Abstract
Multiple myeloma (MM), a plasma cell malignancy, remains incurable despite the development of new therapies. Curcumin anti-tumor effects were previously characterized in multiple myeloma, however only few MM cell lines were included in these studies. Since myeloma is a heterogeneous disease it is important to address the impact of myeloma molecular heterogeneity in curcumin cell death induction. In the present study, a large panel of human myeloma cell lines (HMCLs) (n = 29), representing the main molecular MM subgroups, was screened for curcumin sensitivity. We observed that curcumin cell death induction was heterogeneous, of note 16 HMCLs were highly sensitive to curcumin (LD50 < 20.5 μM), 6 HMCLs exhibited intermediate LD50 values (20.5 μM ≤ LD50 < 32.2 μM) and only 7 HMCLs were weakly sensitive (35 < LD50 < 56 μM). Cell lines harboring the t(11;14) translocation were less sensitive (median LD50 32.9 μM) than non-t(11;14) (median LD50 17.9 μM), which included poor prognosis t(4;14) and t(14;16) cells. Interestingly, curcumin sensitivity was not dependent on TP53 status. For the first time we showed that primary myeloma cells were also sensitive, even those displaying del(17p), another poor prognosis factor. We also unravel the contribution of anti-apoptotic Bcl-2 family molecules in curcumin response. We found that down-regulation of Mcl-1, an essential MM survival factor, was associated with curcumin-induced cell death and its knockdown sensitized myeloma cells to curcumin, highlighting Mcl-1 as an important target for curcumin-induced apoptosis. Altogether, these results support clinical trials including curcumin in association with standard therapy.
Abbreviations
| MM | = | multiple myeloma |
| HMCLs | = | human myeloma cell line(s) |
| CCND | = | Cyclin D |
Introduction
Curcumin (diferuloylmethane) is a pharmacologically safe polyphenol derived from Curcuma longa, its anti-tumor effects have been well described in several types of cancers.Citation1,2 Curcumin has been also reported to inhibit multiple myeloma (MM) cell proliferation and to induce MM cell death.Citation3 These effects were associated with the capacity of curcumin to inhibit NF-kB activation, as well as to down-regulate the expression of IL-6, cyclin D1, Bcl-xL and STAT3 phosphorylation.Citation4-6 All of these studies support the fact that curcumin has an anti-myeloma activity. However, only few myeloma cell lines were studied in the former publications; giving the fact that myeloma is a heterogeneous disease,Citation7 it is important to address the impact of the molecular heterogeneity of myeloma in curcumin cell death induction. In this context, the molecular classification of MM patients takes into account the presence of primary IgH translocations and the universal over-expression of CCND (cyclin D) genes. The main translocation subtypes involves the immunoglobulin heavy chain locus on 14q32.33 with recurrent chromosome partners; they include t(11;14), t(4;14) and t(14;16) [(14;16) or (16;22) or (20;22)] with an overexpression of CCND1, MMSET and cMAF/MAFB genes, respectively.Citation7 Both, MMSET and MAF myeloma subgroups have been associated with poor prognosis.Citation7 Furthermore, del(17p) is universally regarded as a high-risk genetic feature, mainly related to a defect of the TP53 pathway.Citation8 In the present work, a large panel of human myeloma cell lines (HMCLs) (n = 29), representing the main molecular MM subgroups, was studied for curcumin sensitivity. For the first time, curcumin effect was also addressed on primary MM cells. We also unravel the contribution of the anti-apoptotic Bcl-2 family molecules, since they are overexpressed in cancer cells and are associated with resistance to chemotherapy.Citation9,10
Results
