Abstract
The perinatal (prenatal and early neonatal) period is a critical stage for hypothalamic programming of sexual differentiation as well as for the development of energy and metabolic homeostasis. We hypothesized that neonatal treatment with antidiabetic drug biguanide metformin would positively modify regulation of growth hormone – IGF-1 – insulin signaling pathway slowing down aging and improving cancer preventive patterns in rodents. To test this hypothesis male and female 129/Sv mice were s.c. injected with metformin (100 mg/kg) at the 3rd, 5th and 7th days after birth. Metformin-treated males consumed less food and water and their body weight was decreased as compared with control mice practically over their entire lifespan. There were no significant differences in age-related dynamics of food and water consumption in females and they were heavier than controls. The fraction of mice with regular estrous cycles decreased with age and demonstrated a tendency to decrease in the females neonatally treated with metformin. Neonatal exposure to metformin practically failed to change the extent of hormonal and metabolic parameters in blood serum of male and female mice. In males, neonatal metformin treatment significantly increased the mean life span (+20%, P < 0.05) and slightly increased the maximum life span (+3.5%). In females, the mean life span and median in metformin-treated groups were slightly decreased (−9.1% and −13.8% respectively, P > 0.05) in comparison to controls, whereas mean life span of last 10% survivors and maximum life span were the same as in controls. Almost half (45%) of control male mice and 71.8% male mice neonatally exposed to metformin survived up to 800 d of age, the same age was achieved by 54.3% of mice in control female group and 30% of metformin-treated females (P < 0.03). Thus, neonatal metformin exposure slows down aging and prolongs lifespan in male but not in female mice.
Introduction
Findings in GHRKO (growth hormone receptor knockout), Ames and Snell dwarfs and other GH-related mouse mutants provided evidence that absence of a hormone's effects can produce major benefits in terms of health and life expectancy.Citation1,2 This leads to a conclusion that the normal levels and physiological actions of GH are not optimal for disease-free survival or for longevity. Since GH is key determinant of circulating levels of insulin-like growth hormone-1 (IGF-1) and it also increases insulin levels as a secondary consequence of its anti-insulinemic actions, conclusions concerning GH and normal aging extend to the roles of IGF-1 and insulin in this process. It is important to emphasize that the negative association of somatotropic (GH, IGF-1) signaling and longevity discovered in mutant, gene knockout and transgenic mice also applies to genetically normal mice as well as to other species. Growth rate and adult body size, key markers of GH and IGF-1 actions, as well as plasma IGF-1 levels are negatively correlated with lifespan in comparisons with mice from different stocks or inbred strains, as well as with individual animals from a genetically heterogeneous population.Citation2,3
However, even with this evidence it may be difficult to envisage how physiological effects of hormones within normal ranges of their concentration can be detrimental for something as fundamental as longevity. The most likely explanation for this paradox can be found in the concept of antagonistic pleiotropy which emerged from analysis of genetic control of aging.Citation4 Antagonistic pleiotropy explains how genes which exert positive effects on growth, development, sexual maturation and fertility, key elements of the evolutionary fitness, i.e., probability to produce offspring will be selected for and persists in the population even if they have detrimental effects during later stages of life history. Along with other anabolic hormones, GH and its actions fit well with this concept of co-existence of early beneficial effects with late detrimental ones. In juveniles, GH promotes somatic growth, sexual maturation and various aspects of gonadal function, while in adults it increases the risk of cancer, insulin resistance and diabetes. Somatotropic signaling would not have been suppressed or eliminated by natural selection on the basis of these detrimental effects because the force of natural selection declines with age and is presumably negligible during the post-reproductive period, and in the sense of evolutionary fitness promotion of growth, development and fertility far outweigh the risks of late life disease including cancer.Citation2
It have been shown that perinatal (prenatal and early neonatal) period is a critical stage for sexual dimorphism differentiation as well as for the development of energy balance and metabolic circuit.Citation5-7 Barker's hypothesis of links between poor perinatal environment such as undernutrition, and increased susceptibility to diseases has attracted substantial attention during the last years.Citation8 We hypothesized that early life metformin treatment might have long-term late effects, and in this paper we present results show that neonatal metformin exposure to have a significant sex dependent impact on life span, longevity and tumorigeneis in mice.
