9 Prominent fibrosis and inflammation are characteristic.
Historically, the prognosis of this tumor has been dismal, with patients surviving approximately weeks to months. We searched for all English-language case reports for adult patients (>18 y of age; we have included one case of 17 y old who had an amplification of ABL-tyrosine kinase) with RMC that was metastatic at or shortly after the time of diagnosis, published since 2003, and gathered available data on these cases pertaining to chemotherapy protocols and survival since the time of the diagnosis (). We only included patients from the case reports or case series who had data for chemotherapy use, survival post diagnosis and or response to therapy. Our search returned 34 unique cases published during that time, out of which only 17 cases met our above mentioned criteria for inclusion in the .Citation1,Citation10-17 The median survival calculated for all included patients in the was 10.75 months with an average of 10.9 months. We observed that the majority of patients who had any meaningful response (complete or partial response) were treated with cisplatin based chemotherapy (survival appeared to be more extended with PCG regimen when compared to the rest). Majority of these patients had right sided kidney RMC. The observation of positive response to cisplatin appears to be consistent with the previously published notion that renal medullary carcinoma may respond to platinum-based agents.Citation18,10 While these regimens have improved survival compared to the original studies, they still universally fail to produce a durable response or cure.
Table 1. Relevant cases with renal medullary carcinomas and their survival
The difficulty in achieving durable responses for this tumor may reflect our poor understanding of its pathophysiology. A report by Swartz et al.Citation19 has shown a strong expression of Vascular Endothelial Growth Factor (VEGF), and Hypoxia Inducible Factor (HIF) in RMC.Citation19 These findings formed the basis for a pathophysiologic theory, which proposes that the low oxygen tension in the renal medulla, especially in patients with sickle cell hemoglobinopathies, promotes the expression of HIF. Under normal circumstances, HIF leads to upregulation of p53, ultimately causing apoptosis. In the setting of deficient or absent p53, however, HIF instead leads to VEGF expression, which may help tumor vascularization and growth. In a separate report, Yang et al. suggested presence of hypoxia related and unrelated genetic abnormalities in significant number of cases, and hinted their role in the pathogenesis of RMC.Citation20 Recent studies looking into the cytogenetic abnormalities of RMC have failed to identify any consistent abnormalities across cases, and have also brought into question the aforementioned theory by Swartz on the grounds of cases in caucasian patients without hemoglobinopathies.Citation21,22 Gao et al., had recently shown involvement of genes in signaling pathways such as phosphatidylinositol 3-kinasePIK3C2G), cell cycle control (CDC25D and CDC73), and histone synthesis (HIST2H3D) based on molecular analysis of 11 patient specimens in a retrospective fashion. Although mechanism of pathogenesis still remains largely elusive, these alterations may become probable targets for therapy in the future.Citation23
It is both the lack of complete understanding of the disease and rarity of desirable response to treatment that led us to our treatment approach. The fact that RMC is probably sensitive to platinum based chemotherapy, coupled with the low expression/absence of RRM1 in his tumor; may have contributed to the complete remission of our patient's disease when treated with PCG chemotherapy regimen. Whether the low RRM1 expression facilitated a strong response to gemcitabine component in PCG is difficult to determine. The decision to attempt the use of everolimus for maintenance therapy, however, was entirely guided and deemed feasible based on the information regarding loss of PTEN expression gained from IHC profiling of the tumor. It is well known that PTEN deletion causes upregulation of PI3K-AKT pathway which plays a significant role in proliferation and progression of cancer and since mTOR is a downstream signal for the PI3K-AKT pathway, blocking this mTOR signal may cause decrease in tumor proliferation.Citation24 There is literature supporting PTEN deletion being a marker for predicting response to everolimus in other cancer types such as prostate cancer and that was our rationale for using everolimus in this patient.Citation24
Conclusion
We acknowledge that molecular profiling with next generation sequencing is not feasible, nor is it necessary for every patient with a malignant tumor. However, for these rare tumor types, especially those in which treatment guidelines are not well established, comprehensive molecular characterization may provide an invaluable insight for choosing a more personalized therapeutic approach. The information from molecular profiling can be used to guide maintenance therapy with targeted agents, which are better tolerated than chemotherapy and may provide a road to prolonged survival in patients with this deadly disease in this current era of personalized medicine.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethical Statement
The authors complied with patient confidentiality. An informed consent was obtained by the patient prior to preparing this manuscript.