Analysis of curcumin sensitivity in MM molecular subgroups
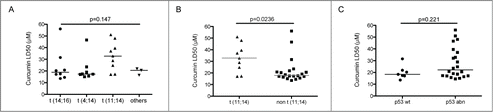
Curcumin cell death effect was tested in a large collection of HMCLs (n=29) encompassing the main molecular myeloma subtypes; after 24 h treatment cell death was determined by Apo2.7 staining followed by FACS analysis. We then calculated the LD50 values, defined as the concentration sufficient to kill 50% of cells. We found that cell death induced by curcumin was heterogeneous among the main myeloma subgroups ( and ) with a trend of the t(11,14) HMCLs to be less sensitive (median LD50 32.9 μM) than the other groups (), which was confirmed when opposing them to all non t(11;14) HMCLs (median LD50 17.9 μM) (p = 0.023) (). Of note, non-t(11;14) HMCLs included t(4;14) and t(14;16), both subgroups known to be of poor prognosis.Citation8 Interestingly, taking all HCMLs together (median LD50=20.5 μM), we found that most HMCLs were efficiently killed by curcumin; we observed that 16 HMCLs were highly sensitive (LD50 < 20.5 μM), and 6 HMCLs exhibited intermediate LD50 values (20.5 μM ≤ LD50 < 32.2 μM); only 7 HMCLs exhibited the highest LD50 values (32,2 < LD50 < 56 μM). Moreover, curcumin sensitivity was not dependent on TP53 status, since LD50 were not statistically different between HMCLs exhibiting either wild type (median LD50 18.4 μM) or abnormal TP53 (mutated, truncated or deleted)Citation12 (median LD50 22.15 μM) (p=0.221) (). All together these results demonstrated that curcumin has an efficient in vitro anti-myeloma activity that covers particularly the non t(11;14) subgroups without been affected by TP53 status.
Table 1. HMCLs' characteristics and sensitivity to curcumin
Figure 1. Curcumin induced cell death of myeloma cells belonging to the main molecular myeloma subgroups. (A) HMCL (n = 29) were treated with curcumin for 24 h. Cell death was measured by FACS analysis of Apo2.7 stained cells. LD50 values were calculated from at least 3 independent experiments. HMCLs were classified according to translocation subtypes. Statistical analysis was performed using Kruskall-Wallis test. Curcumin LD50 of HMCLs were analyzed according to (B) the presence or absence of t(11,14) translocation () or (C) TP53 status (). Statistical analysis was performed using Mann-Whitney test.
Primary plasma cells were sensitive to Curcumin
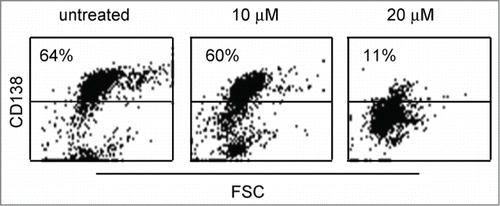
We next examined curcumin effect on primary MM cells, to this aim CD138 positive cells obtained from 9 MM or secondary plasma cell leukemia patients were treated with 10 and 20 μM curcumin during 24h, cell death was evaluated by the loss of CD138 expression.Citation12,13 As shown in , dot plots illustrated the loss of CD138 staining in primary cells from sample 4 under curcumin treatment. We found that primary cells, from MM or secondary plasma cell leukemia, were efficiently killed by 20 μM curcumin (). However, 10 μM curcumin allowed distinguishing sensitive (5, 6, 8 and 9), and weakly sensitive (1, 3, 4 and 7) samples (). Of note, 2 (1 and 4) out of 4 weakly sensitive samples harbored the t(11;14) translocation. Interestingly, primary cells obtained from sample 3 were efficiently killed by 20 μM curcumin (98%) while they were not very sensitive to 20 μM melphalan (13% cell death). It is worth noting that primary cells of 2 (samples 8 and 9) out of 3 patients displaying del(17p) were efficiently killed by 10 μM curcumin.
Table 2. Sensitivity of primary myeloma cells to curcumin
Figure 2. Primary myeloma cells were killed by curcumin. Primary cells (CD 138+) obtained from sample 4 were treated 24 h with the indicated doses of curcumin. Cells were then stained with an anti-CD138-PE mAb. The loss of CD138 staining was representative of cell death. Cell death percentage was calculated relative to untreated cells.