The concept of calorie resriction (CR) mimetics involving pharmacological interventions that produce physiological and anti-aging effects similar to CR is now being intensively explored.Citation9-12 It was suggested to use biguanide antidiabetic drugs (phenformin, buformin and metformin) as a potential anti-aging treatment.Citation13-19 The antidiabetic drugs were observed to reduce hyperglycemia and produce the following effects: improved glucose utilization; reduced free fatty acid utilization, gluconeogenesis, blood serum lipids, insulin and IGF-1, and reduced body weight both in humans and experimental animals.Citation13-19 The use of phenformin in humans has been limited the last 2 decades because of a potential association with lactic acidosis. Widely used antidiabetic biguanide, metformin (dimethylbiguanide) do not increase risk for lactic acidosis or for increased lactate levels in type 2 diabetes,Citation20 but has some adverse effects, including renal insufficiency in some patients,Citation21 vitamin B12 deficiency,Citation22 and gastrointestinal disturbances.Citation23 In female transgenic HER-2/neu mice, metformin was slown to slow down aging and tumor developmentCitation24,25 However in female outbred SHR mice metfromin significantly increased life span but failed to inhibit spontaneous tumorigenesis.Citation26 Smith et al.Citation27 have shown that treatment with metformin did not influence life span of male F344 rats. Recently we described sex dependent effects of meformin on some parameters of aging, life span and spontaneous tumorigenesis in inbred 129/Sv mice.Citation28 In the present paper we show dependant differences in effects of neonatal administration of metformin in the same mouse strain.
Results
Age-related body weight dynamics
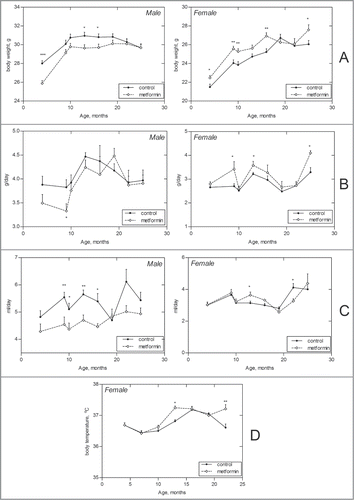
Male 129/Sv mice were heavier than females over their entire life span both in control group and in the one neonatally treated with metformin groups (). The body weight of males exposed to metformin was lower than that males in control group practically during the whole period of observation whereas females treated with metformin had more fast body weight gain and were heavier than those in control group ().
Age-related dynamics of food and water consumption
Food consumption gradually increased between the 4th and 13–20th months of age in males of both control and metformin-treated groups and then slightly decreased. However, metformin-treated males consumed less food and drink less water than control mice practically over their entire life span. At the same time there was increase in food and water consumption in females, significant at some ages ().
Age-related dynamics of body temperature
The body temperature was measured only in females. It decreased between 6th and 9th month of life and was practically at the same level until the age of 24 months. Temperature was elevated in metformin-treated groups as compared to controls at the age of 15 months but was lower than that in control group at the age of 24 months ().
Age-related dynamics of estrous function in mice
The length of estrous cycles in the control mice slightly increased at the age of 19 months whereas in mice neonatally exposed to metformin it did not significantly change with age (). The fraction of mice with regular estrous cycles decreased with age and revealed a tendency to decrease in the females treated with metformin as compared to the non-treated one.
Table 1. Effect of neonatal treatment with metformin on parameters of estrous function in female 129/Sv mice
Metabolic and hormonal parameters in mice treated with metformin
The level of serum insulin was lower whereas the level of serum IGF-1, cholesterol and nitric oxide was increased in 3-month-old control females in comparison to control males ().
Table 2. Some metabolic and hormonal parameters in control male and female 129/Sv mice at various age
No age-related difference was revealed in the levels of glucose, total cholesterol, triglycerides, insulin and some other metabolic and hormonal parameters between 3 and 9-month-old male control mice but levels of malonic dialdehyde, IGF-1 and nitric oxide (NO) in 9-month-old control males were higher in comparison to these in 3-month-old males. In 9-month-old control females the level of malonic dialdehyde and IGF-1 were increased as compared with 3-month–old females whereas the level of cholesterol was decreased. Neonatal exposure to metformin practically failed to change the extent of hormonal and metabolic parameters in blood serum of male and female mice ().
Table 3. Effect of neonatal metformin treatment on some metabolic and hormonal parameters in 3-month-old male and female 129/Sv mice neonatally treated and non-treated with metformin
Survival and longevity of 129/Sv mice
Survival dynamics in control and metformin-treated male and female mice are shown in and in . It is worthy to note that there is no difference in life span distributions between male and female controls (P-value is 0.571, χ2 = 0.3). Metformin treatment evidently produced somewhat different effects on survival of male and female mice, since the difference in life span distributions of males and females under metformin action became statistically significant (P-value is 0.0474, χ2 = 3.9).
Table 4. Effect of neonatal treatment with metformin on survival of 129/Sv mice
Figure 2. Survival (A) and tumor yield (B) curves in male and female 129/Sv mice neonatally treated and non-treated with metformin.