Authors Contribution
JSL and SMR- manuscript preparation and both contributed equally to be co-first authors; ZG and NES- manuscript preparation, critical review of the article; SLH, MK and JJD provided critical review of the article; MJ-manuscript preparation, critical review/revision of the article and patient care.
References
- Maroja Silvino MC, Venchiarutti Moniz CM, Munhoz Piotto GH, Siqueira S, Galapo Kann A, Dzik C. Renal medullary carcinoma response to chemotherapy: a referral center experience in Brazil. Rare Tumors 2013; 5:e44; PMID:24179656
- Hakimi AA, Koi PT, Milhoua PM, Blitman NM, Li M, Hugec V, Dutcher JP, Ghavamian R. Renal medullary carcinoma: the Bronx experience. Urology 2007; 70:878-82; PMID:18068443; http://dx.doi.org/10.1016/j.urology.2007.06.1124
- Simpson L, He X, Pins M, Huang X, Campbell SC, Yang XJ, Perlman EJ, Bergan RC. Renal medullary carcinoma and ABL gene amplification. J Urol 2005; 173:1883-8; PMID:15879768; http://dx.doi.org/10.1097/01.ju.0000158448.56888.09
- Liu Q, Galli S, Srinivasan R, Linehan WM, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol 2013; 37:368-74; PMID:23348212; http://dx.doi.org/10.1097/PAS.0b013e3182770406
- Calderaro J, Moroch J, Pierron G, Pedeutour F, Grison C, Maille P, Soyeux P, de la Taille A, Couturier J, Vieillefond A, et al. SMARCB1INI1 inactivation in renal medullary carcinoma. Histopathology 2012; 61:428-35; PMID:22686875; http://dx.doi.org/10.1111/j.1365-2559.2012.04228.x
- Davis CJ, Mostofi FK, Sesterhenn IA. Renal medullary carcinoma. The seventh sickle cell nephropathy. Am J Surg Pathol 1995; 19:1-11; PMID:7528470; http://dx.doi.org/10.1097/00000478-199501000-00001
- O’Donnell PH, Jensen A, Posadas EM, Bridge JA, Yeldandi AV, Yang XJ, Stadler WM, Al-Ahmadie H. Renal medullary-like carcinoma in an adult without sickle cell hemoglobinopathy. Nat Rev Urol 2010; 7:110-4; http://dx.doi.org/10.1038/nrurol.2009.255
- Gupta R, Billis A, Shah RB, Moch H, Osunkoya AO, Jochum W, Hes O, Bacchi CE, de Castro MG, Hansel DE, et al. Carcinoma of the collecting ducts of Bellini and renal medullary carcinoma: clinicopathologic analysis of 52 cases of rare aggressive subtypes of renal cell carcinoma with a focus on their interrelationship. Am J Surg Pathol 2012; 36:1265-78; PMID:22895263; http://dx.doi.org/10.1097/PAS.0b013e3182635954
- Avery RA, Harris JE, Davis CJ, Borgaonkar DS, Byrd JC, Weiss RB. Renal medullary carcinoma: clinical and therapeutic aspects of a newly described tumor. Cancer 1996; 78:128-32; PMID:8646708; http://dx.doi.org/10.1002/(SICI)1097-0142(19960701)78:1%3c128::AID-CNCR18%3e3.0.CO;2-1
- Bell MD. Response to paclitaxel, gemcitabine, and cisplatin in renal medullary carcinoma. Pediat Blood Cancer 2006; 47:228; PMID:16514612; http://dx.doi.org/10.1002/pbc.20780
- Gangireddy V, Liles GB, Sostre GD, Coleman T. Response of metastatic renal medullary carcinoma to carboplatinum and Paclitaxel chemotherapy. Clin Genitourin Cancer 2012; 10:134-9; PMID:22409864; http://dx.doi.org/10.1016/j.clgc.2012.01.003
- Rathmell WK, Monk JP. High-dose-intensity MVAC for advanced renal medullary carcinoma: report of three cases and literature review. Urology 2008; 72:659-63; PMID:18649931; http://dx.doi.org/10.1016/j.urology.2008.05.009
- Watanabe IC, Billis A, Guimaraes MS, Alvarenga M, de Matos AC, Cardinalli IA, Filippi RZ, de Castro MG, Suzigan S. Renal medullary carcinoma: report of seven cases from Brazil. Mod Pathol: Off J U S Can Acad Pathol, Inc 2007; 20:914-20; PMID:17643096; http://dx.doi.org/10.1038/modpathol.3800934
- Anne M, Sammartino D, Chaudhary S, Bhuiya T, BM. Renal medullary carcinoma masquerading as bilateral breast carcinoma category: case report. Vol 4. 3 ed. World J Oncol 2013; 169-72.