Early caspase-3 activation and Mcl-1 decrease were associated with cell death induced by curcumin
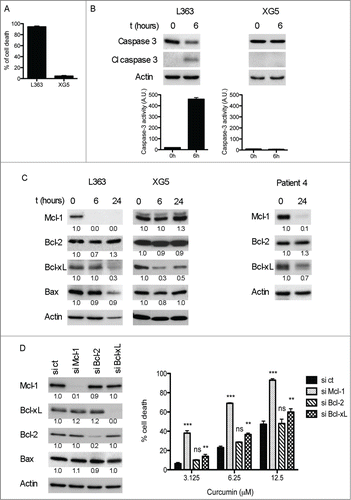
Although apoptosis induction by curcumin has been studied in different cancer models as well as in myeloma cells,Citation1-6 any report included the study of Mcl-1 regulation, the main survival protein for myeloma cells.Citation14 Thus, to further investigate the molecular mechanism by which curcumin induced cell death, we examined 2 HMCLs, a highly sensitive (L363) and a poorly sensitive one (XG5) (). We treated both cell lines with 15 μM curcumin for the indicated times, this concentration induced cell death in L363 but not in XG5 cells (). We demonstrated that curcumin induced apoptosis via the mitochondrial pathway, as evidenced by the activation of caspase-3 as early as 6 hours in the sensitive L363 cells (). No caspase-3 activation was observed in XG5, according to the absence of cell death. We then studied curcumin effect on the main anti-apoptotic Bcl-2 members expression. The main difference between both cell lines studied was the important early decrease of Mcl-1 in the highly sensitive L363 cells (, left panel), while it remained unmodified in the absence of cell death in XG5 cells (, middle panel). Besides, Bcl-xL was down-regulated in both cell lines (), independently of curcumin cell death induction, suggesting that Bcl-xL is probably not critical for curcumin cell death in MM cells. Finally, Bcl-2 protein level remained unchanged in both cell lines, as well as the pro-apoptotic member Bax. In addition, we performed the same analysis in primary CD138+ purified cells (sample 4), similar modifications of the Bcl-2 anti-apoptotic molecules were observed after 24h curcumin treatment, confirming the drastic Mcl-1 decrease in curcumin killed cells (, right panel). Altogether these results indicated that the early Mcl-1 downregulation is a hallmark of curcumin induced cell death in myeloma cells. Then, to unravel the role of Mcl-1, Bcl-2 and Bcl-xL in curcumin induced cell death we next performed transient knock-down of these anti-apoptotic members. Giving the fact that HMCLs are difficult to transfect, we chose LP1 cells, in which siRNA transfection did not induce cell death.Citation15 Efficient silencing of targeted molecules was assessed by western blotting analysis (, left panel). As shown in (right panel), knockdown of Mcl-1 rendered myeloma cells significantly more sensitive to curcumin, even to lower doses (3.125 μM). On the contrary, knockdown of Bcl-2 had no effect on cell death induced by this agent, in agreement with the absence of Bcl-2 modification under curcurmin treatment (). Besides, Bcl-xL silencing exhibited a minor effect (2.3 cell death fold increase at 3.125 μM) in curcumin death response compared to that of Mcl-1 silencing (6.3 cell death fold increase at 3.125 μM). Thus, these data support an important role of Mcl-1 in curcumin cell death compared to its other anti-apoptotic relatives.