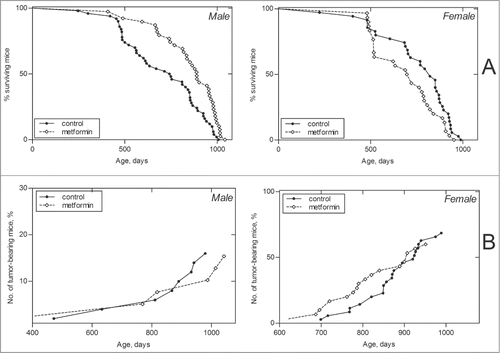
In males, metformin treatment significantly increased the mean life span of all mice (+20%, P < 0.05) but change the mean life span of long-living individuals (last 10% of survivors), and slightly increased the maximum life span (+3.5%; ). In females, the mean life span and median of this parameter in metformin-treated animals were slightly decreased (−9.1% and −13.8%, respectively) in comparison to the controls, whereas the mean life span of last 10% survivors and maximum life span were the same as in the control (). However, it deserves mentioning that until the age of 800 d 45% of control males and 71.8% of males neonatally exposed to metformin mice survived, and 54.3% of mice in the control female group and 30% of metformin-treated females (P < 0.03; Fischer's exact test).
Table 5. Parameters of the life span of male 129/Sv mice neonatally treated and non-treated with metformin
Estimated parameters of the Gompertz model for the group of metformin treated male mice show significant decrease of initial mortality (β) and mortality rate doubling time (MRDT). Metformin treatments increased mean life span in the group of males (long living ones as well). Estimated parameters of the Gompertz survival model for the group of metformin treated females show significant increase in initial mortality and MRDT. Parameter α of the Gompertz model, which is interpreted as the rate of aging, was 1.5 times lower in male control group as compared to females, and increased by 1.7 times in males subjected to metformin treatment vs. control males, whereas in females it was decreased by 1.2 times ().
According to the log-rank test the conditional life span distributions of 129/Sv mice in early life treated with metformin was significantly different from the control population. The difference is much more significant in male mice (χ2 = 11.8; p = 0.000601) than in females (χ2 = 3.7; p = 0.0555). According to the estimated parameters of the Cox's regression modelCitation29 neonatal metformin treatments increased the relative risk of death in female mice and decreased it in males compared to the respective intact groups. The changes are significant in males and females. Cox's regression model parameters were for males: β = −0.767; exp(β) = 0.464; se(β) = 0.228; p = 0.00075; and for females: β = 0.246; exp(β) = 1.28; se(β) = 0.128; p = 0.056.
Spontaneous tumor development in 129/Sv mice
The dynamics of age-related increase in spontaneous tumor development is represented in . The mean life span of tumor-bearing mice was increased by 4% in males exposed to metformin and was unchanged in females (). According to the log-rank test the conditional life span distributions of tumor-bearing 129/Sv mice treated with metformin differed significantly from the control groups. In males: χ2 = 1.8; p = 0.177; in females: χ2 = 3.2; p = 0.0739. According to the estimated parameters of the Cox's regression model metformin treatment significantly increased the relative risk of death in female mice compared to the respective control group: β = 0.286; exp(β) = 1.33; se(β) = 0.161; p = 0.076.
Table 6. Parameters of tumorigenesis in 120/Sv mice neonatally treated and non-treated with metformin
According to the long-rank testCitation29 there were no significant differences in age-related distributions of the total tumors occurrence in control and metformin-treated groups of both sexes. The first tumor-bearing male mouse in the metformin-treated group died at the age of 408 d and at the 472 d in the control male group. Total tumor incidence in effective control male mice (survived by the time of the death from the first tumor in the experiment) was 14.6% and 15% in the metformin-treated animals (). There was no significant difference in the incidence of malignant or benign tumors of any localization between the control and metformin-treated male mice groups.
In the female mice the total tumor incidence was similar in the control and metformin-treated groups. Benign vascular tumors developed most frequently in female 129/Sv mice (), in line with oncological characteristics of this murine strainCitation30 and its incidence did not differ between metformin and control groups. There was no significant difference in the incidence of any other tumors between mice treated or not treated with metformin. In female tumor-free mice metformin significantly decreased the mean life span of long lived animals. In tumor-free males metformin treatments significantly increased mean life span of all animals as well as mean life span of long lived mice compared to the control tumor-free subgroup. Life-span distributions of tumor-free males subjected to metformin treatment differed significantly from the respective control group (χ2 = 9.7; p = 0.00184). According to the estimated parameters of the Cox's regression modelCitation31 metformin treatment significantly decreased the relative risk of death in tumor-free male mice compared to the control group: β = – 0.74; exp(β) = 0.477; se(β) = 0.222; p = 0.0023).