- Noguera-Irizarry WG, Hibshoosh H, Papadopoulos KP. Renal medullary carcinoma: case report and review of the literature. Am J Clin Oncol 2003; 26:489-92; PMID:14528077; http://dx.doi.org/10.1097/01.coc.0000037663.61643.5A
- Assad L, Resetkova E, Oliveira VL, Sun W, Stewart JM, Katz RL, Caraway NP. Cytologic features of renal medullary carcinoma. Cancer 2005; 105:28-34; PMID:15593260; http://dx.doi.org/10.1002/cncr.20764
- Schaeffer EM, Guzzo TJ, Furge KA, Netto G, Westphal M, Dykema K, Yang X, Zhou M, Teh BT, Pavlovich CP. Renal medullary carcinoma: molecular, pathological and clinical evidence for treatment with topoisomerase-inhibiting therapy. BJU Int 2010; 106:62-5; PMID:20002663; http://dx.doi.org/10.1111/j.1464-410X.2009.09139.x
- Strouse JJ, Spevak M, Mack AK, Arceci RJ, Small D, Loeb DM. Significant responses to platinum-based chemotherapy in renal medullary carcinoma. Pediat Blood Cancer 2005; 44:407-11; PMID:15602719; http://dx.doi.org/10.1002/pbc.20292
- Swartz MA, Karth J, Schneider DT, Rodriguez R, Beckwith JB, Perlman EJ. Renal medullary carcinoma: clinical, pathologic, immunohistochemical, and genetic analysis with pathogenetic implications. Urology 2002; 60:1083-9; PMID:12475675; http://dx.doi.org/10.1016/S0090-4295(02)02154-4
- Yang XJ, Sugimura J, Tretiakova MS, Furge K, Zagaja G, Sokoloff M, Pins M, Bergan R, Grignon DJ, Stadler WM, et al. Gene expression profiling of renal medullary carcinoma: potential clinical relevance. Cancer 2004; 100:976-85; PMID:14983493; http://dx.doi.org/10.1002/cncr.20049
- Debelenko LV, Raimondi SC, Daw N, Shivakumar BR, Huang D, Nelson M, Bridge JA. Renal cell carcinoma with novel VCL-ALK fusion: new representative of ALK-associated tumor spectrum. Mod Pathol: Off J U S Can Acad Pathol, Inc 2011; 24:430-42; PMID:21076462; http://dx.doi.org/10.1038/modpathol.2010.213
- Gatalica Z, Lilleberg SL, Monzon FA, Koul MS, Bridge JA, Knezetic J, Legendre B, Sharma P, McCue PA. Renal medullary carcinomas: histopathologic phenotype associated with diverse genotypes. Hum Pathol 2011; 42:1979-88; PMID:21733559; http://dx.doi.org/10.1016/j.humpath.2011.02.026
- Jianjun Gao, Hui Yao, Xiaoping Su, Priya Rao, Padmanee Sharma, Jose A. Karam, Christopher G. Wood, Tannir NM. Molecular characterization of renal medullary carcinoma (RMC). J Clin Oncol 32:5s, 2014 (suppl; abstr 4586); http://dx.doi.org/10.1200/JCO.2013.49.4757
- Templeton AJ, Dutoit V, Cathomas R, Rothermundt C, Bartschi D, Droge C, Gautschi O, Borner M, Fechter E, Stenner F, et al. Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 0808). Eur Urol 2013; 64:150-8; PMID:23582881; http://dx.doi.org/10.1016/j.eururo.2013.03.040