Figure 3. Curcumin cell death was associated with Mcl-1 decrease and Caspase-3 activation. (A) L363 (highly sensitive) and XG5 (poorly sensitive) cells were treated during 24 h with 15 μM curcumin, cell death was determined by Apo 2.7 staining. Data represent the mean ± SD of 3 independent experiments. (B) Caspase-3 protein levels and activity were determined on cell lysates. Data represent mean ± SD of 3 independent experiments. (C) Cells were treated with curcumin for the indicated times and the expression of Bcl-2 family molecules was assessed by protein gel blotting. Primary purified CD138+ cells (p4 sample) were treated 24 h with 15 μM curcumin, which induced 82% of cell death. (D) Transient knock-down of Mcl-1, Bcl-2 and Bcl-xL was performed on LP1 cells. After 48 h of transfection cells were treated with curcumin for the next 24 h and subjected to Apo 2.7 staining. Efficient silencing of the 3 anti-apoptotic proteins is shown by immunoblotting in the left panel. Data represent mean ± SD of 3 independent experiments. *** = P < 0.001; ** = P < 0.01; * = P < 0.05 and ns = P > 0.05. Statistical analysis was performed using 2-way Anova test.
Discussion
The present study demonstrated that curcumin was able to induce cell death of HMCLs belonging to the main MM subtypes. Taking into account myeloma molecular heterogeneity, we found that most sensitive HMCLs were spread out over the t(14;16) and t(4;14) subgroups, which over-express MAF and MMSET genes, respectively. Strikingly, HMCLs displaying t(11;14) were less sensitive when compared to the non t(11;14) HMCLs (median LD50 32.9 μM and LD50 17.9 μM, respectively). This finding could be related to the fact that t(11;14) subgroup is considered as a particular entity regarding its apoptotic machinery. In fact, t(11;14) subgroup that over-express CCND1 has a low expression of pro-apoptotic Bcl-2 family members, namely: multidomain effectors (Bax/Bak) and BH3-only (Puma, Bik and Bad) proteins.Citation16 Therefore, it is possible that this feature may influence the less efficient apoptotic response triggered by curcumin, Additionally the higher curcumin sensitivity of t(4;14) and t(14;16) subgroups is a relevant finding, since these translocations are regarded as poor prognosis factors.Citation8 Our in vitro study also showed that curcumin sensitivity was not affected by TP53 status, which is not the case for melphalan,Citation17 a frontline myeloma treatment, efficient to induce long lasting remission, but which does not cure myeloma patients.Citation18 We also demonstrated for the first time that primary MM cells obtained from patients either at diagnosis or relapse were efficiently killed by curcumin. It is worth noting that primary cells of 2 out of 3 patients displaying del(17p) (samples 4, 8 and 9) were efficiently killed by 10 μM curcumin. This finding is important because del(17p) is also regarded as a high-risk genetic feature, mainly related to a defect of the TP53 pathway.Citation19 Moreover, it also reflects the results obtained in HMCLs and is in agreement with the study performed in melanoma cells, in which curcumin induced similar cell death of either wild type or mutated p53 cell lines.Citation20
We showed that curcumin cell death triggered an early caspase-3 activation and pointed out to anti-apoptotic Mcl-1 as an essential target of curcumin. Indeed, an early decrease of Mcl-1 was observed in curcumin sensitive cells, either in cell lines or in primary cells, and Mcl-1 knockdown significantly sensitized myeloma cells to this polyphenol. Sung et al. proposed that Bcl-xL decrease was one of the mechanisms that explained curcumin effect;Citation6 however, we observed the decrease of this protein not only during curcumin cell death induction (L363) but also in the absence of cell death (XG5). Thus, we could hypothesize that Bcl-xL decrease had less impact in curcumin cell death than Mcl-1, as supported by our results of transient knockdown experiments. Furthermore, the pharmacologically safeness of curcumin has been proved and supported a double-blind study in MGUS and smoldering MM patients; this trial proposed that curcumin might have a potential to slow the disease process in those patients.Citation21 Additionally, it has been shown that curcumin not only potentiated the antitumor effects of bortezomib and thalidomide, but also overcame chemoresistance to conventional myeloma therapy.Citation6 Incidentally, we can also report the case of a 42 year-old male who was diagnosed with a symptomatic de novo multiple myeloma in 2002, IgG kappa, M-spike 53 g/L at diagnosis. He was treated with the VAD regimen (vincristin, doxorubicin and dexamethasone) as induction therapy followed by tandem autologous stem cell transplantation prepared by high-dose melphalan. Following high-dose therapy, he was in partial response (M-spike 18 g/L). Progression was documented in july 2006, with an M-component of 30 g/L. He received lenalidomide and dexamethasone from july 2006 until may 2011. This salvage therapy induced a very good partial response, and the M-spike was 2 g/L in may 2011. Lenalidomide and dexamethasone were stopped based on patient's decision. In January 2012, he started oral curcumin, at the dose of 1.5 g/day. He is now receiving daily curcumin for 27 months, and the M-spike is not detectable in the serum electrophoresis, but immunofixation is remaining positive (P.Moreau, unpublished data). All these findings might support clinical trials including curcumin in association with standard therapy, as chemoresistance remains a major challenge in myeloma treatment.