Discussion
Back in 2010 we have shown for the first time sex differences in metformin effect on survival, life span and spontaneous carcinogenesis in 129/Sv mice.Citation28 The long-term treatment of 129/Sv mice with metformin (100 mg/kg in drinking water) started at the age of 3 months has slightly modified the food consumption failing to influence body weight dynamics, decreased the mean life span of male mice by 13.4% and slightly increased the mean life span of female mice (by 4.4%). Metformin treatment failed to influence spontaneous tumor incidence in male 129/Sv mice, decreased the incidence of malignancies in female mice by 3.5 times. We observed inversion of sex response to metformin treatment when the drug was administered neonatally (). The reasons for these differences are dubious at present. There are several possible explanations for these observations. One of them could relate to fundamental differences in mechanism of aging in males and females. It is difficult to explain these differences by the dose and route of administration variations. It was shown practically similar patterns of biodistribution ofCitation14 C-metformin in mice in 1 hour after a single intraperitoneal injection or gavage, and in mice allowed to drink during 5 d oral metformin dissolved in tap water. However serum levels of metformin was higher in mice received the drug with drinking water during 5 d than that after a single treatment.Citation31 It seems clear that it is impossible to administrate metformin to neonatal mice with drinking water – the pups use dam's milk.
Table 7. Effect of gender and time of start metformin treatment on biomarkers, life span and tumorigenesis in 129/Sv mice
Another possible reason of observed differences related to sex peculiarities of physiology in the targets of drugs, including metformin. This question needs a special discussion. We believe neonatal treatment with metformin to have reprogrammed hypothalamic control of energy and reproductive homeostasis in different ways in males and females. Two sets of observations should be taken into consideration for the assessment of this problem.
Firstly, it was shown that there is an impairment of glucose tolerance and elevated fasting glucose level in adult male offspring of mice dams treated with metformin (300 mg/kg) from the 1st to the 18th day of pregnancy whereas for female offspring this effect was not observed.Citation32 Body weight was increased in offspring of female mice prenatally exposed to metformin during the time when mice were kept both at regular diet and at the high fat diet whereas in male offspring body weight was increased at the high fat diet period. The gene set enrichment analysis has shown that respiratory electron transport is enriched in metformin exposed male offspring. The authors suggested that prenatal metformin exposure reprogrammed central regulation of energy balance in the body.Citation32
Secondly, it is worthy to note that early life growth hormone treatment shortened longevity and decreased cellular stress resistance in long-lived Ames dwarf mice.Citation33 These observations strongly suggest that deficiency of growth hormone in dwarf mice during development is important for their exceptional longevity and that diminished levels of GH signal during early development are critical to the longevity.
Several years ago, it was originally suggested to use antidiabetic biguanides as mimetics of calorie restriction (CR) and a potential anti-aging treatment.Citation13 In a number of studies it was shown that administration of antidiabetic biguanides (phenformin, buformin and metformin) started at adult age increased life span and suppressed spontaneous and induced tumorigenesis.Citation2,Citation34–38 It is worthy to note that male and female adult 129/Sv mice demonstrated divergent reaction to the same dose of metformin. Metformin failed to increase life span of male F344 rats.Citation27
Inhibitor of mammalian target of rapamycin (mTOR) increased survival of females to a larger extent than in male hybrid mice.Citation39 It is worthy to note that the mean life span extension was observed in female mice (+20.4%) but was not increased in male S6K1−/− ones.Citation40 Deletion of ribosomal S6 protein kinase 1 (S6K1), a component of the nutrient-responsive mTOR signaling pathway, led to increased life span in mice and to resistance to age-related pathologies, such as bone, immune, and motor dysfunction and loss of insulin sensitivity.Citation40 Deletion of S6K1 induced gene expression patterns similar to those seen in CR or with pharmacological activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK), a conserved regulator of the metabolic response to CR. The mean life span of oldest 10% survivors and maximum life span were also increased only in females. There was no difference in the incidence of macroscopic tumors in S6K1−/− and wild type mice.
