Obesity, which results from imbalanced energy intake and energy expenditure, is well recognized as a key risk factor for various metabolic and cardiovascular diseases as well as certain types of cancer. Obesity is characterized by excessive expansion of white adipose tissue (WAT) and impaired thermogenic function in brown adipose tissue (BAT). In addition to WAT and BAT, recent studies reveal the presence of a subset of adipocytes in WAT that, in response to environmental or hormonal stimulation, become “brown-like” or “beige” cells. Over the past decade, great progress has been made on our understanding of the anti-obesity benefits of the beige cells and the mechanisms regulating the “beigeing” process at the transcriptional level.Citation1 However, the upstream signaling pathways regulating the transcriptional machinery involved in the regulation of adipogenesis, thermogenesis, and adipocyte function remain to be fully characterized.
Accumulating evidence has been obtained in recent years to indicate that the mechanistic target of rapamycin complex 1 (mTORC1) plays a key role in the regulation of fat metabolism. Inhibition of the mTORC1 signaling pathway promotes triacylglycerol lipolysis, increases free fatty acid release, enhances uncoupling protein 1 (UCP1) expression and thermogenesis, and improves obesity, inflammation and insulin resistance.Citation2
The precise mechanisms regulating mTORC1 signaling and action, especially in vivo, remain elusive. Two recent studies showed that mTOR directly phosphorylates Grb10 (Growth factor receptor-bound protein-10),Citation3,4 a Src homology 2 (SH2)- and pleckstrin homology (PH)-domain containing adaptor protein originally identified by us and others as a negative regulator of insulin and IGF-1 signaling.Citation5 Grb10 expression in adipose tissues is greatly induced by cold exposure. In addition, fat-specific knockout of Grb10 suppressed lipolytic and thermogenic gene expression, reduced energy expenditure, and exacerbated diet-induced obesity and insulin resistance,Citation6 uncovering Grb10 as an important regulator of adipose tissue metabolism and energy homeostasis.
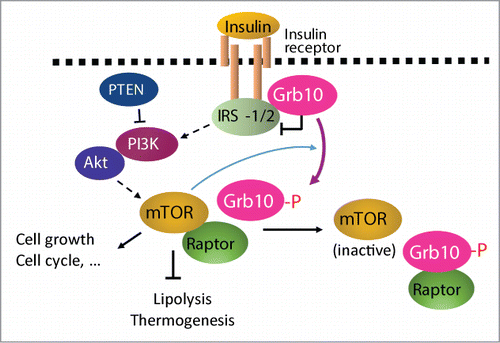
How does Grb10 regulate lipid metabolism and thermogenesis in vivo? Grb10-deficiency led to a marked increase in S6 kinase phosphorylation in adipose tissues,Citation6 suggesting that Grb10 may promote lipolysis and thermogenesis by negative regulation of the mTORC1 signaling pathway. Consistent with this, overexpression of Grb10 inhibits not only insulin- but also leucine-stimulated S6 kinase phosphorylation,Citation6 revealing that Grb10 is able to inhibit the mTORC1 signaling pathway via a PI3K/Akt-independent mechanism. Grb10 interacts with both the insulin receptor and raptor, a positive regulator of mTORC1, and the interaction is inhibited or stimulated, respectively, by mTOR-mediated phosphorylation, demonstrating a feedback mechanism to regulate the mTORC1 signaling pathwayCitation6 and (). The identification of Grb10 as a critical regulator of mTORC1 signaling, lipid metabolism, thermogenesis, and the development of beige fat should shed new light on the development of pharmacological tools to treat obesity and obesity-induced metabolic diseases.
Figure 1. Feedback regulation of PI3K/Akt/mTORC1 signaling by Grb10. Grb10 inhibits PI3K/Akt/mTORC1 pathway by binding to tyrosine phosphorylated insulin receptor (IR). Sustained activation of the mTORC1 leads to Grb10 phosphorylation at Ser501/503, which promotes Grb10 dissociation from the IR to interact with raptor, an activator of mTOR. The interaction of Grb10 with raptor prevents raptor from binding to mTOR and thus suppresses mTORC1 signaling. By phosphorylation-dependent interaction with IR and raptor, Grb10 dynamically regulates the signaling pathways downstream of IR, thus selectively regulating mTORC1-mediated biological events such as lipid metabolism, cell growth, and cell cycle.
It should be noted that in addition to functioning as a central regulator of protein synthesis and lipid metabolism, the mTORC1 signaling pathway also plays key roles in many other important biological processes such as cell growth, proliferation, and cell cycle.Citation7 Thus, it is not surprising that this signaling is subjected to tight regulation of multiple oncogenes and tumor suppressors. Comprehensive meta-analysis of available microarray data reveals that Grb10 levels are decreased in many tumor types compared to normal tissue counterparts.Citation4 In addition, there is a significant negative correlation between Grb10 and PTEN expression in tumor samples.Citation4 Thus, Grb10 deficiency, which results in abnormal PI3K/Akt/mTORC1 activation, might provide growth and survival advantages for tumor cells, leading to cancer development. The finding that Grb10 negatively regulates the PI3K/Akt/mTORC1 signaling pathway via a phosphorylation-dependent mechanism suggests that Grb10 might be a tumor suppressor that is regulated by mTORC1. Since abnormal activation of the PI3K/Akt/mTORC1 signaling pathway may promote tumors or cancer, enhancing the expression levels of Grb10 may provide a promising new treatment in cancer therapy. Further investigations on tissue-specific roles of Grb10, its expression, and the mechanisms of Grb10 action should thus guide new approaches for obesity-related metabolic diseases and cancer.
References
- Rosen ED, et al. Cell 2014; 156(1-2):20–44; PMID:24439368; http://dx.doi.org/10.1016/j.cell.2013
- Lamming DW, et al. Cell metabolism 2013; 18(4):465–9; PMID:23973332; http://dx.doi.org/10.1016/j.cmet.2013.08.002
- Hsu PP, et al. Science 2011; 332(6035):1317–22; PMID:21659604; http://dx.doi.org/10.1126/science.1199498
- Yu Y, et al. Science 2011; 332(6035):1322–6; PMID:21659605; http://dx.doi.org/10.1126/science.1199484
- Lim MA, et al. Front Biosci 2004; 9:387–403; PMID:14766376.
- Liu M, et al. Cell metabolism 2014; 19(6):967–80; PMID:24746805; http://dx.doi.org/10.1016/j.cmet.2014.03.018
- Kelsey I, et al. Science signaling 2013; 6(294):pe31; PMID:24065143; http://dx.doi.org/10.1126/scisignal.2004632
