Tumor progression requires the activation of neovascularization—angiogenesis—a process orchestrated by both tumor and stromal cells within the tumor mass. Therefore, targeting this process demonstrates an impact in tumor growth and progression and is currently used as a therapeutic arm to fight certain types of cancer. The initial hypothesis was that the anti-angiogenic therapy would not induce resistance, because it is specific against endothelial cells that do not exhibit genetic instability. However, experimental and clinical evidence has shown that the benefits of this therapy are often mild and transient.Citation1 Therefore, resistance to anti-angiogenic therapies does indeed occur, involving both tumor cells and stromal components with different relative contribution in each cancer type.
Among tumor responses to therapy, it is essential to distinguish between refractoriness, sometimes called intrinsic resistance, and acquired resistance that emerges overtime. Intrinsic resistance is characterized by indifference on the part of the tumor to anti-angiogenic therapy, as there is no response to treatment. This type of resistance has been described in patients treated with either antibodies (bevacizumab), small molecule TKIs (sunitinib, sorafenib…) or traps (aflibercept), and is typically characterized by tumors that continue to grow in the face of therapy.Citation1
Mechanistically, intrinsic resistance typically includes tumors that are capable of expressing multiple pro-angiogenic factors upfront, from the beginning of their progression. In these tumors, anti-VEGF/R therapy is not fully effective because it is not able to fully block all the variety of factors that promote angiogenesis. Another molecular mechanism of intrinsic resistance is the dis-regulation of the HIF pathway that consequently produces overexpression of several pro-angiogenic genes, thereby reducing the efficacy of anti-VEGF/R angiogenic therapy.Citation2
Therefore, overcoming anti-angiogenic resistance is a crucial step in the future development of anti-angiogenic therapies. Several strategies have been postulated to fight resistance, including multi-pathway inhibitors or multi-combination of anti-angiogenic drugs that target different pathways that can revert resistance.
In the latest issue of OncoTarget, Mésange and colleaguesCitation3 compared 2 colorectal (CRC) cell lines with a clearly distinct sensitivity to anti-VEGF antibody, bevacizumab (Bev). On one hand, the Bev-sensitive DLD1 CRC cells and their derived tumors respond to Bev with decreased vessel density and impaired tumor growth. On the other hand, the Bev-resistant HT-29 CRC cell line shows a non-significant reduction of vessel density and a minor reduction of tumor size. Furthermore, the authors describe a very distinct hypoxia tolerance between these 2 cell types, with the Bev-resistant cells being significantly more tolerant to survive in hypoxic conditions than the Bev-sensitive ones. This survival phenotype could be associated to mTOR upregulation, as Bev-resistant cells showed increased accumulation of phospho-S6 as a readout for mTOR activity. Therefore, the study from Mésange et al. describes a dual mechanism of resistance to Bev in these CRC cells, implicating both tumor-extrinsic effects (vascular trimming resistance), and tumor-intrinsic effects (hypoxia tolerance and prosurvival signaling) (, left and middle).
Figure 1. Anti-VEGF vs Multi-angiokinase inhibition of CRC. Bevacizumab sensitive (left) and resistance (middle) responses are depicted, together with Nintedanib effects (right) on CRC tumor cells and vasculature. Resistance pathways (black); inhibited pathways (red).
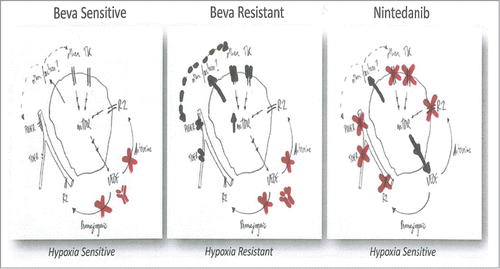
In an attempt to therapeutically impinge on the resistance to Bev in these CRC cells, Mésange et al. evaluated a multi-target angiokinase small molecule inhibitor, nintedanib (BIBF1120), which inhibits VEGFR1,2,3, FGFR1,2,3, PDGFRa,b, and Flt3.Citation4 This inhibitor exhibited anti-tumor efficacy in both the Bev-sensitive DLD1 tumors and the Bev-resistant HT29 tumors, suggesting its multi-kinase inhibitory spectrum allowed for broader efficacy in a variety of CRC tumor types. Indeed, Nintedanib exhibited potent anti-angiogenic efficacy with vascular trimming and increased tumor necrosis in both tumor types, but more importantly, it also exerted anti-tumor direct effects in blocking proliferation and survival in both Bev-sensitive and resistant tumors. Therefore, Nindetanib demonstrates pleiotropic anti-tumoral effects, targeting both in the vascular (stromal) components (antiangiogenic effects) together with tumor cell components (direct antitumoral effects) (, right).
Overall, the data presented in Mésange et al. together with previous studiesCitation5 exemplify the benefit of targeting several pro-angiogenic pathways with multi-target angiokinase inhibitors that are currently being developed. Nevertheless, several issues are to be raised and addressed. First, a secondary unwanted effect of the multi-targeting drugs can be the possible increase of off-target effects that could lead to more clinical toxicity when used in the real life setting in patients. Indeed, there is evidence of an increased toxicity profile of multi-target vs mono-target drugs. Secondly, several preclinical studies have demonstrated enhanced benefit of multi-target antiangiogenic drugs when compared to mono-target ones.Citation3,5 Nevertheless, a proportion of initially Bev-sensitive patients could benefit of a mono-target treatment without having to cope with the added toxicity of an upfront multi-targeting. Some of them remain responsive even beyond progression to a first line combination of chemotherapy and Bev. In addition, a number of multi-target angiokinase inhibitors have failed in the clinical setting in first and second line in combination with chemotherapy.Citation6 This combination app-roach may have failed for differentreasons, including different spectra of multi-target angiokinase inhibition and another compound of this class, regorafenib as a single agent, has shown significant yet limited efficacy in the third or fourth line setting in patients with tumors resistant to Bev.Citation7 Therefore, patient selection would be key in this situation in order to select patients that would respond to an anti-VEGF/R monotherapy from the patients that would need a multi-target angiokinase therapy or those who would not respond to any currently available antiangiogenic therapy. Obviously, many efforts are currently underway to unveil these elusive biomarkers of response to anti-angiogenic therapies, both for patient selection and for mono-target or multi-target antiangiogenic treatment and for designing more active combinations.
References
- Rini BI, et al. Lancet Oncology 2009; 10:992-1000; PMID:19796751; http://dx.doi.org/10.1016/S1470-2045(09)70240-2
- Rini BI. Clin Cancer Res 2010; 16:1348-54; PMID:20179240; http://dx.doi.org/10.1158/1078-0432.CCR-09-2273
- Mésange P, et al. Oncotarget 2014; Jan 20. [Epub ahead of print] 5(13):4709-21; PMID:25015210
- Hilberg F, et al. Cancer Res 2008; 68(12):4774-82; PMID:18559524; http://dx.doi.org/10.1158/0008-5472.CAN-07-6307
- Allen E, et al. Clin Cancer Res 2011; 17(16):5299-310; PMID:21622725; http://dx.doi.org/10.1158/1078-0432.CCR-10-2847
- Tabernero J, et al. Clin Cancer Res 2013; 19(9):2541-50; PMID:23532888; http://dx.doi.org/10.1158/1078-0432.CCR-13-0107
- Grothey A, et al. Lancet 2013; 381(9863):303-12; PMID:23177514; http://dx.doi.org/10.1016/S0140-6736(12)61900-X