Comparison of effects of metformin on various biomarkers of aging in different strains of mice shows notable similarity in observed patterns.Citation34,35 It was demonstrated that the body weight and food consumption were unchanged or slightly decreased at some periods of life in metformin-treated rodents. The body temperature was similar in control and metformin-treated mice. In females of 3 strains (129/Sv, HER-2/neu and SHR) exposed to metformin the slowing down of age-related disturbances in estrus function has been detected. Similar to women with polycystic ovary syndrome, metformin improves menstrual regularity, leading to spontaneous ovulation, and enhances the induction of ovulation with clomiphene citrate.Citation41
Treatment with metformin started after puberty failed to influence metabolic parameters inmale 129/Sv mice and resulted in some improvements in female SHR and HER-2/neu mice. Metformin inhibited tumorigenesis in female 129/Sv and HER-2/neu mice and did not affect it in male 129/Sv and female SHR mice. In adult female 129/Sv mice treatment with metformin inhibited development of malignant tumors and increased the total incidence of benign angiogenic tumors. These data correspond to observations that metformin can increase VEGF expression, intratumoral microvascular density and reduces necrosis thus promoting the angiogenic phenotype and increasing tumorigenic progression.Citation42
Martin-Montalvo et al.Citation38 supplied male C57BL/6 mice with ad libitum diet with supplementation of 0.1% (1,000 ppm) or 1% (10,000 ppm) of metformin starting from the age of 54 weeks for the remainder of their lives. The mean life span of mice treated with 0.1% of the drug in diet was increased by 5.83% as compared to the relevant control group, whereas the dose 1% was toxic and reduced the mean life span by 14.4%. Diet supplementation with metformin increased lifespan by 4.15% in another strain of mice, B6C3F1. No numerical data on maximal lifespan of mice of any group were presented in the paper. It was observed that C57BL/6 mice were lighter than those in control group between the age of 72 to 90 weeks but heavier than these mice at the age of 124 weeks. These effects were not observed in male B6C3F1 mice. There were no significant differences in pathologies observed in both strains of mice fed with diet including 0.1% metformin. However as calculated from the data presented by these authors in their Table S1, diet with 1% metformin led to significant reduction of liver cancers incidence (3.3% in the metformin group and 26.5% in control group, P < 0.001). Male C57BL/6 mice given metformin have lower rates of cataract. The authors stressed that treatment with metformin mimics some of the benefits of calorie restriction such as improved physical performance, prevention of the onset of metabolic syndrome: improvement of glucose-tolerance test, increase of insulin sensitivity, and reduction of low-density lipoprotein and cholesterol levels without a decrease in caloric intake. At the molecular level metformin increased AMP-activated protein kinase activity and antioxidant protection, resulting in reduction of both oxidative damage accumulation and chronic inflammation.Citation43-45 Metformin also exerts CR-like genomic and metabolic responses which were interpreted as induction of association with longevity pathways in mice.
Very interesting hypothesis on sex difference in life span and key role of mTOR in this fenomen has been proposed by Blagosklonny's papers.Citation46–48 It was stressed that aging is driven by the mTOR pathway, which is stimulated by food, growth factors, including insulin and IGF-1, androgens and some other factors. Females are have less body size and weight than males that could be related to a higher activity of mTOR pathway in males. As it was shown by Leontieva et al.,Citation46 male C57Bl/6Ncr mice had significantly higher fasted serum insulin levels and higher insulin response to re-feeding when compared with females. The serum insulin level was also higher in our 3-month-old male 129/Sv mice than in females (). We also observed the decrease in the sex difference in insulin level in 9-month-old male and female 129/Sv mice as compared with the 3-month-old animals. Leontieva et al.Citation46 have shown that both pS6 (a marker of mTORC1) and p-AKT (a marker mTORC2) were more active in young males as compared with young females. These observations can explain robust growth and faster aging in male species. Reprogramming of the regulation of mTOR pathway in females induced by neonatal treatment with metformin leads to more fast growth of young females and faster aging of them in comparison with intact control female mice.
Our results alongside with recent findings of mTOR signaling pathway involvement in regulation of agingCitation44,46 and evidence of significant life span extension of mammals with rapamycin and calorie restrictionCitation10,12,18,39 suggest that mimetic of calorie restriction, anti-diabetic biguanide metformin may be rapidly contemplated for pharmacological intervention at a population level taking in consideration sex and age-associated peculiarities of this drug's effect.
Material and Methods
Animals
All studies was planned and performed according to the regulations of European Convention for the Protection of Vertebral Animals Used for Experimental and Other Scientific Purposes. CETS No. 123 and were approved by the N.N. Petrov Research Institute of Oncology Ethic Committee. During the study, animals were under veterinarian monitoring for any sigтs of morbidity and all efforts were taken for alleviation suffering. Inbred 129/Sv male and female mice were breed at the animal facility of N.N. Petrov Research Institute of Oncology.
Experimental design
For mating, a male mouse was introduced to female one before the onset of the dark time and removed from the cage in the morning. The offspring were subjected to 3 subcutaneous injections with metformin (1,1-Dimethylbiguanide hydrochloride, MP Biomedicals, France) on the 3rd, 5th and 7th days after birth in a single dose of 100 mg/kg of body weight. Metformin was dissolved ex tempore in sterile bidistillated water (“DALKHIMPHARM,” Russia) and administered in a volume of 0.1 ml. Control offspring was treated with the solvent without metformin according to the same schedule. Pups were with dams until the age of 4 weeks and then males and females were separately kept 5–7 in polypropylene cages (30 × 21 × 10 cm) under standard light/dark regimen (12 hours light :12 hours darkness) at 22 ± 2°C, and received standard laboratory chowCitation49 and tap water ad libitum. Once a week all mice were palpated for detection of tumor mass appearance. Once a month all mice were weighed and, simultaneously, the amount of consumed food and water was measured, and the rate of the consumed water (ml) and food (g) per mouse were calculated. Once in every 3 months, daily for 2 weeks vaginal smears of the females were cytologically examined to estimate the estrous function. In the same period, rectal body temperatures of female mice were measured with an electronic thermometer, TPEM (KMIZ, Russia). At the age of 3 and 9 months some number of male and female mice from the control group and the group treated with metformin on the 3rd, 5th and 7th days of postnatal life at the age of 3 months were sacrificed by decapitation after overnight starvation. Samples of serum were obtained and stored at the −20°C for subsequent analyses. Other animals were observed until their natural deaths. The date of each death was registered, and the mean life span, the age at which 90% of the animals died, and the maximum life span were estimated.