Material and Methods
Human myeloma cell lines and primary myeloma cells
All (HMCLs) were previously characterized.Citation12,22 The HMCLs BCN; NAN1, −3, −7, −8 and −9; SBN; and XG1, −2, −5, −6, −7 and −11 were derived in Nantes or Montpellier laboratories in the presence of interleukin-6 (IL-6). KMS11, KMS12-PE and KMM1 HMCLs were kindly provided by Dr. Otsuki (Kurashiki, Japan), JJN3 was kindly provided by Dr. Van Riet (Brussels, Belgium), JIM3 was kindly provided by Dr. MacLennan (Birmingham, UK), Karpas 620 was kindly provided by Dr. Karpas (Cambridge, UK) and MM1S was kindly provided by Dr. Rosen (Chicago, IL). AMO1, LP1, L363, NCI-H929, SKMM2, U266 and OPM2 were purchased from DSMZ (Braunsweig, Germany). All HMCLs were cultured in RPMI1640 supplemented with 5% fetal calf serum (FCS). Cell cultures from BCN, NAN, SBN and XG cells were additionally supplemented with 3 ng/mL IL-6. Blood or bone marrow samples from patients were collected after informed consent at the Department of Hematology, University Hospital of Nantes. Experiments with primary myeloma cells were carried out using unpurified or purified (CD138+) myeloma cells, depending on the myeloma infiltration and the total number of cells. Chromosome abnormalities were assessed by fluorescence in situ hybridization (FISH).
Reagents and antibodies
Curcumin was purchased from Sigma-Aldrich (C7727) and resuspended in DMSO (50 mM). Anti-APO2.7–phycoerythrin (PE) (IM2088U), anti-CD138–PE (A54190) and control immunoglobulin G1 (IgG1)–PE (555749) monoclonal antibodies (mAbs) were purchased from BD Biosciences.
Cell death measurement
After 24 h of curcumin treatment, cell death of HMCLs was quantified by APO2.7-PE staining followed by flow cytometry analysis, using a FACSCalibur and Cell Quest software (Becton Dickinson). Solvent DMSO did not affect cell viability, since concentrations equivalent to those present in the highest doses of curcumin (dilution 1/600) did not induce cell death. Cell death of primary myeloma cells was determined by the measurement of the loss of CD138 staining, as previously described.Citation12,13 Fluorescence was analyzed on FACSCalibur (Cytocell, SFR Bonamy).
Western blotting analysis
Cells were collected and lysed in lysis buffer containing 10 mM Tris, pH 7.6, 150 mM NaCl, 5 mM EDTA and 1% TritonX100, 50 μg/ml pefabloc and 2 μg/ml aprotinin. Cell pellets were disrupted by 10 min sonication and lysates were obtained by centrifugation at 12,000 g for 30 min at 4°C. Equal amount of total proteins were resolved by SDS-PAGE and immunoblotted using antibodies against Mcl-1 (Santa Cruz, sc-819), Bcl-2 (Dako, M0887), Bcl-xL (Transduction Lab, 610209), Caspase-3 (Santa Cruz, sc-7272), Bax (Enzo Life Sciences, ADI-AAM-14-E) and Actin (Millipore, MAB 1501), used as a loading control. Quantification of bands was done using ImageJ software. The basal level of each cell line or patient was considered as 1.