Biochemical parameters
Metabolic parameters were assessed in blood serum. Glucose concentration was calculated electrochemically by means of express analyzer (i-STAT, Abbot) using CG8+ cartridges. Hemoglobin (HGB) and glycosylated hemoglobin (gHGB) concentration were determined electrophoretically using Becman Coulter, Inc.. reagent kits. Total cholesterol, triglycerides’ and malonic aldehyde (MDA) concentrations, superoxide-dysmutase (SOD) and catalaze activities were processed using Stat Fax 3300 analyzer and Spinreact reagent kits according to standard instructions. Threeiodine-tyronin (T3), thyroxine (T4), vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF-1) and nitrogen oxide (NO) concentrations were assessed by means of immune enzyme assay (ELISA) using Cusabio and R&B Systems reagent kits according to standard procedures.
Pathomorphological examination
All animals were autopsied. All tumors, as well as tissues and organs with suspected tumor development were excised and fixed in 10% neutral formalin. After routine histological processing the tissues were embedded in paraffin. Five–7 μm thin histological sections were stained with haematoxylin and eosine and were microscopically examined. Tumors were classified according to International Agency for Research on Cancer recommendations.Citation50
Statistics
Experimental results were statistically processed by methods of variation statistics with the use of STATGRAPH statistic program kit. The significance of the discrepancies was estimated according to the Student t-criterion, Fischer exact method, χ2, and non-parametric Wilcoxon-Mann-Whitney. Student-Newman-Keuls method was used for all pairwise multiple comparisons. Coefficient of correlation was estimated by Spearman method.Citation51 Differences in tumor incidence were evaluated by the Mantel-Haenszel log-rank test. Parameters of Gompertz model were estimated using maximum likelihood method, non-linear optimization procedureCitation52 and self-written code in ‘Matlab’; confidence intervals for the parameters were obtained using the bootstrap method.Citation53
For experimental group the Cox regression modelCitation29,63 was used to estimate relative risk of death and tumor development under the treatment compared to the control group: h(t,z) = h0(t) exp(zβ), where h(t,z) and h0(t) denote the conditional hazard and baseline hazard rates, respectively, β is the unknown parameter for treatment group, and z takes values 0 and 1, being an indicator variable for 2 samples − the control and treatment group.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are very thankful to Dr. TV Pospelova for critical reading of the manuscript and valuable comments.
Funding
This article was supported in part by grant no. NSh-16.120.11.6383 from The President of Russian Federation and grant no. 11–04–00347а from the Russian Foundation for Basic Research.
References
- Bartke A, Chandrashekar V, Dominici F, Turyn D, Kinney B, Steger R, Kopchick JJ. Insulin-like growth factor 1 (IGF-1) and aging: controverses and new insights. Biogerontology 2003; 4:1-8; PMID:12652183; http://dx.doi.org/10.1023/A:1022448532248
- Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol 2013; 87:201-23; PMID:23434537; http://dx.doi.org/10.1016/j.critrevonc.2013.01.005
- Yaun R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, et al. Aging in inbred stains of mice: study design and interim report of median lifespans and circulating IGF1 levels. Aging Cell 2009; 8:277-87; PMID:19627267; http://dx.doi.org/10.1111/j.1474-9726.2009.00478.x
- Williams G. Pleiotropy, natural selection, and evolution of senescence. Evolution 1957; 11:398-411; http://dx.doi.org/10.2307/2406060
- Levin BE. Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Philos Trans R Soc London B Biol Ser 2006; 361:1107-21; http://dx.doi.org/10.1098/rstb.2006.1851
- Nugent BM, Tobet SA, Lara HE, Lucion AB, Wilson ME, Rexabarren SE, Paredes AH. Hormonal programming across the life span. Horm Metab Res 2012; 44:577-86; PMID:22700441; http://dx.doi.org/10.1055/s-0032-1312593
- Vickers MH. Early life nutrition, epigenetics and programming of late life disease. Nutrients 2014; 6.16502178; http://dx.doi.org/10.3390/nu6062165
- Barker DJ. The fetal and infant origin of adult disease. BMJ 1990; 3901:1111; http://dx.doi.org/10.1136/bmj.301.6761.1111
- Hadley EC, Dutta C, Finkelstein J, Harris TB, Lane MA, Roth GS, Sherman SS, Starke-Reed PE. Human implications of caloric restriction's effect on laboratory animals: an overview of opportunities for research. J Gerontol Ser A 2001; 56A (Special issue I):5-6; http://dx.doi.org/10.1093/gerona/56.suppl_1.5
- Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R. Calorie restriction mimetics: an emerging research field. Aging Cell 2006; 5:97-108; PMID:16626389; http://dx.doi.org/10.1111/j.1474-9726.2006.00202.x
- Mattson MP, Duan W, Lee J, Guo Z, Roth GS, Ingram DK, Lane MA. Progress in the development of caloric restriction mimetic dietary supplements. J Anti-Aging Med 2001; 4:225-32; http://dx.doi.org/10.1089/109454501753249993
- Spindler SR. Caloric restriction: from soup to nuts. Ageing Res Rev 2010; 9:324-53; PMID:19853062; http://dx.doi.org/10.1016/j.arr.2009.10.003
- Dilman VM. Development, Aging and Disease. A New Rationale for an Intervention. Chur: Harwood Academic Publishers 1994.
- Dilman VM, Anisimov VN. Effect of treatment with phenformin, dyphenylhydantoin or L-DOPA on life span and tumor incidence in C3HSn mice. Gerontology 1980; 26:241-5; PMID:7390164; http://dx.doi.org/10.1159/000212423
- Anisimov VN. Carcinogenesis and Aging. Vol. 2. Boca Raton: CRC Press, 1987.
- Anisimov VN, Semenchenko AV, Yashin AI. Insulin and longevity: antidiabetic biguanides as geroprotectors. Biogerontology 2003; 4:297-307; PMID:14618027; http://dx.doi.org/10.1023/A:1026299318315
- Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug Discov Today 2007; 12:218-24; PMID:17331886; http://dx.doi.org/10.1016/j.drudis.2007.01.004
- Blagosklonny MV. Prevention of cancer by inhibiting aging. Cancer Biol Ther 2008; 7:1520-4; PMID:18769112; http://dx.doi.org/10.4161/cbt.7.10.6663
- Berstein LM. Biguanides: An Expansion to Practical Oncology (past and present). St.Petersburg: Aesculap, 2010.
- Kruse JA. Review: metformin does not increase risk for lactic acidosis or increase lactate levels in type 2 diabetes. ACP J Club 2004; 141:7; PMID:15230555
- Nisbet JC, Strurtevant JM, Prins JB. Metformin and serious adverse effects. Med J Aust 2004; 180:53-4; PMID:14723582
- Vidal-Alaball J, Butler CC. Reduced serum vitamin B-12 in patients taking metformin. BMJ 2010; 340:c2198; PMID:20488912; http://dx.doi.org/10.1136/bmj.c2198
- Krentz AJ, Ferner RE, Balley CJ. Comparative tolerability profiles of oral antidiabetic agents. Drug Safety 1994; 11:223-41; PMID:7848543; http://dx.doi.org/10.2165/00002018-199411040-00002
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2neu transgenic mice. Exp Gerontol 2005; 40:685-93; PMID:16125352; http://dx.doi.org/10.1016/j.exger.2005.07.007
- Anisimov VN, Egormin PA, Piskunova TS, Popovich IG, Tyndyk ML, Yurova MV, Zabezhinski MA, Anikin IV, Karkach AS, Romanyukha AA. Metformin extends life span of HER-2neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle 2010; 9:188-97; PMID:20016287; http://dx.doi.org/10.4161/cc.9.1.10407
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MN, Kovalenko IG, Poroshina TE, et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle 2008; 7:2769-73; PMID:18728386; http://dx.doi.org/10.4161/cc.7.17.6625
- Smith DL, Elam CF, Mattison JA, Lane MA, Roth GS, Ingram DK, Allison DB. Metformin supplementation and life span in Fischer-344 rats. J Gerontol Biol Sci 2010; 65A:468-74; http://dx.doi.org/10.1093/gerona/glq033
- Anisimov VN, Piskunova TS, Popovich IG Zabezhinski MA, Tyndyk ML, Egormin PA, Yurova MV, Rosenfeld SV, Semenchenko AV, Kovalenko IG, et al. Gender differences in metformin effects on aging, life span and spontaneous tumorigenesis in 129Sv mice. Aging (Albany, NY) 2010; 2:945-57; PMID:21164223
- Cox DR, Oakes D. Analysis of Survival Data. London: Chapman & Hall, 1996.