Caspase-3 activity
Caspase-3 activity was detected on cell lysates as previously described.Citation23 Briefly, caspase-3 activity was measured by following the cleavage of 50 μmol/L Ac-DEVD-AMC peptide in the presence of cell lysates. The fluorescence of the cleaved substrate was determined every 15 min using a spectrophotometer (Fluorolite 1000, Dynatech Laboratories) set at an excitation wavelength of 365 nm and emission wavelength of 465 nm.
siRNA Transfection
LP1 myeloma cells were transfected with 100 pmol siRNA using RNAimaxTM Reagent (Invitrogen, 13778075) according to the manufacturer's instructions; after 48 h cells were treated with curcumin for the following 24 h. siRNAs scramble, Mcl-1 (L-004501), Bcl-2 (L-003307) and Bcl-xL (L-003458) were purchased from Dharmacon.
Statistical analysis
Statistical analysis was performed with Kruskall-Wallis, Mann-Whitney or 2 way Anova test using GraphPad Prism software, where P < 0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Ligue Contre le Cancer Grand Ouest.
References
- Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res 2008; 18:7283-92; PMID:18794115; http://dx.doi.org/10.1158/0008-5472.CAN-07-6246
- Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol 2005; 5:700-13; PMID:16023083; http://dx.doi.org/10.1016/j.bcp.2005.04.043
- Bharti AC, Shishodia S, Reuben J, Weber D, Alexanian R, Raj-Vadhan S, Estrov Z, Talpaz M, Aggarwal BB. Nuclear factor-kB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood 2004; 103:3175-84; PMID:15070700; http://dx.doi.org/10.1182/blood-2003-06-2151
- Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human myeloma cells. J Immunol 2003; 171:3863-71; PMID:14500688; http://dx.doi.org/10.4049/jimmunol.171.7.3863
- Park J, Ayyappan V, Bae EK, Lee C, Kim BS, Kim BK, Lee YY, Ahn KS, Yoon SS. Curcumin in combination with bortezomib synergistically induced apoptosis in human multiple myeloma U266 cells. Mol Oncol 2008; 2:317-26; PMID:19383353; http://dx.doi.org/10.1016/j.molonc.2008.09.006
- Sung B, Kunnumakkara GS, Sethi G, Anand P, Gluha S, Aggarwal BB. Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and botezomib against human multiple myeloma in nude mice model. Mol Cancer Ther 2009; 8:959-70; PMID:19372569; http://dx.doi.org/10.1158/1535-7163.MCT-08-0905
- Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, Epstein J, Yaccoby S, Sawyer J, Burington B, et al. The molecular classification of multiple myeloma. Blood 2006; 108:2020-28; PMID:16728703; http://dx.doi.org/10.1182/blood-2005-11-013458
- Chesi M, Bergsagel PL. Molecular pathogenesis of multiple myeloma: basic and clinical updates. Int J Hematol 2013; 97:313-23; PMID:23456262; http://dx.doi.org/10.1007/s12185-013-1291-2
- Johnson SK, Heuck CJ, Albino AP, Qu P, Zhang Q, Shaughnessy JD Jr. The use of molecular-base risk stratification and pharmacogenomics for outcome prediction and personalized therapeutic management of multiple myeloma. Int J Hematol 2011; 94:321-33; PMID:22002477; http://dx.doi.org/10.1007/s12185-011-0948-y
- Wuillème-Toumi S, Robillard N, Gomez P, Moreau P, Le Gouill S, Avet-Loiseau H, Harousseau JL, Amiot M, Bataille R. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia 2005; 9:1248-52; PMID:15902294; http://dx.doi.org/10.1038/sj.leu.2403784