- Piskunova TS, Yurova MN, Ovsjannikov AI, Semenchenko AV, Zabezhinski MA, Popovich IG, Wang Z-Q, Anisimov VN. Deficiency in poly(ADP-ribose) polymerase-1 (PARP-1) accelerates aging and spontaneous carcinogenesis in mice. Current Gerontol Geriatr Res 2008; 754190:11; http://dx.doi.org/10.1155/2008/754190; PMID:19415146
- Quin BJ, Dallos M, Kitagawa H, Kunnumakkara AB, Memmott RM, Hollander MC, Gills JJ, Dennis PA. Inhibition of lung tumorigenesis by metformin is associated with decreased plasma IGF-I and diminished receptor tyrosine kinase signaling. Cancer Prev Res (Phila) 2013; 6:801-10; PMID:23771523; http://dx.doi.org/10.1158/1940-6207.CAPR-13-0058-T
- Salomaki H, Vahatal LH, Laurila K, Jappinen NT, Penmttinen A-M, Ailanen L, Ilyazadeh J, Pesonen U, Koulu M. Prenatal metformin exposure in mice programs the metabolic phenotype of the offspring during a high fat diet at adulthood. PLoS One 8(2):e56594; PMID:23457588; http://dx.doi.org/10.1371/journal.pone.0056594
- Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice.FASEB J 2010; 24:5073-9; http://dx.doi.org/10.1096/fj.10-163253
- Anisimov VN. Metformin for aging and cancer prevention. Aging (Albany NY) 2010; 2:760-74; PMID:21084729
- Anisimov VN. Metformin: do we finally have an antiaging drug? Cell Cycle 2013; 12:3483-9; PMID:24189526; http://dx.doi.org/10.4161/cc.26928
- Anisimov VN. Do metformin a real anticarcinogen? A critical reappraisal of experimental data. Ann Transl Med 2014; 2(6); http://dx.doi.org/10.3978/j.issn.2305-5839.2014.06.02
- Martin-Castillo B, Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Metformin and cancer. Doses, mechanisms and the dandelion and hermetic phenomena. Cell Cycle 2010; 9:1057-64; PMID:20305377; http://dx.doi.org/10.4161/cc.9.6.10994
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun 2013; 4:2192; PMID:23900241; http://dx.doi.org/10.1038/ncomms3192
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogenous mice. Nature 2009; 460:392-6; PMID:19587680
- Selman C, Tullet JMA, Wieser D, Irvine E, Lingard SJ, Cloudhury AI, Claret M, Al-Quassab H, Carmignac D, Ramadani F, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 2009; 326:140-4; PMID:19797661; http://dx.doi.org/10.1126/science.1177221
- Awartani, KA, Cheung AP. Metformin and polycystic ovary syndrome: a literature review. J Obstet Gynecol Can 2002; 24:393-401.
- Orecchioni S, Reggiani F, Talarico G, Mancuso P, Calleri A, Gregato G, Labanca V, Noonan DM, Dallaglio K, Albini A, et al. The biguanides metformin and phenformin inhibit angiogenesis, local and metastatic growth of breast cancer by targeting both neoplastic and microenvironment cells. Int J Cancer 2014; Sep 6. PMID:25196138; http://dx.doi.org/10.1002/ijc.29193
- Parkhitko AA, Favorova OO, Khabibullin DI, Anisimov VN, Henske EP. Kinase mTOR: regulation and role in maintenance of cellular homeostasis, tumor development, and aging. Biochemistry (Moscow) 2014; 79(2):88-101; http://dx.doi.org/10.1134/S0006297914020023
- Menendez JA, Joven J. Energy metabolism and metabolic sensors in stem cells: the metabolistem crossroads of aging and cancer. Adv Exp Med Biol 2014; 824:117-39; PMID:25038997; http://dx.doi.org/10.1007/978-3-319-07320-0_10
- Andrzejewski S, Gravel S-P, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab 2014; 2:12; PMID:25184038; http://dx.doi.org/10.1186/2049-3002-2-12
- Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Mechanistic or mammalian target of rapamycin (mTOR) may determine robustness in young male mice at the cost of accelerated aging. Aging (Albany NY).2012; 4(12):899-916; PMID:23443503
- Blagosklonny MV. Big mice die young but large animals live longer. Aging (Albany NY) 2013; 5(4):227-33; PMID:23603822
- Blagosklonny MV. M(o)TOR of aging: MTOR as a universal molecular hypothalamus. Aging (Albany NY) 2013; 5(7):490-4; PMID:23872658
- Anisimov VN, Popovich IG, Zabezhinski MA. Methods of evaluating the effect of of pharmacological drugs on aging and life span in mice. Methods Mol Biol 2007; 371:227-36; PMID:17634585; http://dx.doi.org/10.1007/978-1-59745-361-5_17
- Turusov VS, Mohr U (Eds) Pathology of Tumours in Laboratory Animals. Volume 2. Tumours of the Mouse (2nd ed). IARC Sci Publ 111, Lyon: IARC 1994.
- Goubler EV. Computing Methods of Pathology Analysis and Recognition. Leningrad: Meditsina, 1978.
- Fletcher R. Practical Methods of Optimization (2nd ed.). New York: Wiley 1987.
- Davison AC, Hinkley DV. Bootstrap Methods and Their Application, Cambridge: Cambridge University Press, 1997.
- Cox D. Regression models and life-tables (with discussion). J Royal Stat Soc Series B (Methodological) 1972; 34:187-220.