- Azmi AS, Wang Z, Philip PA, Mohammad RM, Sarkar FH. Emerging Bcl-2 inhibitors for the treatment of cancer. Expert Opin Emerg Drugs 2011; 1:59-70; PMID:20812891; http://dx.doi.org/10.1517/14728214.2010.515210
- Surget S, Chiron D, Gomez-Bougie P, Descamps G, Ménoret E, Bataille R, Moreau P, Le Gouill S, Amiot M, Pellat-Deceunynck C. Cell Death via DR5, but not DR4, is regulated by p53 in myeloma cells. Cancer Res 2012; 17:4562-73; PMID:22738917; http://dx.doi.org/10.1158/0008-5472.CAN-12-0487
- Jourdan M, Ferlin M, Legouffe E, Horvathova M, Liautard J, Rossi JF, Wijdenes J, Brochier J, Klein B. The myeloma cell antigen syndecan-1 is lost by apoptotic myeloma cells. Br J Haematol 1998; 100:637-46; PMID:9531328; http://dx.doi.org/10.1046/j.1365-2141.1998.00623.x
- Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JL, Bataille R, Amiot M. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood 2002; 100:194-99; PMID:12070027; http://dx.doi.org/10.1182/blood.V100.1.194
- Bodet L, Gomez-Bougie P, Touzeau C, Dousset C, Descamps G, Maïga S, Avet-Loiseau H, Bataille R, Moreau P, Le Gouill S, et al. ABT-737 is highly effective against molecular subgroups of multiple myeloma. Blood 2011; 118: 3901-10; PMID:21835956; http://dx.doi.org/10.1182/blood-2010-11-317438
- Gomez-Bougie P, Amiot M. Apoptotic machinery diversity in multiple myeloma molecular subtypes. Front Immunol 2013; 4:1-6; PMID:23355837; http://dx.doi.org/10.3389/fimmu.2013.00467
- Surget S, Lemieux-Blanchard E, Maïga S, Descamps G, Le Gouill S, Moreau P, Amiot M, Pellat-Deceunynck C. Bendamustine and melphalan kill myeloma cells similarly through reactive oxygen species production and activation of the p53 pathway and do not overcome resistance to each other. Leuk Lymphoma 2014; Epub ahead of print; PMID:24308434
- Moreau P, Avet-Loiseau H, Harousseau JL, Attal M. Current trends in autologous stem-cell transplantation for myeloma in the era of novel therapies. J Clin Oncol 2011; 9:1898-06; PMID:21482979; http://dx.doi.org/10.1200/JCO.2010.32.5878
- Lodé L, Eveillard M, Trichet V, Soussi T, Wuillème S, Richebourg S, Magrangeas F, Ifrah N, Campion L, Traullé C et al. Mutations in TP53 are exclusively associated with del(17p) in multiple myeloma. Haematologica 2010; 95:1973-76; PMID:Can't; http://dx.doi.org/10.3324/haematol.2010.023697
- Bush JA, Cheung KJ Jr, Li G. Curcumin induces apoptosis in human melanoma cells through a Fas/receptor/caspase-8 pathway independent of p53. Exp Cell Res 2001; 2:305-14; PMID:11716543; http://dx.doi.org/10.1006/excr.2001.5381
- Golombick T, Diamond TH, Manoharan A, Ramakrishna R. Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: A randomized, double-blind placebo controlled cross-over 4g study and an open-label 8g extension study. Am J Hematol 2012; 5:455-60; PMID:11716543 ; http://dx.doi.org/10.1002/ajh.23159
- Moreaux J, Klein B, Bataille R, Descamps G, Maïga S, Hose D, Goldschmidt H, Jauch A, Rème T, Jourdan M et al. A high-risk signature for patients with multiple myeloma established from the molecular classification of human myeloma cell lines. Haematologica 2011; 4:574-82; PMID:21173094; http://dx.doi.org/10.3324/haematol.2010.033456
- Gomez-Bougie P, Wuillème-Toumi S, Ménoret E, Trichet V, Robillard N, Philippe M, Bataille R, Amiot M. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res 2007; 67:5418-24; PMID:17545623; http://dx.doi.org/10.1158/0008-5472.CAN-06-4322
